Dayang Chem (Hangzhou) Co.,Ltd.
Dayangchem's R&D center can offer custom synthesis according to the contract research and development services for the fine chemicals, pharmaceutical, biotechnique and some of the other chemicals. DayangChem can provide different quantities
Cas:1277-49-2
Min.Order:1 Kilogram
FOB Price: $3.0
Type:Lab/Research institutions
inquirySimagchem Corporation
Welcome to Simagchem, your partner in China as a premier supply of bulk specialty chemicals for industry and life science. We introduce experienced quality product and exceptional JIT service with instant market intelligence in China to benefit our
Cas:1277-49-2
Min.Order:0 Metric Ton
Negotiable
Type:Manufacturers
inquiryAlity Chemical Corporation
The above product is Ality Chemical's strong item with best price, good quality and fast supply. Ality Chemical has been focusing on the research and production of this field for over 14 years. At the same time, we are always committed to providi
Chemwill Asia Co., Ltd.
Our main production base is located in Xuzhou industry park. We are certified both to the ISO 9001 and ISO 14001 Standards, have a safety management system in place.Our R&D team masters core technology for process-design of target building block
Cas:1277-49-2
Min.Order:5 Kiloliter
FOB Price: $1.2 / 5.0
Type:Manufacturers
inquiryZhuozhou Wenxi import and Export Co., Ltd
WITH US,YOUR MONEY IN SAFE,YOUR BUSINESS IN SAFE 1)Quick Response Within 12 hours; 2)Quality Guarantee: All products are strictly tested by our QC, confirmed by QA and approved by third party lab in China, USA, Canada, Germany, UK, Italy, France et
Cas:1277-49-2
Min.Order:1 Kilogram
FOB Price: $139.0 / 210.0
Type:Trading Company
inquiryShanghai Minstar Chemical Co., Ltd
Product Name: 1-(Ferrocenyl)ethanol CAS: 1277-49-2 MF: C12H14FeO10* MW: 230.08 EINECS: 625-176-1 Mol File: 1277-49-2.mol 1-(Ferrocenyl)ethanol Structure 1-(Ferrocenyl)ethanol Chemical Properties Melting point 76-79 °C(lit.
Cas:1277-49-2
Min.Order:1 Gram
Negotiable
Type:Lab/Research institutions
inquiryShanghai Upbio Tech Co.,Ltd
1.In No Less 10 years exporting experience. you can 100% received goods 2.Lower Price with higher quality 3,Free sample 4,We are sincerely responsible for the "product quality" and "After Service" Upbio is Specializ
Cas:1277-49-2
Min.Order:1 Gram
Negotiable
Type:Lab/Research institutions
inquiryShandong Hanjiang Chemical Co., Ltd.
Hello, dear friend! I'm Hansen and Allen from China. Welcome to my lookchem mall! The following is a brief introduction of our company's products and services. If you are interested in our products, please contact us by emai
Cas:1277-49-2
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryHebei Nengqian Chemical Import and Export Co., LTD
Our advantages: 1. All inquiries will be replied within 12 hours. 2. Dedication to quality, supply & service. 3. Strictly on selecting raw materials. 4. Reasonable & competitive price, fast lead time. 5. Sample is available for your eva
Cas:1277-49-2
Min.Order:1 Kilogram
FOB Price: $9.0 / 99.0
Type:Trading Company
inquiryAfine Chemicals Limited
Company Introduction 1. Established in 2005, with two independent business divisions: Fine chemicals division; Pharmaceutical division. 2. Main product: Optical brightener Textile auxiliary Dye stuff Pigments
Cas:1277-49-2
Min.Order:1 Gram
Negotiable
Type:Lab/Research institutions
inquirySHANGHAI T&W PHARMACEUTICAL CO., LTD.
A substitute for perfluorooctanoic acid, mainly used as a surfactant, dispersant, additive, etc Appearance:White solid or Colorless liquid Purity:99.3 % We will ship the goods in a timely manner as required We can provide relevant documents acc
Cas:1277-49-2
Min.Order:4 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryHangzhou J&H Chemical Co., Ltd.
J&H CHEM R&D center can offer custom synthesis according to the contract research and development services for the fine chemicals, pharmaceutical, biotechnique and some of the other chemicals. J&H CHEM has some Manufacturing base in Jia
Hebei Sankai Chemical Technology Co., Ltd
1. Product advantages High purity, all above 98.5%, no impurities after dissolution We will test each batch to ensure quality OEM and private brand services designed for free Various cap colors available We can also provide MT1 peptide powd
Cas:1277-49-2
Min.Order:1 Kilogram
FOB Price: $65.0 / 75.0
