Hunan Wistar Imp. & Exp. Co., Ltd.
The company serves as a key global supplier of statins intermediates, which has a solid industrial foundation in the field of statins for lipid-lowering drugs, and holds a leading position in the market. Leveraging extensive experience in research an
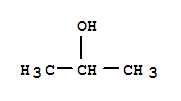
Cas:125971-95-1
Min.Order:25 Kilogram
Negotiable
Type:Trading Company
inquiryZhejiang Haizhou Pharmaceutical Co., Ltd.
Cas:125971-95-1
Min.Order:0
Negotiable
Type:Trading Company
inquiryHangzhou Dingyan Chem Co., Ltd
Items Standard Result Appearance A white or almost white crystalline powder A white crystalline powder Water
Cas:125971-95-1
Min.Order:1 Gram
FOB Price: $100.0 / 500.0
Type:Manufacturers
inquirySimagchem Corporation
Welcome to Simagchem, your partner in China as a premier supply of bulk specialty chemicals for industry and life science. We introduce experienced quality product and exceptional JIT service with instant market intelligence in China to benefit our
Cas:125971-95-1
Min.Order:0 Metric Ton
Negotiable
Type:Manufacturers
inquirySinoway Industrial Co., Ltd.
Assay: 99% up; Stable supply with over 50Mt annually; Appearance:white powder Storage:room temprature Package:25kg/drum Application:Intermediate of Atrovastatin Calcium Port:Beijing/Shanghai/Shenzhen/Hangzhou
Cas:125971-95-1
Min.Order:1 Kilogram
FOB Price: $75.0 / 85.0
Type:Trading Company
inquiryHebei yanxi chemical co.,LTD.
Sleeping pills and stability.This product is white or white crystalline powder;Odourless and slightly bitter taste.Almost insoluble in water, soluble in hydrochloric acid.In acid or alkali and heat hydrolysis, oral drug under the action of gastric
Cas:125971-95-1
Min.Order:10000 Gram
FOB Price: $1300.0
Type:Manufacturers
inquiryHenan Allgreen Chemical Co.,Ltd
high quality Appearance:White or off-white Solid Storage:Sealed, dry, microtherm , avoid light and smell. Package:According to the demand of customer Application:Organic synthesis Transportation:by air or by sea Port:shanghai
Cas:125971-95-1
Min.Order:1 Kilogram
Negotiable
Type:Manufacturers
inquiryAlity Chemical Corporation
The above product is Ality Chemical's strong item with best price, good quality and fast supply. Ality Chemical has been focusing on the research and production of this field for over 14 years. At the same time, we are always committed to providi
Cas:125971-95-1
Min.Order:1
Negotiable
Type:Other
inquiryXi'an Xszo Chem Co., Ltd.
1. Factory price and high quality must be guaranteed, base on 8 years of production and R&D experience2. Free samples will be provided,ensure specifications and quality are right for customer3. Customers will receive the most professional technical s
Cas:125971-95-1
Min.Order:1 Gram
FOB Price: $0.1
Type:Manufacturers
inquiryDayang Chem (Hangzhou) Co.,Ltd.
As a leading manufacturer and supplier of chemicals in China, DayangChem not only supply popular chemicals, but also DayangChem’s R&D center offer custom synthesis services. DayangChem can provide different quantities of custom synthesis
Cas:125971-95-1
Min.Order:1 Kilogram
FOB Price: $3.0
Type:Lab/Research institutions
inquiryChemwill Asia Co., Ltd.
Our main production base is located in Xuzhou industry park. We are certified both to the ISO 9001 and ISO 14001 Standards, have a safety management system in place.Our R&D team masters core technology for process-design of target building block
Cas:125971-95-1
Min.Order:5 Kiloliter
FOB Price: $1.2 / 5.0
Type:Manufacturers
inquiryHubei DiBo chemical co., LTD
Name:Atorvastatin Acetonide tert-Butyl Ester CAS no:125971-95-1 Grade:Medical, scientific research, export Molecular formula:C40H47FN2O5 Molecular weight:654.81 Product Quality 12 years of chemical raw materials Mature operation of the indu
Cas:125971-95-1
Min.Order:25 Kilogram
FOB Price: $1.0 / 3.0
Type:Other
inquiryHenan Tianfu Chemical Co., Ltd.
Product Name: tert-Butyl (4R,6R)-2-[[[6-(2-4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)pyrrol-1-yl]ethyl]-2,2-dimethyl-1,3-dioxan-4-yl]acetate Synonyms: tert-Butyl 2-((4R,6R)-6-(2-(2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenyl-cara
Cas:125971-95-1
Min.Order:1 Metric Ton
FOB Price: $480.0
Type:Lab/Research institutions
inquiryLeader Biochemical Group
About Product Technical Details
Cas:125971-95-1
Min.Order:1 Kilogram
FOB Price: $1.0 / 3.0
Type:Lab/Research institutions
inquiryHebei Nengqian Chemical Import and Export Co., LTD
Our advantages: 1. All inquiries will be replied within 12 hours. 2. Dedication to quality, supply & service. 3. Strictly on selecting raw materials. 4. Reasonable & competitive price, fast lead time. 5. Sample is available for your eva
Cas:125971-95-1
Min.Order:1 Kilogram
FOB Price: $9.0 / 99.0
Type:Trading Company
inquiryWuhan Circle Star Chem-medical Technology co.,Ltd.
1,we produce and sell good chemicals around the world. 2,our success rate is about 95%. this means, if customer order is accepted, the probability that the customer will obtain the ordered substances, is 95%. 3,our staff consists of highly qualified
