-
Name
alpha-Methylcinnamaldehyde
- EINECS 202-938-8
- CAS No. 101-39-3
- Article Data50
- CAS DataBase
- Density 1.015 g/cm3
- Solubility Insoluble in water, but soluble in organic solvents such as ethanol.
- Melting Point
- Formula C10H10O
- Boiling Point 254.604 °C at 760 mmHg
- Molecular Weight 146.189
- Flash Point 90.278 °C
- Transport Information
- Appearance yellow liquid
- Safety 24/25
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Cinnamaldehyde,a-methyl- (6CI,7CI,8CI);2-Methyl-3-phenyl-2-propen-1-al;2-Methyl-3-phenyl-2-propenal;2-Methyl-3-phenylacrolein;2-Methyl-3-phenylacrylaldehyde;2-Methyl-3-phenylpropenal;2-Methylcinnamic aldehyde;Methylcinnamaldehyde;NSC22283;NSC 49286;a-Methylcinnamic aldehyde;alpha-methyl cinnamaldehyde;alpha-Methylcinnamaldehyde;
- PSA 17.07000
- LogP 2.28880
Synthetic route

| Conditions | Yield |
|---|---|
| With potassium phosphate; carbon dioxide In dimethyl sulfoxide at 90℃; for 48h; | 92% |
| With 2,6-dicarboxypyridinium chlorochromate In acetonitrile for 0.366667h; Heating; | 86% |
| With trifluorormethanesulfonic acid; 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione In 1,4-dioxane at 20℃; for 0.433333h; | 85% |

| Conditions | Yield |
|---|---|
| With Wilkinson's catalyst; boron trifluoride diethyl etherate In toluene at 30 - 80℃; Inert atmosphere; | 90% |

| Conditions | Yield |
|---|---|
| potassium fluoride on basic alumina In PEG400 at 30℃; for 4h; | 89% |
| With KF-Al2O3 In PEG-400 at 30℃; for 4h; | 89% |
| With sodium hydroxide at 0 - 20℃; for 6h; | 89% |

| Conditions | Yield |
|---|---|
| With N,N,N,N,-tetramethylethylenediamine; α,α′-bis(2-pyridyl(tert-butyl)phosphino)-o-xylene; hydrogen; palladium diacetate In toluene at 60℃; under 3750.38 Torr; for 16h; Autoclave; | 89% |
-
-
78-85-3
2-methylpropenal

-
-
591-50-4
iodobenzene

-
A
-
838-87-9
α-benzyl-cinnamaldehyde

-
B
-
101-39-3
2-methyl-3-phenyl-2-propenal

| Conditions | Yield |
|---|---|
| With 3-amino-3-methylbutanoic acid; palladium diacetate; silver trifluoroacetate In acetic acid at 80℃; for 24h; Reagent/catalyst; Schlenk technique; Inert atmosphere; | A 45% B 13% |


-
-
101-39-3
2-methyl-3-phenyl-2-propenal

| Conditions | Yield |
|---|---|
| With 4-acetylamino-2,2,6,6-tetramethylpiperidine-1-oxoammonium tetrafluoroborate In acetonitrile at 40℃; for 72h; | 44% |
-
-
63163-76-8
(E)-1--2-methyl-1-propen

-
-
774-48-1
(diethoxymethyl)benzene

-
-
101-39-3
2-methyl-3-phenyl-2-propenal

| Conditions | Yield |
|---|---|
| With titanium tetrachloride In dichloromethane at -78℃; for 16h; | 30% |
| With titanium tetrachloride In dichloromethane at -78℃; for 5h; Product distribution; also reaction with benzaldehyde; | 30% |
-
-
60218-91-9
3-hydroxy-2-methyl-3-phenyl-propionaldehyde

-
-
101-39-3
2-methyl-3-phenyl-2-propenal

| Conditions | Yield |
|---|---|
| With sodium acetate at 120 - 130℃; |
-
-
35811-93-9
3-chloro-2-methylcinnamaldehyde

-
-
101-39-3
2-methyl-3-phenyl-2-propenal

| Conditions | Yield |
|---|---|
| With zinc In ethanol |
-
-
7422-93-7
1,1-dimethyl-2-propylidenehydrazine

-
-
100-52-7
benzaldehyde

-
-
101-39-3
2-methyl-3-phenyl-2-propenal

| Conditions | Yield |
|---|---|
| Multistep reaction; |

| Conditions | Yield |
|---|---|
| (i) BF3-Et2O, (ii) aq. HCl; Multistep reaction; |
-
-
50259-54-6
4,4,6-trimethyl-2-(1-methyl-2-phenyl-vinyl)-5,6-dihydro-4H-[1,3]oxazine