Type:Trading Company
inquiryKAISA GROUP INC
1.Applied in food field.it can improve the immune system and prolong life. 2.Appliedin cosmetic field.it can improve the skin care. 3.Applied in pharmaceutical field.it can treat various dieases. 4.Our product quality assurance will make our customer
Cas:1277-49-2
Min.Order:1 Metric Ton
FOB Price: $7.0 / 8.0
Type:Trading Company
inquiryGolden Pharma Co., Limited
GOLDEN PHARMA CO.,LIMITED.is a professional pharmaceutical company,our team have more than 20years expereince in pharmaceutical production and sales. we are a professional technical enterprise specializing in the R & D, production,QA regulation
Win-Win chemical Co.Ltd
Stock products, own laboratory Package:Grams, Kilograms Application:For R&D Transportation:According to customer request Port:Shanghai
Cas:1277-49-2
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryGIHI CHEMICALS CO.,LIMITED
Lower price, sample is available,SDS test documents are available,large stock in warehouseAppearance:White powder Storage:Sealed and preserved Package:200/Kilograms Application:Fine chemical intermediates, used as the main raw material for the synthe
Henan Allgreen Chemical Co.,Ltd
high quality Storage:Sealed, dry, microtherm , avoid light and smell. Package:According to the demand of customer Application:Organic synthesis Transportation:by air or by sea
Aecochem Corp.
Our clients, like BASF,CHEMO,Brenntag,ASR,Evonik,Merck and etc.Appearance:COA Storage:in stock Application:MSDS/TDS
Xi`an Eastling Biotech Co., Ltd.
high purity lowest priceAppearance:solid or liquid Storage:in sealed air resistant place Package:Foil bag; Drum; Plastic bottle Application:Pharma;Industry;Agricultural Transportation:by sea or air Port:Beijing or Guangzhou
Henan Tianfu Chemical Co., Ltd.
1.Our services:A.Supply sampleB.The packing also can be according the customers` requirmentC.Any inquiries will be replied within 24 hoursD.we provide Commerical Invoice, Packing List, Bill of loading, COA , Health certificate and Origin certificate.
Antimex Chemical Limied
Ansciep Chemical is a professional enterprise manufacturing and distributing fine chemicals and speciality chemicals. We have been dedicated to heterocycle compounds and phenyl rings for tens of years. This is our mature product for export. Our quali
Hangzhou Sartort Biopharma Co., Ltd
Best quality with low priceAppearance:yellow to yellow-orange crystalline powder Storage:ln stock Package:25kg/Barrel Application:Chemicals Transportation:Express/Sea/Air Port:Shanghai
Xiamen AmoyChem Co.,Ltd
Amoychem is committed to providing the top-quality chemical products and services Internationally. We offer our customers with friendly, professional service and reliable, high performance products that have been manufactured according to the accredi
Hangzhou ZeErRui Chemical Co., Ltd.
Hangzhou ZeErRui Chemical Co., Ltd. located in Lingang industrial areas, our plant covers an area of 6000 square meters.ZeErRui dedicated to the development, production and marketing of chemicals. We have earned ourselves a good reputation at home
Cas:1277-49-2
Min.Order:1 Gram
Negotiable
Type:Lab/Research institutions
inquiryHANGZHOU TIANYE CHEMICALS CO., LTD.
We product this chemical more than 10 years . We are very experience to export it to many countries, Our superior & stable quality , competitive price gain warm reception from our customers. Application:Chemical intermediate
Changchun Artel lmport and Export trade company
Supply top quality products with a reasonable price Application:api
Hangzhou Fandachem Co.,Ltd
1-(Ferrocenyl)ethanol cas 1277-49-2Appearance:white crystalline powder Storage:Store in dry, dark and ventilated place Package:25KG drum Application:intermediate Transportation:by air, by sea, by express
Henan Kanbei Chemical Co.,LTD
factory?direct?saleAppearance:White powder Storage:Sealed and preserved Package:200/Kilograms Application:healing drugs Transportation:By sea Port:Shanghai/tianjin
Shanghai Yuanye Bio-Technology Co., Ltd.
Known for its best quality and competitve price, this chemicals we offered is widely appreciated by our customers.Appearance:White powder Storage:keep sealed and keep from direct light Package:According client's requirements Application:pharmaceutica
Cas:1277-49-2
Min.Order:0
Negotiable
Type:Trading Company
inquirySynthetic route