Cas:125971-95-1
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryZhuozhou Wenxi import and Export Co., Ltd
WITH US,YOUR MONEY IN SAFE,YOUR BUSINESS IN SAFE 1)Quick Response Within 12 hours; 2)Quality Guarantee: All products are strictly tested by our QC, confirmed by QA and approved by third party lab in China, USA, Canada, Germany, UK, Italy, France et
Cas:125971-95-1
Min.Order:1 Kilogram
FOB Price: $139.0 / 210.0
Type:Trading Company
inquiryWuhan Han Sheng New Material Technology Co.,Ltd
Our Advantage: high quality with competitive price High quality standard: BP/USP/EP Enterprise standard All purity customized Fast and safe delivery We have reliable forwarder who can help us deliver our goods more fast and safe. We
Cas:125971-95-1
Min.Order:10 Kilogram
FOB Price: $100.0 / 120.0
Type:Trading Company
inquiryKono Chem Co.,Ltd
Product Name: tert-Butyl (4R,6R)-2-[[[6-(2-4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)pyrrol-1-yl]ethyl]-2,2-dimethyl-1,3-dioxan-4-yl]ace…Appearance:Solid powder Storage:Sealed,light and oxygen resistant Package:aluminum foil bag,carton
Cas:125971-95-1
Min.Order:0
Negotiable
Type:Other
inquiryHenan Sinotech Import&Export Corporation
Prouduct name: Atorvastatin intermediate L-1 CAS: 125971-95-1 Appearance: white to off-white crystalline powder Purity: 99.0%min Appearance: white powder Storage:Store in cool and dry place, away from sun light. Package: 25kg per bag or 2
Cas:125971-95-1
Min.Order:1 Kilogram
FOB Price: $1.0
Type:Other
inquiryHangzhou Sartort Biopharma Co., Ltd
Appearance:White powder Storage:R.T Package:25kg/Barrel Application:Avastatin calcium intermediate Transportation:Express/Sea/Air Port:Any port in China
Cas:125971-95-1
Min.Order:1 Kilogram
FOB Price: $1.0
Type:Lab/Research institutions
inquiryHubei Langyou International Trading Co., Ltd
1. Guaranteed purity; 2. Large quantity in stock; 3. Largest manufacturer; 4. Best service after shipment with email; 5. High quality & competitive price; Appearance:white powder Storage:Store in sealed containers at cool & dr
Cas:125971-95-1
Min.Order:100 Gram
Negotiable
Type:Other
inquiryShanghai Upbio Tech Co.,Ltd
1.No Less 8 years exporting experience. Clients can 100% received goods 2.Lower Price with higher quality 3,Free sample 4,We are sincerely responsible for the "product quality" and "After Service" Upbio is Specialized
Cas:125971-95-1
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryHANWAYS CHEMPHARM CO.,LIMITED
Hanways Chempharm Co., Limited, the former is Hubei Hanways Pharchem CO.,Limited, set up in 2009 in Wuhan, China. We specialize in sourcing and supplying APIs, pharmaceutical intermediates, and fine chemicals for worldwide markets. The founder has d
Cas:125971-95-1
Min.Order:1 Kilogram
FOB Price: $1.0
Type:Trading Company
inquiryHangzhou Huarong Pharm Co., Ltd.
Hangzhou Huarong Pharm Co., Ltd. established since 2009 , has been always focusing on supplying products and services to our clients in the field of small molecule drug. Huarong Pharm has built platforms for the research, development and manufac
Cas:125971-95-1
Min.Order:1 Gram
FOB Price: $1.0 / 2.0
Type:Lab/Research institutions
inquiryQingdao Beluga Import and Export Co., LTD
Beluga chemical professional supply (+/-)-2,5-dimethoxy-4-bromo-amphetamine hydrobromide CAS 53581-53-6 Why choose Beluga chemical 1. Beluga Chemical has a professional RESEARCH and development team and strong technical force to ensure technical
Cas:125971-95-1
Min.Order:1 Gram
Negotiable
Type:Lab/Research institutions
inquiryShandong Hanjiang Chemical Co., Ltd.
Hello, dear friend! I'm Hansen and Allen from China. Welcome to my lookchem mall! The following is a brief introduction of our company's products and services. If you are interested in our products, please contact us by emai
Cas:125971-95-1
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryHebei Mojin Biotechnology Co.,Ltd
Hebei Mojin Biotechnology Co., Ltd, Our company is a professional chemical raw materials and chemical reagents research and development production enterprises. We have several production line,So we can control the lowest price. We also have several
Cas:125971-95-1
Min.Order:25 Gram
FOB Price: $90.0 / 100.0
Type:Trading Company
inquiryWuhan Zenuo Biological Medicine Technology Co Ltd
Product Name: tert-Butyl (4R,6R)-2-[[[6-(2-4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)pyrrol-1-yl]ethyl]-2,2-dimethyl-1,3-dioxan-4-yl]acetate Synonyms: tert-Butyl 2-((4R,6R)-6-(2-(2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenyl-ca
Cas:125971-95-1
Min.Order:100 Gram
Negotiable
Type:Lab/Research institutions
inquiryHenan Wentao Chemical Product Co., Ltd.
Henan Wentao Chemical Product Co.,Ltd is Located in Zhengzhou High-tech Development Zone with import and export license, We passed ISO 9001:2008 as well, Henan Wentao has developed more than 1000 compounds, which are widely used in the fields of prod
Cas:125971-95-1
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquirySynthetic route