-
-
101-39-3
2-methyl-3-phenyl-2-propenal

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate |
-
-
61668-36-8
bromo-2 trimethylsilyloxy-1 propene-1

-
-
100-52-7
benzaldehyde

-
A
-
32398-66-6
1-phenylbut-2-yn-1-ol

-
B
-
101-39-3
2-methyl-3-phenyl-2-propenal

| Conditions | Yield |
|---|---|
| With hydrogenchloride; tert.-butyl lithium 1.) THF, pentane, -70 deg C, 14.5 h; 2.) THF, pentane, -70 deg C, 20 min, then 0 deg C, 20 min; 3) THF, pentane, water 0 deg C, 30 min; Yield given. Multistep reaction. Yields of byproduct given; |

-
-
100-52-7
benzaldehyde

-
-
82215-51-8
4-((E)-2-Phenylsulfanyl-propenyl)-morpholine

-
-
101-39-3
2-methyl-3-phenyl-2-propenal

| Conditions | Yield |
|---|---|
| With ammonia; lithium 1.) ether, THF, -65 deg C, 15 min, 2.) ether, THF, -65 deg C, 1 h; Yield given. Multistep reaction; |
-
-
64-17-5
ethanol

-
-
7732-18-5
water

-
-
100-52-7
benzaldehyde

-
-
123-38-6
propionaldehyde

-
-
101-39-3
2-methyl-3-phenyl-2-propenal


-
-
100-52-7
benzaldehyde

-
-
123-38-6
propionaldehyde

-
A
-
101-39-3
2-methyl-3-phenyl-2-propenal

-
B
-
65-85-0
benzoic acid

-
-
60218-91-9
3-hydroxy-2-methyl-3-phenyl-propionaldehyde

-
-
101-39-3
2-methyl-3-phenyl-2-propenal

| Conditions | Yield |
|---|---|
| at 120 - 130℃; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: (i) NaH, 1,2-dimethoxy-ethane, (ii) /BRN= 471223/ 2: NaBH4 View Scheme | |
| Multi-step reaction with 2 steps 1: (i) KOtBu, THF, (ii) /BRN= 471223/ 2: NaBH4 View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: POCl3 2: Zn / ethanol View Scheme | |
| Multi-step reaction with 2 steps 1: lithium hexamethyldisilazane / tetrahydrofuran / -78 - 20 °C / Inert atmosphere 2: palladium diacetate; N,N,N,N,-tetramethylethylenediamine; α,α′-bis(2-pyridyl(tert-butyl)phosphino)-o-xylene; hydrogen / toluene / 16 h / 60 °C / 3750.38 Torr / Autoclave View Scheme |
-
-
1504-55-8
2-methyl-3-phenylprop-2-en-1-ol

-
A
-
1197-33-7
2-methyl-3-phenyl-acrylonitrile

-
B
-
101-39-3
2-methyl-3-phenyl-2-propenal

| Conditions | Yield |
|---|---|
| With ammonia; oxygen In tetrahydrofuran at 120℃; under 4560.31 Torr; for 5h; |
-
-
1504-55-8, 39924-63-5, 55131-20-9
(E)-3-phenyl-2-methyl-2-propenol

-
-
101-39-3
2-methyl-3-phenyl-2-propenal

| Conditions | Yield |
|---|---|
| With silica gel; palladium at 90℃; for 24h; chemoselective reaction; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: pyrrolidine; water; trifluoroacetic acid / acetonitrile / 48 h / 50 °C / Inert atmosphere 2: acetonitrile / 12 h / 20 °C / Inert atmosphere View Scheme |
-
-
86129-99-9
α-Methylcinnamaldehyde N-phenylimine

-
A
-
101-39-3
2-methyl-3-phenyl-2-propenal

-
B
-
62-53-3
aniline

| Conditions | Yield |
|---|---|
| With (R)-1,1'-binaphthyl-2,2'-phosphoric acid; yttrium(III) trifluoromethanesulfonate In 1,2-dichloro-ethane at 20℃; for 18h; |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water; dimethyl sulfoxide at 25 - 28℃; for 1h; pH=9.5; | A n/a B 100 mg |
-
-
100-52-7
benzaldehyde

-
-
123-38-6
propionaldehyde

-
A
-
60218-91-9
3-hydroxy-2-methyl-3-phenyl-propionaldehyde

-
B
-
101-39-3
2-methyl-3-phenyl-2-propenal

| Conditions | Yield |
|---|---|
| With 4-oxalocrotonate tautomerase from Pseudomonasputida mt-2, F50V mutant In aq. phosphate buffer at 20℃; for 5h; pH=7.6; Catalytic behavior; Time; Aldol Condensation; Enzymatic reaction; |