| Conditions | Yield |
|---|---|
| With bis(1,5-cyclooctadiene)diiridium(I) dichloride; C39H45FeN2O2PS; hydrogen; sodium methylate In methanol at 40℃; for 12h; Reagent/catalyst; Solvent; Autoclave; Large scale; | 99% |
| With LiAlH4 In diethyl ether LiAlH4 (10.54 mmol) added in portion to soln. of acetylferrocene (4.38 mmol) (0°C) under N2; stirred (room temp., 3 h);; cooled to 0°C; H2O added; ether added; org. layer sepd.; aq. layer extd. with ether; combined org. layers dried over Na2SO4; filtered; concd.; | 97% |
| With potassium tert-butylate; hydrogen; tetracarbonyl(2-(diphenylphosphino)-ethylamine)chromium(0) In diethylene glycol dimethyl ether at 120℃; under 37503.8 Torr; for 14h; Autoclave; | 97% |
-
-
12093-10-6
ferrocenecarboxaldehyde

-
-
1277-49-2
1-ferrocenylethanol

| Conditions | Yield |
|---|---|
| With LiAlH4 In diethyl ether Ar-atmosphere; addn. of acetylferrocene to excess LiAlH4 (15-20°C, stirring); addn. of satd. aq. NH4Cl (cooling to 0-5°C), washing of org. layer (water), drying (Na2SO4), solvent removal; | 93% |

| Conditions | Yield |
|---|---|
| With samarium diiodide In tetrahydrofuran N2-atmosphere; molar ratio SmI2:ferrocene derivative:water=2.6:1:11, 25°C, 10 min; addn. of ice water, filtration over SiO2, concn.; | 92% |

| Conditions | Yield |
|---|---|
| With methylmagnesium bromide In not given Wittig reaction;; | A 6% B 90% |
| With CH3MgBr In not given Wittig reaction;; | A 6% B 90% |

| Conditions | Yield |
|---|---|
| In diethyl ether reflux; | 85.3% |

| Conditions | Yield |
|---|---|
| In methanol lithium borohydride (5.0 equiv.) added to a soln. of the ferrocenyl ketone (1.0 equiv.) in methanol, stirred at room temp. overnight (16 h), water added; extracted with ether, dried (MgSO4), concentrated in vacuo, crude product purified by flash column chromy. on silica gel (TLC Rf 0.66 (1:3 acetone-hexane)), elem. anal.; | A 2% B 77% |


| Conditions | Yield |
|---|---|
| In ethanol Irradiation (UV/VIS); UV-photolysis in abs. ethanol; mechanism discussed;; | A 4% B 9% C 4% D 58% |
| In ethanol Irradiation (UV/VIS); UV-photolysis in abs. ethanol, presence of H2O; mechanism discussed;; | A n/a B 0% C n/a D n/a |


| Conditions | Yield |
|---|---|
| In acetonitrile addn. of Zn complex to soln. of ferrocene derivative in CH3CN at room temp., strirred for 45 min at room temp., addn. of soln. of FeCl3*6H2O, stirred for 20 min; extraction with CH2Cl2, dried (MgSO4), evapn. of solvent, chromy. (silica gel / CCl4/Et2O: 5/2); | 50% |

| Conditions | Yield |
|---|---|
| With aluminum oxide In n-heptane ambient temp., neutral Al2O3;; | A 6% B 3% C 44% |

-
-
16853-85-3
lithium aluminium tetrahydride

-
-
1271-55-2
acetylferrocene

-
A
-
1271-51-8
vinyl ferrocene

-
B
-
1277-49-2
1-ferrocenylethanol

| Conditions | Yield |
|---|---|
| In tetrahydrofuran room temp., 16 h; |

-
-
7647-01-0
hydrogenchloride

-
-
191876-57-0
(C5H5)Fe(C5H4CH(CH3)OCH2COOCH3)