-
-
125971-95-1
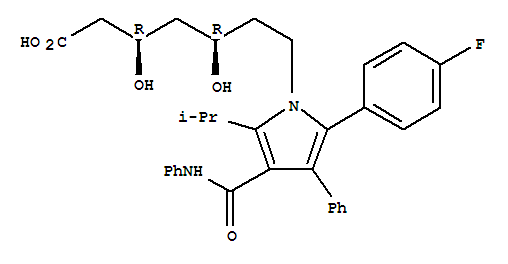
tert-butyl (4R,6R)-6-{2-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)pyrrol-1-yl]ethyl}-2,2-dimethyl-1,3-dioxane-4-acetate

| Conditions | Yield |
|---|---|
| With palladium 10% on activated carbon; hydrogen | 97% |
-
-
125971-86-0, 125995-13-3
tert-butyl [(4R,6R)-6-aminoethyl-2,2-dimethyl-1,3-dioxan-4-yl]acetate

-
-
125971-96-2
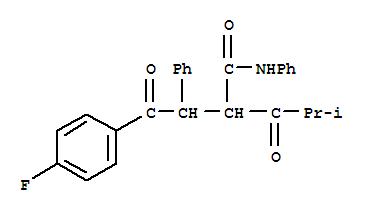
2-[2-(4-fluorophenyl)-2-oxo-1-phenylethyl]-4-methyl-3-oxopentanoic acid phenylamide

-
-
125971-95-1
tert-butyl (4R,6R)-6-{2-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)pyrrol-1-yl]ethyl}-2,2-dimethyl-1,3-dioxane-4-acetate

| Conditions | Yield |
|---|---|
| With Trimethylacetic acid In toluene at 105 - 110℃; for 1h; Industrial scale; | 96.5% |
| With tetra(n-butyl)ammonium hydrogensulfate; diisopropylamine; Trimethylacetic acid at 78 - 85℃; for 40h; Paal-Knorr Pyrrole Synthesis; | 82.3% |
| With Trimethylacetic acid In tetrahydrofuran; n-heptane; toluene Heating; | 75% |
-
-
1331869-19-2
C26H22FNO3

-
-
125971-86-0, 125995-13-3
tert-butyl [(4R,6R)-6-aminoethyl-2,2-dimethyl-1,3-dioxan-4-yl]acetate

-
-
125971-95-1
tert-butyl (4R,6R)-6-{2-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)pyrrol-1-yl]ethyl}-2,2-dimethyl-1,3-dioxane-4-acetate

| Conditions | Yield |
|---|---|
| With 1,8-diazabicyclo[5.4.0]undec-7-ene; Trimethylacetic acid In cyclohexane at 80 - 84℃; for 25h; Concentration; Reagent/catalyst; Paal-Knorr Pyrrole Synthesis; Dean-Stark; Reflux; | 79% |
| With Trimethylacetic acid In tetrahydrofuran; hexane; toluene at 110℃; for 30h; Paal-Knorr pyrrole synthesis; Inert atmosphere; |
-
-
1173184-80-9
tert-butyl 2-((4R,6S)-6-(2-iodoethyl)-2,2-dimethyl-1,3-dioxan-4-yl)acetate

-
-
125971-95-1
tert-butyl (4R,6R)-6-{2-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)pyrrol-1-yl]ethyl}-2,2-dimethyl-1,3-dioxane-4-acetate

| Conditions | Yield |
|---|---|
| With 18-crown-6 ether; potassium carbonate In acetonitrile Reflux; | 65% |

-
-
460-00-4
1-Bromo-4-fluorobenzene

-
-
125971-95-1
tert-butyl (4R,6R)-6-{2-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)pyrrol-1-yl]ethyl}-2,2-dimethyl-1,3-dioxane-4-acetate

| Conditions | Yield |
|---|---|
| With potassium acetate; palladium diacetate at 150℃; for 15h; Heck Reaction; Inert atmosphere; | 44% |
-
-
124401-38-3
4-methyl-3-oxo-N-phenylpentanamide

-
-
125971-95-1
tert-butyl (4R,6R)-6-{2-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)pyrrol-1-yl]ethyl}-2,2-dimethyl-1,3-dioxane-4-acetate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: β-alanine; glacial acetic acid / hexane / Heating 2: 3-ethyl-5-(2-hydroxyethyl)-4-methylthiazolium bromide; triethylamine / ethanol / Heating 3: pivalic acid / tetrahydrofuran; toluene; heptane / Heating View Scheme | |
| Multi-step reaction with 3 steps 1.1: L-proline / Inert atmosphere 2.1: 3,4-dimethyl-5-(2-hydroxyethyl)thiazolium iodide; potassium phosphate / acetonitrile / 16 h / 120 °C / Inert atmosphere; Glovebox; Schlenk technique; Sealed tube 2.2: 4 h / 120 °C / Inert atmosphere; Gas phase; Schlenk technique; Sealed tube 3.1: potassium acetate; palladium diacetate / 15 h / 150 °C / Inert atmosphere View Scheme |
-
-
100-52-7
benzaldehyde

-
-
125971-95-1
tert-butyl (4R,6R)-6-{2-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)pyrrol-1-yl]ethyl}-2,2-dimethyl-1,3-dioxane-4-acetate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: β-alanine; glacial acetic acid / hexane / Heating 2: 3-ethyl-5-(2-hydroxyethyl)-4-methylthiazolium bromide; triethylamine / ethanol / Heating 3: pivalic acid / tetrahydrofuran; toluene; heptane / Heating View Scheme |
-
-
125971-57-5
4-methyl-3-oxo-N-phenyl-2-(phenylmethylene)pentanamide