-
-
1199-77-5
α-methylcinnamic acid

-
A
-
1504-55-8
2-methyl-3-phenylprop-2-en-1-ol

-
B
-
101-39-3
2-methyl-3-phenyl-2-propenal

| Conditions | Yield |
|---|---|
| With D-Glucose; Bacillus subtilis glucose dehydrogenase; Segniliparus rugosus carboxylic acid reductase; nicotinamide adenine dinucleotide phosphate; dimethyl sulfoxide; ATP; magnesium chloride In aq. phosphate buffer at 30℃; for 18h; pH=7.5; Enzymatic reaction; |
-
-
30457-88-6
2-benzyl-2-propenal

-
-
101-39-3
2-methyl-3-phenyl-2-propenal

| Conditions | Yield |
|---|---|
| With palladium on silica; hydrogen at 80℃; under 375.038 Torr; Reagent/catalyst; Pressure; Flow reactor; |
alpha-Methylcinnamaldehyde Consensus Reports
alpha-Methylcinnamaldehyde Specification
The alpha-Methylcinnamaldehyde, with the CAS registry number 101-39-3, is also known as 3-Phenyl-2-methyl acrolein. It belongs to the product categories of Aldehydes; C10 to C21; Carbonyl Compounds. Its EINECS number is 202-938-8. This chemical's molecular formula is C10H10O and molecular weight is 146.19. What's more, its systematic name is 2-Methyl-3-phenylacrylaldehyde. Its classification code is Skin / Eye Irritant. This chemical should be sealed and stored in a cool and ventilated place. Moreover, it should be protected from heat and fire. It has been used in essence for household and personal care chemicals.
Physical properties of alpha-Methylcinnamaldehyde are: (1)ACD/LogP: 2.319; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 2.32; (4)ACD/LogD (pH 7.4): 2.32; (5)ACD/BCF (pH 5.5): 34.05; (6)ACD/BCF (pH 7.4): 34.05; (7)ACD/KOC (pH 5.5): 434.82; (8)ACD/KOC (pH 7.4): 434.82; (9)#H bond acceptors: 1; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 2; (12)Polar Surface Area: 17.07 Å2; (13)Index of Refraction: 1.563; (14)Molar Refractivity: 46.805 cm3; (15)Molar Volume: 144.036 cm3; (16)Polarizability: 18.555×10-24cm3; (17)Surface Tension: 36.9 dyne/cm; (18)Density: 1.015 g/cm3; (19)Flash Point: 90.278 °C; (20)Enthalpy of Vaporization: 49.204 kJ/mol; (21)Boiling Point: 254.604 °C at 760 mmHg; (22)Vapour Pressure: 0.017 mmHg at 25°C.
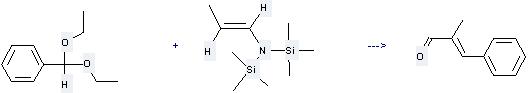
Preparation: this chemical can be prepared by N,N-bis(trimethylsilyl)-(E)-propenylamine and diethoxymethyl-benzene at the temperature of -78 °C. This reaction will need reagent TiCl4 and solvent CH2Cl2 with the reaction time of 16 hours. The yield is about 30%.
Uses of alpha-Methylcinnamaldehyde: it can be used to produce 2-methyl-3-phenyl-acrylonitrile by heating. It will need reagents NH2OH·HCl, NaI and solvent acetonitrile with the reaction time of 100 min. The yield is about 75%.

When you are using this chemical, please be cautious about it as the following:
This chemical is irritating to eyes, respiratory system and skin. When using it, you must avoid contact with skin and eyes.
You can still convert the following datas into molecular structure:
(1)SMILES: O=CC(=Cc1ccccc1)C
(2)Std. InChI: InChI=1S/C10H10O/c1-9(8-11)7-10-5-3-2-4-6-10/h2-8H,1H3
(3)Std. InChIKey: VLUMOWNVWOXZAU-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| rabbit | LD50 | skin | > 5gm/kg (5000mg/kg) | Food and Cosmetics Toxicology. Vol. 13, Pg. 853, 1975. | |
| rat | LD50 | oral | 2050mg/kg (2050mg/kg) | Food and Cosmetics Toxicology. Vol. 13, Pg. 853, 1975. |
Related Products
- alpha-Methylcinnamaldehyde
- 10139-47-6
- 101395-04-4
- 10139-58-9
- 10139-84-1
- 101398-43-0
- 101403-24-1
- 1014-05-7
- 10140-65-5
- 10140-87-1
- 10141-00-1
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View