-
A
-
1277-49-2
1-ferrocenylethanol

| Conditions | Yield |
|---|---|
| In diethyl ether addn. of aq. HCl (1:1) to Fe-complex soln., stirring (room temp., 24 h); TLC (SiO2); product mixt. not sepd., detd. by NMR spectroscopy; |

-
-
16940-66-2
sodium tetrahydroborate

-
-
1271-55-2
acetylferrocene

-
A
-
1271-51-8
vinyl ferrocene

-
B
-
1277-49-2
1-ferrocenylethanol

| Conditions | Yield |
|---|---|
| In methanol room temp., 16 h; |


| Conditions | Yield |
|---|---|
| In water decompn. in H2O at 20-40°C;; | A <1 B n/a |
| In water decompn. in H2O at 20-40°C;; | A <1 B n/a |

| Conditions | Yield |
|---|---|
| With acetic acid In benzene | |
| With CH3COOH In benzene |


| Conditions | Yield |
|---|---|
| With water In water reaction with Na at -78°C or with Mg at -10°C, hydrolysis at ambient temp.; yield of products depends on conditions;; |


-
-
1277-49-2
1-ferrocenylethanol

| Conditions | Yield |
|---|---|
| With water; sodium carbonate In water -78°C;; |

-
-
1277-49-2
1-ferrocenylethanol

| Conditions | Yield |
|---|---|
| With hydroxide In water alkaline hydrolysis;; | |
| With OH(1-) In water alkaline hydrolysis;; |

| Conditions | Yield |
|---|---|
| In benzene | |
| In benzene | |
| In benzene |


-
-
1277-49-2
1-ferrocenylethanol

| Conditions | Yield |
|---|---|
| With water In water | |
| With water In tetrahydrofuran; water Kinetics; 50°C, 50 Vol.-% H2O; | |
| With H2O In water; acetone Kinetics; 10-40 Vol.-% H2O, 50°C; |

| Conditions | Yield |
|---|---|
| byproducts: C10H9FeCOCH3, (C5H5Fe(CO)2)2; | |
| byproducts: C10H9FeCOCH3, (C5H5Fe(CO)2)2; |

-
-
1277-49-2
1-ferrocenylethanol

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride byproducts: C10H9FeCOCH3, (C5H5Fe(CO)2)2; | |
| With LiAlH4 byproducts: C10H9FeCOCH3, (C5H5Fe(CO)2)2; |

-
-
1277-49-2
1-ferrocenylethanol

| Conditions | Yield |
|---|---|
| With water |

-
-
1277-49-2
1-ferrocenylethanol

| Conditions | Yield |
|---|---|
| With LiAlH4 11-28 % retention of configuration; | |
| With LiAlH4 In diethyl ether addn. of LiAlH4 to starting complex soln. in diethyl ether at 0°C under N2, reaction for 1 h; addn. of dil. NaOH or water at 0 or 25°C; |

-
B
-
1277-49-2
1-ferrocenylethanol

| Conditions | Yield |
|---|---|
| With water In diethyl ether; water | |
| With H2O In diethyl ether; water |

-
-
1277-49-2
1-ferrocenylethanol

| Conditions | Yield |
|---|---|
| With methanol In methanol Kinetics; methanolysis at 0°C; IR; |

-
-
1277-49-2
1-ferrocenylethanol

| Conditions | Yield |
|---|---|
| With water In acetone byproducts: vanillin; heating in aq. acetone; | >99 |

-
-
1277-49-2
1-ferrocenylethanol

| Conditions | Yield |
|---|---|
| With silica gel In not given byproducts: p-hydroxybenzaldehyde; hydrolysis during an attempt of purification on a SiO2 column; | >99 |
-
-
207301-27-7
(C6H5)CN4H(CH(CH3)(C5H4)Fe(C5H5))(1+)*BF4(1-)=[C6H5CN4H(CH(CH3)(C5H4)Fe(C5H5))]BF4

-
A
-
18039-42-4
5-Phenyl-1H-tetrazole

-
B
-
1277-49-2
1-ferrocenylethanol

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water refluxing (5-10 min), cooling to 5 to 7°C; ppt. filtration off, washing (hot pentane), Fe-compd. obtaining from pentane extracts by drying, tetrazole remaining in the solid residue; |
-
-
1277-49-2
1-ferrocenylethanol