-
-
125971-95-1
tert-butyl (4R,6R)-6-{2-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)pyrrol-1-yl]ethyl}-2,2-dimethyl-1,3-dioxane-4-acetate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 3-ethyl-5-(2-hydroxyethyl)-4-methylthiazolium bromide; triethylamine / ethanol / Heating 2: pivalic acid / tetrahydrofuran; toluene; heptane / Heating View Scheme | |
| Multi-step reaction with 2 steps 1.1: 3,4-dimethyl-5-(2-hydroxyethyl)thiazolium iodide; potassium phosphate / acetonitrile / 16 h / 120 °C / Inert atmosphere; Glovebox; Schlenk technique; Sealed tube 1.2: 4 h / 120 °C / Inert atmosphere; Gas phase; Schlenk technique; Sealed tube 2.1: potassium acetate; palladium diacetate / 15 h / 150 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 2 steps 1: 3-ethyl-5-(2-hydroxyethyl)-4-methyl-1,3-thiazolium bromide; triethylamine / Reflux 2: Trimethylacetic acid / toluene; hexane; tetrahydrofuran / Reflux View Scheme |
-
-
62-53-3
aniline

-
-
125971-95-1
tert-butyl (4R,6R)-6-{2-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)pyrrol-1-yl]ethyl}-2,2-dimethyl-1,3-dioxane-4-acetate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 96 percent / toluene / Heating 2: β-alanine; glacial acetic acid / hexane / Heating 3: 3-ethyl-5-(2-hydroxyethyl)-4-methylthiazolium bromide; triethylamine / ethanol / Heating 4: pivalic acid / tetrahydrofuran; toluene; heptane / Heating View Scheme |

-
-
125971-95-1
tert-butyl (4R,6R)-6-{2-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)pyrrol-1-yl]ethyl}-2,2-dimethyl-1,3-dioxane-4-acetate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 96 percent / toluene / Heating 2: β-alanine; glacial acetic acid / hexane / Heating 3: 3-ethyl-5-(2-hydroxyethyl)-4-methylthiazolium bromide; triethylamine / ethanol / Heating 4: pivalic acid / tetrahydrofuran; toluene; heptane / Heating View Scheme |
-
-
459-57-4
4-fluorobenzaldehyde

-
-
125971-95-1
tert-butyl (4R,6R)-6-{2-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)pyrrol-1-yl]ethyl}-2,2-dimethyl-1,3-dioxane-4-acetate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 2) NEt3 / 1) ethyl thiazolium catalyst / or with methyl thiazolium catalyst; 1) EtOH 2: 75 percent / pivalic acid / toluene; heptane; tetrahydrofuran / Heating View Scheme | |
| Multi-step reaction with 2 steps 1: 3-ethyl-5-(2-hydroxyethyl)-4-methyl-1,3-thiazolium bromide; triethylamine / Reflux 2: Trimethylacetic acid / toluene; hexane; tetrahydrofuran / Reflux View Scheme |
-
-
125971-94-0
2-((4R, 6R)-6-cyanomethyl-2,2-dimethyl-1,3-dioxan-4-yl)acetic acid tert-butyl ester

-
-
125971-95-1
tert-butyl (4R,6R)-6-{2-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)pyrrol-1-yl]ethyl}-2,2-dimethyl-1,3-dioxane-4-acetate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 95 percent / H2, ammonia / Molybdenum doped Raney nickel / methanol / 2585.7 Torr 2: 75 percent / pivalic acid / toluene; heptane; tetrahydrofuran / Heating View Scheme | |
| Multi-step reaction with 2 steps 1: hydrogen; nickel; ammonia / methanol 2: Acidic conditions View Scheme | |
| Multi-step reaction with 2 steps 1: ammonia; hydrogen / methanol / 6 h / 30 - 40 °C / 2250.23 - 3000.3 Torr / Autoclave 2: Trimethylacetic acid / tetrahydrofuran; n-heptane / 60 h / Reflux View Scheme | |
| Multi-step reaction with 2 steps 1: hydrogen; ammonia / cyclohexane; water / 30 - 40 °C / 3750.38 Torr / Autoclave 2: Trimethylacetic acid; diisopropylamine; tetra(n-butyl)ammonium hydrogensulfate / 40 h / 78 - 85 °C View Scheme |
-
-
805242-36-8
1-[2-((4R,6R)-6-tert-butoxycarbonylmethyl-2,2-dimethyl-[1,3]dioxan-4-yl)ethyl]-5-(4-fluorophenyl)-2-isopropyl-4-phenyl-1H-pyrrole-3-carboxylic acid

-
-
62-53-3
aniline

-
-
125971-95-1
tert-butyl (4R,6R)-6-{2-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)pyrrol-1-yl]ethyl}-2,2-dimethyl-1,3-dioxane-4-acetate

| Conditions | Yield |
|---|---|
| With O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In DMF (N,N-dimethyl-formamide) at 50 - 60℃; Product distribution / selectivity; |

-
-
62-53-3
aniline

-
-
125971-95-1
tert-butyl (4R,6R)-6-{2-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)pyrrol-1-yl]ethyl}-2,2-dimethyl-1,3-dioxane-4-acetate

| Conditions | Yield |
|---|---|
| In benzene at 70℃; Product distribution / selectivity; |

-
-
62-53-3
aniline

-
-
125971-95-1
tert-butyl (4R,6R)-6-{2-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)pyrrol-1-yl]ethyl}-2,2-dimethyl-1,3-dioxane-4-acetate

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In tetrahydrofuran at 55 - 60℃; Product distribution / selectivity; |

-
-
125971-86-0, 125995-13-3
tert-butyl [(4R,6R)-6-aminoethyl-2,2-dimethyl-1,3-dioxan-4-yl]acetate