-
-
17465-86-0, 130322-69-9, 130322-70-2
γ-cyclodextrin

| Conditions | Yield |
|---|---|
| In water molar ratio cyclodextrin : ferrocene = 1:2, addn. of crystals of ferrocene to an aq. soln. of cyclodextrin at 60°C with stirring; washed with water and THF, recrystn. from water or aq. alcohol; elem. anal.; | 100% |
-
-
1277-49-2
1-ferrocenylethanol

| Conditions | Yield |
|---|---|
| In water molar ratio cyclodextrin : ferrocene = 1:2, addn. of crystals of ferrocene to an aq. soln. of cyclodextrin at 60°C with stirring; washed with water and THF, recrystn. from water or aq. alcohol; elem. anal.; | 100% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide; trifluoroacetic acid In acetone addn. of 1.1 equiv. of mercapto-acid to Fe-complex soln., cooling to 0°C, addn. of CF3COOH, standing (room temp., overnight); addn. of 5% aq. KOH, treatment with CH2Cl2, addn. of few drops H3PO4 to aq. layer, extn. into CH2Cl2, shaking of extract with H2O, drying (MgSO4), evapn., recrystn. (hexane/C6H6 or EtOAc, not specified); elem. anal.; | 99% |
| With ammonium cerium (IV) nitrate In nitromethane at 20℃; Inert atmosphere; | 65% |
| With CF3COOH or CH3COOH | <=93 |

| Conditions | Yield |
|---|---|
| With samarium diiodide In tetrahydrofuran N2-atmosphere; molar ratio SmI2:ferrocene derivative:water=3.6:1:5.5, refluxing for 50 min; chromy. (SiO2, AcOEt/hexane=1:1); elem. anal.; | 99% |

| Conditions | Yield |
|---|---|
| With pyridine at 20℃; Inert atmosphere; | 99% |
| With catalyst: DMAP In triethylamine acetic anhydride (196 mmol) and DMAP added to soln. of 1-ferrocenylethanol (38.5 mmol); stirred (room temp., 17 h); solvent removed; dild. with ether (200 ml) and H2O (100 ml); org. layer sepd.; dried over Na2SO4; filtered; concd.; | 93% |
| With pyridine for 24h; Darkness; | 76% |
| With dmap; triethylamine In dichloromethane at 0 - 20℃; for 2.5h; |

| Conditions | Yield |
|---|---|
| Stage #1: 1-ferrocenylethanol With n-butyllithium In tetrahydrofuran; hexane for 0.0833333h; Inert atmosphere; Stage #2: chloroformic acid ethyl ester In tetrahydrofuran; hexane for 0.0833333h; Inert atmosphere; Stage #3: aniline Further stages; | A 98% B n/a |


| Conditions | Yield |
|---|---|
| With copper(II) sulfate In toluene byproducts: H2O; in boiling toluene;; | 97% |
| With potassium hydrogensulfate In benzene byproducts: H2O; in boiling benzene;; | 90% |
| In neat (no solvent) byproducts: H2O; 210°C, 20 Torr;; | 84% |
-
-
95-14-7
1,2,3-Benzotriazole

-
-
1277-49-2
1-ferrocenylethanol

-
-
101006-59-1, 654083-40-6, 654083-57-5
1-N-(1-ferrocenyl-1-methyl)ethylbenzotriazole

| Conditions | Yield |
|---|---|
| With tetrafluoroboric acid In dichloromethane; water addn. of aq. HBF4 to a mixt. of iron complex and heterocycle in CH2Cl2, stirring for 5 min; addn. of Et2O, water, ascorbic acid, sepn., washing org. phase with coldwater, evapn. drying over CaCl2; elem. anal.; | 97% |
| With HBF4 In dichloromethane treatment of 1-ferrocenylethanol with benzotriazole in CH2Cl2 in the presence of 45% aq. HBF4 at room temp. for several minutes ((J. Organomet. Chem. 580 (1999) 26); | 93% |
| With tetrafluoroboric acid In dichloromethane 20°C; |