-
-
125971-95-1
tert-butyl (4R,6R)-6-{2-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)pyrrol-1-yl]ethyl}-2,2-dimethyl-1,3-dioxane-4-acetate

| Conditions | Yield |
|---|---|
| With 2-Methylbutanoic acid In n-heptane; toluene for 22h; Product distribution / selectivity; Heating / reflux; | |
| With Trimethylacetic acid In n-heptane; toluene for 25h; Heating / reflux; | |
| With 2-Methylbutanoic acid In n-heptane; toluene for 48h; Product distribution / selectivity; Heating / reflux; |

-
-
125971-86-0, 125995-13-3
tert-butyl [(4R,6R)-6-aminoethyl-2,2-dimethyl-1,3-dioxan-4-yl]acetate

-
-
125971-95-1
tert-butyl (4R,6R)-6-{2-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)pyrrol-1-yl]ethyl}-2,2-dimethyl-1,3-dioxane-4-acetate

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; n-heptane; oenanthic acid; toluene for 8h; Heating / reflux; |
-
-
914222-70-1
(4R-cis)-1,1-dimethylethyl-[6-(2-aminoethyl)-2,2-dimethyl-1,3-dioxan-4-yl]acetate

-
-
125971-96-2
2-[2-(4-fluorophenyl)-2-oxo-1-phenylethyl]-4-methyl-3-oxopentanoic acid phenylamide

-
-
67-63-0
isopropyl alcohol

-
A
(4R-cis)-1,1-dimethylethyl 6-[2]2-(-fluorophenyl)-5-(1-methylethyl)-3-phenyl-4-[(phenylamino)carbonyl]-1H-pyrrol-1-yl ]ethyl]-2,2-dimethyl-1,3-dioxane-4-acetate -
(4R-cis)-1,1-dimethylethyl 6-[2]2-(-fluorophenyl)-5-(1-methylethyl)-3-phenyl-4-[(phenylamino)carbonyl]-1H-pyrrol-1-yl ]ethyl]-2,2-dimethyl-1,3-dioxane-4-acetate

-
B
-
125971-95-1
tert-butyl (4R,6R)-6-{2-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)pyrrol-1-yl]ethyl}-2,2-dimethyl-1,3-dioxane-4-acetate

| Conditions | Yield |
|---|---|
| In n-heptane; toluene |

-
-
125971-86-0, 125995-13-3
tert-butyl [(4R,6R)-6-aminoethyl-2,2-dimethyl-1,3-dioxan-4-yl]acetate

-
-
125971-96-2
2-[2-(4-fluorophenyl)-2-oxo-1-phenylethyl]-4-methyl-3-oxopentanoic acid phenylamide

-
-
67-63-0
isopropyl alcohol

-
-
125971-95-1
tert-butyl (4R,6R)-6-{2-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)pyrrol-1-yl]ethyl}-2,2-dimethyl-1,3-dioxane-4-acetate

| Conditions | Yield |
|---|---|
| In n-heptane; toluene |

-
-
1105067-89-7
tert-butyl [(4S,6R)-6-(2-aminoethyl)-2,2-dimethyl-1,3-dioxane-4-yl]acetate

-
-
125971-96-2
2-[2-(4-fluorophenyl)-2-oxo-1-phenylethyl]-4-methyl-3-oxopentanoic acid phenylamide

-
A
-
1105067-90-0
tert-butyl (4S,6R)-6-{2-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)pyrrol-1-yl]ethyl}-2,2-dimethyl-1,3-dioxane-4-acetate

-
B
-
125971-95-1
tert-butyl (4R,6R)-6-{2-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)pyrrol-1-yl]ethyl}-2,2-dimethyl-1,3-dioxane-4-acetate

| Conditions | Yield |
|---|---|
| With Trimethylacetic acid In n-heptane; toluene for 19h; Heating; Title compound not separated from byproducts.; |

-
-
125971-86-0, 125995-13-3
tert-butyl [(4R,6R)-6-aminoethyl-2,2-dimethyl-1,3-dioxan-4-yl]acetate

-
-
125971-96-2
2-[2-(4-fluorophenyl)-2-oxo-1-phenylethyl]-4-methyl-3-oxopentanoic acid phenylamide

-
A
-
1116118-82-1
((4R,6R)-6-{2-[2-((4R,6R)-6-{2-[2-(4-fluoro-phenyl)-5-isopropyl-3-phenyl-4-phenylcarbamoyl-pyrrol-1-yl]-ethyl}-2,2-dimethyl-[1,3]dioxan-4-yl)-acetylamino]-ethyl}-2,2-dimethyl-[1,3]dioxan-4-yl)-acetic acid tert-butyl ester

-
B
-
125971-95-1
tert-butyl (4R,6R)-6-{2-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)pyrrol-1-yl]ethyl}-2,2-dimethyl-1,3-dioxane-4-acetate

| Conditions | Yield |
|---|---|
| Stage #1: tert-butyl [(4R,6R)-6-aminoethyl-2,2-dimethyl-1,3-dioxan-4-yl]acetate; 2-[2-(4-fluorophenyl)-2-oxo-1-phenylethyl]-4-methyl-3-oxopentanoic acid phenylamide In toluene at 90 - 100℃; for 1 - 1.5h; Heating / reflux; Stage #2: Trimethylacetic acid In toluene Product distribution / selectivity; Heating / reflux; | |
| Stage #1: tert-butyl [(4R,6R)-6-aminoethyl-2,2-dimethyl-1,3-dioxan-4-yl]acetate; 2-[2-(4-fluorophenyl)-2-oxo-1-phenylethyl]-4-methyl-3-oxopentanoic acid phenylamide In tetrahydrofuran; n-heptane; toluene at 40 - 50℃; for 1 - 1.5h; Heating / reflux; Stage #2: Trimethylacetic acid In tetrahydrofuran; n-heptane; toluene for 35h; Product distribution / selectivity; Heating / reflux; | |
| Stage #1: tert-butyl [(4R,6R)-6-aminoethyl-2,2-dimethyl-1,3-dioxan-4-yl]acetate; 2-[2-(4-fluorophenyl)-2-oxo-1-phenylethyl]-4-methyl-3-oxopentanoic acid phenylamide In tetrahydrofuran; toluene at 40 - 50℃; for 1 - 1.5h; Heating / reflux; Stage #2: Trimethylacetic acid In tetrahydrofuran; toluene for 24h; Product distribution / selectivity; Heating / reflux; |


-
-
125971-86-0, 125995-13-3
tert-butyl [(4R,6R)-6-aminoethyl-2,2-dimethyl-1,3-dioxan-4-yl]acetate

-
-
125971-95-1
tert-butyl (4R,6R)-6-{2-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)pyrrol-1-yl]ethyl}-2,2-dimethyl-1,3-dioxane-4-acetate

| Conditions | Yield |
|---|---|
| Stage #1: +/-(4-fluoro-alpha-(2-methyl-1-oxopropyl)-gama-oxo-N-beta-diphenyl benzenebutaneamine); tert-butyl [(4R,6R)-6-aminoethyl-2,2-dimethyl-1,3-dioxan-4-yl]acetate With Trimethylacetic acid In tetrahydrofuran; n-heptane; toluene for 40 - 50h; Heating / reflux; Stage #2: With sodium hydrogencarbonate In tetrahydrofuran; n-heptane; water; toluene pH=7; Product distribution / selectivity; | |
| Stage #1: +/-(4-fluoro-alpha-(2-methyl-1-oxopropyl)-gama-oxo-N-beta-diphenyl benzenebutaneamine); tert-butyl [(4R,6R)-6-aminoethyl-2,2-dimethyl-1,3-dioxan-4-yl]acetate With Trimethylacetic acid In tetrahydrofuran; hexane; toluene for 40 - 50h; Heating / reflux; Stage #2: With sodium hydrogencarbonate In tetrahydrofuran; hexane; water; toluene pH=7; Product distribution / selectivity; |
-
-
1331869-16-9
(R)-N,N-diallyl-5-(benzyloxy)-3-hydroxypentanethioamide

-
-
125971-95-1
tert-butyl (4R,6R)-6-{2-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)pyrrol-1-yl]ethyl}-2,2-dimethyl-1,3-dioxane-4-acetate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 11 steps 1.1: 2,6-dimethylpyridine / dichloromethane / 3 h / 0 - 20 °C 2.1: diethyl ether / 4.5 h / 0 - 20 °C 3.1: tetrahydrofuran; diethyl ether / -78 °C 3.2: -78 °C 4.1: tetrabutyl ammonium fluoride / tetrahydrofuran / 3.5 h / 0 - 20 °C 5.1: sodium tetrahydroborate; diethyl methoxy borane / tetrahydrofuran; methanol / 10 h / -80 °C 6.1: toluene-4-sulfonic acid / acetone / 4 h / 20 °C 7.1: 20% palladium hydroxide on carbon; hydrogen / ethyl acetate / 24 h / 60 °C / 760.05 Torr 8.1: dmap; triethylamine / dichloromethane / 4 h / 0 - 20 °C 9.1: sodium azide / N,N-dimethyl-formamide / 6 h / 20 °C 10.1: water; triphenylphosphine / tetrahydrofuran / 2 h / 50 °C 11.1: Trimethylacetic acid / tetrahydrofuran; hexane; toluene / 30 h / 110 °C / Inert atmosphere View Scheme |
-
-
1347738-07-1
(R)-N,N-diallyl-5-(benzyloxy)-3-((tert-butyldimethylsilyl)oxy)pentanethioamide

-
-
125971-95-1
tert-butyl (4R,6R)-6-{2-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)pyrrol-1-yl]ethyl}-2,2-dimethyl-1,3-dioxane-4-acetate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 10 steps 1.1: diethyl ether / 4.5 h / 0 - 20 °C 2.1: tetrahydrofuran; diethyl ether / -78 °C 2.2: -78 °C 3.1: tetrabutyl ammonium fluoride / tetrahydrofuran / 3.5 h / 0 - 20 °C 4.1: sodium tetrahydroborate; diethyl methoxy borane / tetrahydrofuran; methanol / 10 h / -80 °C 5.1: toluene-4-sulfonic acid / acetone / 4 h / 20 °C 6.1: 20% palladium hydroxide on carbon; hydrogen / ethyl acetate / 24 h / 60 °C / 760.05 Torr 7.1: dmap; triethylamine / dichloromethane / 4 h / 0 - 20 °C 8.1: sodium azide / N,N-dimethyl-formamide / 6 h / 20 °C 9.1: water; triphenylphosphine / tetrahydrofuran / 2 h / 50 °C 10.1: Trimethylacetic acid / tetrahydrofuran; hexane; toluene / 30 h / 110 °C / Inert atmosphere View Scheme |