| Conditions | Yield |
|---|---|
| byproducts: (CH3)2NH; (Ar); mixing for 3 h at 80-90°C; fractionation under deep vac.; TLC; column chromy. (silica gel); elem. anal.; | 96.5% |

| Conditions | Yield |
|---|---|
| byproducts: (C2H5)2NH; (Ar); mixing for 3 h at 80-90°C; fractionation under deep vac.; TLC; column chromy. (silica gel); elem. anal.; | 96.5% |

| Conditions | Yield |
|---|---|
| With CF3COOH or CH3COOH | 96% |
| With tetrafluoroboric acid In dichloromethane; water at 20℃; for 0.0833333h; | 83% |
-
-
1277-49-2
1-ferrocenylethanol

-
-
996-50-9
N,N-diethyl-1,1,1-trimethylsilanamine

-
A
-
1271-51-8
vinyl ferrocene

| Conditions | Yield |
|---|---|
| In neat (no solvent) byproducts: (C2H5)2NH, H2O, (CH3)3SiOSi(CH3)3; under argon; equimolar amts of diethylaminotrimethylsilane and the Fe complex are heated to 80-90°C; reaction mixt. distd. under vac.; vinylferrocene recrystd. from hexane; products identified by chromy., IR, and mass spectrometry; | A 4% B 96% |
| In toluene byproducts: (C2H5)2NH, H2O, (CH3)3SiOSi(CH3)3; under argon; 0.131 mol of diethylaminotrimethylsilane and 0.048 mol of the Fe complex in toluene are heated for 4 h at 120-125°C while distilling off the liberated Et2NH; after cooling, the pptd. crystals of vinylferrocene are sepd.; filtrate distd. under vac.; vinylferrocene recrystd. from hexane; products identified by chromy., IR, and mass spectrometry; | A 83% B 17% |
| In neat (no solvent) byproducts: (C2H5)2NH, H2O, (CH3)3SiOSi(CH3)3; under argon; 0.104 mole of diethylaminotrimethylsilane and 0.02 mole of the Fe complex are heated to 60-65°C; reaction mixt. distd. under vac.; vinylferrocene recrystd. from hexane; products identified by chromy., IR, and mass spectrometry; | A 25% B 75% |


| Conditions | Yield |
|---|---|
| With perchloric acid In dichloromethane byproducts: H2O; addn. of acid to soln. of Fe-complex and phosphine in CH2Cl2 with vigorous stirring, stirring (20°C, 30 min), addn. of ether, keeping (10-15 h, 5-8°C), pptn.; filtration, washing (dry ether), crystn. (EtOH) or repptn. from soln. in acetone (ether); elem. anal.; | 96% |
-
-
1277-49-2
1-ferrocenylethanol

-
-
15217-42-2
sodium benzotriazolate

-
-
101006-59-1, 654083-40-6, 654083-57-5
1-N-(1-ferrocenyl-1-methyl)ethylbenzotriazole

| Conditions | Yield |
|---|---|
| With tetrafluoroboric acid In dichloromethane; water byproducts: NaBF4; addn. of aq. HBF4 to mixt. of sodium benzotriazolide and Fe complex in CH2Cl2 with rapid stirring; maintaining, 1 h; evapn.; addn. of ether; washing ethereal soln. (water); drying over Na2SO4; evapn.; crystn. (hexane); elem. anal.; | 96% |

| Conditions | Yield |
|---|---|
| In dichloromethane all manipulations under Ar atm.; to soln. of ferrocenyl compd. added equiv. amt. of soln. of B compd. in CH2Cl2, stirred at room temp. for 3.5 h; quenched with aq. NH4Cl, org. layer sepd., aq. layer extd. with CH2Cl2,combined org. extracts dried over anhyd. MgSO4, solvent removed in vac., chromy., elem. anal.; | 95% |

| Conditions | Yield |
|---|---|
| In diethyl ether in cold abs. ether;; | 95% |

| Conditions | Yield |
|---|---|
| In dichloromethane; water aq. HBF4 was added to mixt. of corazol and Fe-complex in CH2Cl2, 15 min,Et2O was added; filtered, washed with ether, dried; elem. anal.; | 94% |