-
-
125971-95-1
tert-butyl (4R,6R)-6-{2-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)pyrrol-1-yl]ethyl}-2,2-dimethyl-1,3-dioxane-4-acetate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 9 steps 1.1: tetrahydrofuran; diethyl ether / -78 °C 1.2: -78 °C 2.1: tetrabutyl ammonium fluoride / tetrahydrofuran / 3.5 h / 0 - 20 °C 3.1: sodium tetrahydroborate; diethyl methoxy borane / tetrahydrofuran; methanol / 10 h / -80 °C 4.1: toluene-4-sulfonic acid / acetone / 4 h / 20 °C 5.1: 20% palladium hydroxide on carbon; hydrogen / ethyl acetate / 24 h / 60 °C / 760.05 Torr 6.1: dmap; triethylamine / dichloromethane / 4 h / 0 - 20 °C 7.1: sodium azide / N,N-dimethyl-formamide / 6 h / 20 °C 8.1: water; triphenylphosphine / tetrahydrofuran / 2 h / 50 °C 9.1: Trimethylacetic acid / tetrahydrofuran; hexane; toluene / 30 h / 110 °C / Inert atmosphere View Scheme |
-
-
1331869-20-5
(R)-tert-butyl 7-(benzyloxy)-5-((tert-butyldimethylsilyl)oxy)-3-oxoheptanoate

-
-
125971-95-1
tert-butyl (4R,6R)-6-{2-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)pyrrol-1-yl]ethyl}-2,2-dimethyl-1,3-dioxane-4-acetate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1: tetrabutyl ammonium fluoride / tetrahydrofuran / 3.5 h / 0 - 20 °C 2: sodium tetrahydroborate; diethyl methoxy borane / tetrahydrofuran; methanol / 10 h / -80 °C 3: toluene-4-sulfonic acid / acetone / 4 h / 20 °C 4: 20% palladium hydroxide on carbon; hydrogen / ethyl acetate / 24 h / 60 °C / 760.05 Torr 5: dmap; triethylamine / dichloromethane / 4 h / 0 - 20 °C 6: sodium azide / N,N-dimethyl-formamide / 6 h / 20 °C 7: water; triphenylphosphine / tetrahydrofuran / 2 h / 50 °C 8: Trimethylacetic acid / tetrahydrofuran; hexane; toluene / 30 h / 110 °C / Inert atmosphere View Scheme |

-
-
125971-95-1
tert-butyl (4R,6R)-6-{2-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)pyrrol-1-yl]ethyl}-2,2-dimethyl-1,3-dioxane-4-acetate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: sodium tetrahydroborate; diethyl methoxy borane / tetrahydrofuran; methanol / 10 h / -80 °C 2: toluene-4-sulfonic acid / acetone / 4 h / 20 °C 3: 20% palladium hydroxide on carbon; hydrogen / ethyl acetate / 24 h / 60 °C / 760.05 Torr 4: dmap; triethylamine / dichloromethane / 4 h / 0 - 20 °C 5: sodium azide / N,N-dimethyl-formamide / 6 h / 20 °C 6: water; triphenylphosphine / tetrahydrofuran / 2 h / 50 °C 7: Trimethylacetic acid / tetrahydrofuran; hexane; toluene / 30 h / 110 °C / Inert atmosphere View Scheme |

-
-
125971-95-1
tert-butyl (4R,6R)-6-{2-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)pyrrol-1-yl]ethyl}-2,2-dimethyl-1,3-dioxane-4-acetate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: toluene-4-sulfonic acid / acetone / 4 h / 20 °C 2: 20% palladium hydroxide on carbon; hydrogen / ethyl acetate / 24 h / 60 °C / 760.05 Torr 3: dmap; triethylamine / dichloromethane / 4 h / 0 - 20 °C 4: sodium azide / N,N-dimethyl-formamide / 6 h / 20 °C 5: water; triphenylphosphine / tetrahydrofuran / 2 h / 50 °C 6: Trimethylacetic acid / tetrahydrofuran; hexane; toluene / 30 h / 110 °C / Inert atmosphere View Scheme |
-
-
125971-95-1
tert-butyl (4R,6R)-6-{2-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)pyrrol-1-yl]ethyl}-2,2-dimethyl-1,3-dioxane-4-acetate

-
-
134395-00-9
(3R,5R)-7-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-phenylcarbamoylpyrrol-1-yl]-3,5-dihydroxyheptanoic acid t-butyl ester

| Conditions | Yield |
|---|---|
| With hydrogenchloride; water In acetonitrile for 13h; Product distribution / selectivity; | 98.9% |
| With hydrogenchloride In methanol; water at 50 - 55℃; | 98% |
| With hydrogenchloride In methanol; water at 0 - 30℃; Industrial scale; | 96.7% |
-
-
125971-95-1
tert-butyl (4R,6R)-6-{2-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)pyrrol-1-yl]ethyl}-2,2-dimethyl-1,3-dioxane-4-acetate

-
-
134523-03-8
lipitor

| Conditions | Yield |
|---|---|
| Stage #1: tert-butyl (4R,6R)-6-{2-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)pyrrol-1-yl]ethyl}-2,2-dimethyl-1,3-dioxane-4-acetate With hydrogenchloride; methanol; water at 35℃; for 3h; Stage #2: With calcium hydroxide In methanol; water; toluene at 70℃; for 2h; Stage #3: In methanol; water at 20 - 78℃; for 24.25h; | 96% |
| Stage #1: tert-butyl (4R,6R)-6-{2-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)pyrrol-1-yl]ethyl}-2,2-dimethyl-1,3-dioxane-4-acetate With hydrogenchloride; water In methanol at 15 - 30℃; for 12h; Stage #2: With sodium hydroxide In methanol; water at 25 - 30℃; for 6h; pH=12; Stage #3: With hydrogenchloride; calcium acetate Product distribution / selectivity; more than 3 stages; | 93.94% |
| Stage #1: tert-butyl (4R,6R)-6-{2-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)pyrrol-1-yl]ethyl}-2,2-dimethyl-1,3-dioxane-4-acetate With hydrogenchloride; water In methanol at 15 - 30℃; for 10 - 12h; Stage #2: With sodium hydroxide In methanol; water at 25 - 30℃; for 4 - 6h; pH=~ 12; Stage #3: With hydrogenchloride; calcium acetate Product distribution / selectivity; more than 3 stages; | 86.5% |
-
-
125971-95-1
tert-butyl (4R,6R)-6-{2-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)pyrrol-1-yl]ethyl}-2,2-dimethyl-1,3-dioxane-4-acetate