-
-
1277-49-2
1-ferrocenylethanol

-
-
101006-58-0
1-ferrocenylmethyl-1H-1,2,3-benzotriazole

-
-
151602-74-3
C6N3H4((CH(CH3)C5H4)Fe(C5H5))2(1+)*BF4(1-)=C6N3H4((CH(CH3)C5H4)Fe(C5H5))2BF4

| Conditions | Yield |
|---|---|
| In dichloromethane aq. soln. of HBF4 addn. to intensively stirred soln. of Fe-compounds, stirring 3-5 min, ether addn.; ppt. filtration off, washing (water, hexane) drying (CaCl2, vacuum); elem. anal., mass spectroscopy; | 94% |


| Conditions | Yield |
|---|---|
| In dichloromethane aq. HBF4 addn. to vigorously stirred soln. of Fe-compounds, 4 min; ppt. filtration off, washing (cold water, pentane), drying (CaCl2, vacuum); elem. anal., mass spectroscopy; | 94% |

-
-
1277-49-2
1-ferrocenylethanol

-
-
135-88-6
N-Phenyl-2-naphthylamine

-
-
93122-45-3
C5H5FeC5H4CH(CH3)N(C6H5)(C10H7)

| Conditions | Yield |
|---|---|
| With HClO4 or HBF4 In dichloromethane byproducts: H2O; aq. HClO4 (70 %) or HBF4 (45 %) added (vigorous stirring) to the Fe-complex and the amine; soln. stirred (30 min, room temp.), addn. of ether; ether soln. washed twice (water), dried (sodium sulfate), solvent removed (vac.); elem. anal.; | 93% |

-
-
1277-49-2
1-ferrocenylethanol

-
-
170468-07-2
N-(α-ferrocenylethyl)azoniaferrocene tetrafluoroborate

| Conditions | Yield |
|---|---|
| In dichloromethane aq. HBF4 addn. to vigorously stirred soln. of Fe-compounds, 5 min, diethyl ether addn., water addn.; pptn. on cooling (0°C), ppt. filtration off, washing (water, ether), drying (CaCl2, vacuum); elem. anal., mass spectroscopy; | 93% |


| Conditions | Yield |
|---|---|
| With potassium hydroxide semihydrate In dimethyl sulfoxide at 70℃; under 9880.66 Torr; for 0.75h; Temperature; Concentration; Reagent/catalyst; Autoclave; | 93% |
| With potassium hydroxide In dimethyl sulfoxide at 70 - 80℃; | 93% |

| Conditions | Yield |
|---|---|
| With ammonium cerium (IV) nitrate In nitromethane at 20℃; Inert atmosphere; | 93% |

| Conditions | Yield |
|---|---|
| With HBF4 or HClO4 In dichloromethane byproducts: H2O; aq. HBF4 (45 %) or HClO4 (70 %) added (vigorous stirring) to the Fe-complex and PhSH; soln. stirred (60 min, 20°C), addn. of ether; ether soln. washed twice (water), dried (sodium sulfate), solvent removed (vac.), recrystn. (ethanol); | 92% |

| Conditions | Yield |
|---|---|
| With HBF4 or HClO4 In dichloromethane byproducts: H2O; aq. HBF4 (45 %) or HClO4 (70 %) added (vigorous stirring) to the Fe-complex and the amine; soln. stirred (30 min, room temp.), addn. of ether; ether soln. washed twice (water), dried (sodium sulfate), solvent removed (vac.); elem. anal.; | 92% |

-
-
1277-49-2
1-ferrocenylethanol

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane byproducts: H2O; addn. of acid to soln. of Fe-complex and base in CH2Cl2 with vigorous stirring, stirring (20°C, 45 min), addn. of ether, keeping (10-15 h, 5-8°C), pptn.; filtration, washing (dry ether), crystn. (EtOH) or repptn. from soln. in acetone (ether); elem. anal.; | 92% |
Related products
Raw Materials
Downstream Products
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View
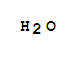





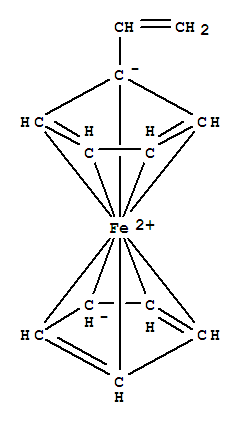

 Xi,
Xi,  Xn
Xn