-
-
340266-37-7
[R-(R*,R*)]-2-(4-fluorophenyl)-β,δ-dihydroxy-5-(1-methylethyl)-3-phenyl-4-[(phenylamino)carbonyl]-1H-pyrrole-1-heptanoic acid; ammonium salt

| Conditions | Yield |
|---|---|
| Stage #1: tert-butyl (4R,6R)-6-{2-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)pyrrol-1-yl]ethyl}-2,2-dimethyl-1,3-dioxane-4-acetate With hydrogenchloride In methanol; water at 50℃; for 10h; Stage #2: With methanol; sodium hydroxide; water at 20 - 60℃; for 10h; Stage #3: With hydrogenchloride; ammonia Product distribution / selectivity; more than 3 stages; | 95% |
-
-
67-56-1
methanol

-
-
125971-95-1
tert-butyl (4R,6R)-6-{2-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)pyrrol-1-yl]ethyl}-2,2-dimethyl-1,3-dioxane-4-acetate

-
-
1353049-81-6
methyl 2-((4R,6R)-6-(2-(2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)-1H-pyrrol-1-yl)ethyl)-2,2-dimethyl-1,3-dioxan-4-yl)acetate

| Conditions | Yield |
|---|---|
| With sodium hydroxide for 7h; Reflux; | 37% |
-
-
125971-95-1
tert-butyl (4R,6R)-6-{2-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)pyrrol-1-yl]ethyl}-2,2-dimethyl-1,3-dioxane-4-acetate

-
-
134523-00-5
atorvastatin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: HCl / tetrahydrofuran; H2O / 20 °C 2: sodium hydroxide / H2O; tetrahydrofuran / 20 °C View Scheme | |
| Stage #1: tert-butyl (4R,6R)-6-{2-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)pyrrol-1-yl]ethyl}-2,2-dimethyl-1,3-dioxane-4-acetate With hydrogenchloride; water In tetrahydrofuran; methanol at 20℃; Stage #2: With sodium hydroxide; water In tetrahydrofuran; methanol at 0 - 20℃; | |
| Stage #1: tert-butyl (4R,6R)-6-{2-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)pyrrol-1-yl]ethyl}-2,2-dimethyl-1,3-dioxane-4-acetate With hydrogenchloride; water In tetrahydrofuran at 25℃; for 8h; Stage #2: With sodium hydroxide; water In tetrahydrofuran for 8h; Stage #3: With phosphoric acid In water; ethyl acetate pH=4.0; Product distribution / selectivity; |
-
-
125971-95-1
tert-butyl (4R,6R)-6-{2-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)pyrrol-1-yl]ethyl}-2,2-dimethyl-1,3-dioxane-4-acetate

-
-
134523-01-6
atorvastatin sodium

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: aq. HCl / methanol 2: aq. NaOH / methanol View Scheme | |
| Stage #1: tert-butyl (4R,6R)-6-{2-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)pyrrol-1-yl]ethyl}-2,2-dimethyl-1,3-dioxane-4-acetate With hydroxylamine hydrochloride In methanol; water; acetone at 55 - 65℃; Industry scale; Stage #2: With sodium hydroxide In methanol; water at 35 - 45℃; Product distribution / selectivity; | |
| Stage #1: tert-butyl (4R,6R)-6-{2-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)pyrrol-1-yl]ethyl}-2,2-dimethyl-1,3-dioxane-4-acetate With hydrogenchloride; water In tetrahydrofuran at 25℃; for 6.25h; Industry scale; Stage #2: With sodium hydroxide In tetrahydrofuran at 31℃; for 14h; Product distribution / selectivity; Cooling; |
-
-
125971-95-1
tert-butyl (4R,6R)-6-{2-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)pyrrol-1-yl]ethyl}-2,2-dimethyl-1,3-dioxane-4-acetate

| Conditions | Yield |
|---|---|
| With hydroxylamine hydrochloride In methanol; water; acetone at 67℃; for 1h; Product distribution / selectivity; Heating / reflux; | |
| With hydroxylamine hydrochloride In water; isopropyl alcohol; acetone at 67℃; for 1h; Product distribution / selectivity; Heating / reflux; |
-
-
125971-95-1
tert-butyl (4R,6R)-6-{2-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)pyrrol-1-yl]ethyl}-2,2-dimethyl-1,3-dioxane-4-acetate

| Conditions | Yield |
|---|---|
| Stage #1: tert-butyl (4R,6R)-6-{2-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)pyrrol-1-yl]ethyl}-2,2-dimethyl-1,3-dioxane-4-acetate With hydrogenchloride In methanol; water at 20 - 26℃; for 6.25h; Stage #2: With sodium hydroxide In methanol; water at 25 - 30℃; for 6h; pH=~ 12; Stage #3: With hydrogenchloride; magnesium acetate In methanol; water at 20 - 55℃; pH=7.8 - 8; | |
| Stage #1: tert-butyl (4R,6R)-6-{2-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)pyrrol-1-yl]ethyl}-2,2-dimethyl-1,3-dioxane-4-acetate With hydrogenchloride; methanol; water at 20 - 25℃; for 3.75h; Stage #2: With sodium hydroxide In methanol; water at 20 - 25℃; for 3h; Stage #3: With hydrogenchloride; magnesium acetate more than 3 stages; |
Related products
Raw Materials
Downstream Products
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View