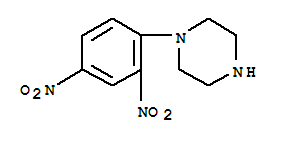
 This product is a nationally controlled contraband, and the Lookchem platform doesn't provide relevant sales information.
This product is a nationally controlled contraband, and the Lookchem platform doesn't provide relevant sales information.
Synthetic route

| Conditions | Yield |
|---|---|
| With water; sodium hydroxide at 100℃; for 1.5h; | 100% |
| With potassium carbonate In ethanol | 97.5% |
| Stage #1: 1-chloro-2,4-dinitro-benzene With sodium hydroxide In water; acetonitrile for 1h; Reflux; Stage #2: With hydrogenchloride In water; acetonitrile Kinetics; Thermodynamic data; Solvent; Temperature; | 97% |

| Conditions | Yield |
|---|---|
| With ammonium hydroxide; sodium sulfite In methanol at 70℃; for 48h; | 100% |
-
-
2687-45-8
triethylamine N-oxide

-
-
97-00-7
1-chloro-2,4-dinitro-benzene

-
A
-
51-28-5
2,4-Dinitrophenol

-
B
-
554-68-7
triethylamine hydrochloride

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide for 24h; | A 98% B 98.5% |
| In N,N-dimethyl-formamide for 24h; Ambient temperature; | A 98% B 98.5% |


| Conditions | Yield |
|---|---|
| With trichloroisocyanuric acid; silica gel; sodium nitrite at 20℃; for 0.25h; | 98% |
| With dinitrogen tetraoxide; ferric nitrate In ethyl acetate for 0.166667h; Heating; | 96% |
| With chromium(III) nitrate; dinitrogen tetraoxide In ethyl acetate for 0.25h; Nitration; reflux; | 95% |

| Conditions | Yield |
|---|---|
| With water; sodium hydroxide at 100℃; for 1.5h; | 98% |
| With potassium hydroxide |
-
-
1184-78-7
trimethylamine-N-oxide

-
-
68-12-2, 33513-42-7
N,N-dimethyl-formamide

-
-
97-00-7
1-chloro-2,4-dinitro-benzene

-
A
-
51-28-5
2,4-Dinitrophenol

-
B
-
593-81-7
trimethylamine hydrochloride

| Conditions | Yield |
|---|---|
| for 24h; Ambient temperature; | A 97% B 98% |

-
-
1184-78-7
trimethylamine-N-oxide

-
-
97-00-7
1-chloro-2,4-dinitro-benzene

-
A
-
51-28-5
2,4-Dinitrophenol

-
B
-
593-81-7
trimethylamine hydrochloride

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide for 24h; Ambient temperature; | A 97% B 98% |


| Conditions | Yield |
|---|---|
| With dimethylbromosulphonium bromide; tetrabutylammonium nitrite In acetonitrile at 20℃; for 24h; regioselective reaction; | 97% |
| With perchloric acid; montmorillonite K10 supported ammonium nitrate at 50℃; for 1.5h; | 95% |
| With Zn(NO3)2*2N2O4 In dichloromethane at 20℃; for 5h; | 95% |

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide; potassium tert-butylate; ammonia In tetrahydrofuran for 0.25h; | 96% |
| With Cumene hydroperoxide; potassium tert-butylate In N,N,N,N,N,N-hexamethylphosphoric triamide at 15 - 20℃; for 1.5h; | 92% |
| With potassium hydroxide; Cumene hydroperoxide In ammonia at -33℃; | 90% |

| Conditions | Yield |
|---|---|
| With triethylamine In water; acetonitrile at 25℃; for 7h; Kinetics; Reagent/catalyst; | A n/a B 96% |
| With triethylamine hydrochloride; triethylamine In water; acetonitrile at 25℃; Rate constant; different Et3N concentrations; | |
| With sodium ethanolate In ethanol at 25℃; Kinetics; Further Variations:; Reagents; Elimination; |

| Conditions | Yield |
|---|---|
| With ammonium cerium(IV) nitrate In acetonitrile at 20℃; for 12h; | A 95% B 3% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In 1,4-dioxane; water for 5h; Kinetics; Product distribution; Further Variations:; pH-values; Heating; | 94% |
-
-
97-00-7
1-chloro-2,4-dinitro-benzene

-
A
-
51-28-5
2,4-Dinitrophenol

-
B
-
54715-57-0
5-chloro-2,4-dinitrophenol

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide; potassium tert-butylate; ammonia In tetrahydrofuran for 0.25h; | A n/a B 93% |
| With potassium tert-butylperoxide In ammonia at -33℃; Mechanism; competition between hydrogen and halogen substitution; other reagent t-butyl hydroperoxide, cumene hydroperoxide, NaOH, t-BuOK; | A 20% B 50% |
| With tert.-butylhydroperoxide; potassium tert-butylate; ammonia In tetrahydrofuran for 0.25h; | A 50% B n/a |

| Conditions | Yield |
|---|---|
| With thiophene; sodium hydrogen sulfate; silica gel for 9h; Heating; | 93% |
-
-
1655-52-3
N-(2,4-dinitro-phenyl)-L-alanine

-
A
-
51-28-5
2,4-Dinitrophenol

-
B
-
4615-69-4
2-methyl-5-nitro-1H-benzimidazole 3-oxide

| Conditions | Yield |
|---|---|
| With sodium hydroxide In 1,4-dioxane; water for 2h; Heating; | A n/a B 88% |

-
-
51-28-5
2,4-Dinitrophenol

| Conditions | Yield |
|---|---|
| With hydrazine hydrate; caesium carbonate at 20℃; for 15h; | 86% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide for 1h; Heating; | 85% |
| With potassium carbonate In dimethyl sulfoxide for 3h; Heating; | 79% |
| With Cumene hydroperoxide; potassium tert-butylate In ammonia at -33℃; | 63% |

| Conditions | Yield |
|---|---|
| With Zn(NO3)2*2N2O4 In ethyl acetate at 20℃; for 4h; | A 85% B 8% |

| Conditions | Yield |
|---|---|
| With ferric nitrate; 1,3-di-n-butyl-imidazolium tetrafluoroborate at 60℃; for 2h; | 82% |
| With Zn(NO3)2*2N2O4 In dichloromethane for 4h; Heating; | 82% |
| With N-Bromosuccinimide; silver nitrate In acetonitrile for 7.5h; Reflux; | 75% |
-
-
108-95-2
phenol

-
A
-
51-28-5
2,4-Dinitrophenol

-
B
-
88-89-1
2,4,6-Trinitrophenol

-
C
-
106-51-4
p-benzoquinone

| Conditions | Yield |
|---|---|
| With NO+*18-crown-6*H(NO3)2- In ethyl acetate for 0.166667h; Nitration; Heating; | A 82% B 8% C 3% |
| With silica-acetate; dinitrogen tetraoxide In ethyl acetate for 0.166667h; Heating; | A 74% B 8% C 10% |

-
-
863033-41-4
2',3',5'-tri-O-acetyl-1-(2,4-dinitrobenzenesulfonyl)inosine

-
-
100-46-9
benzylamine

-
A
-
866325-38-4
1-N-benzyl-2',3',5'-tri-O-acetyladenosine

-
B
-
51-28-5
2,4-Dinitrophenol

| Conditions | Yield |
|---|---|
| Stage #1: 2',3',5'-tri-O-acetyl-1-(2,4-dinitrobenzenesulfonyl)inosine; benzylamine In acetonitrile at -30℃; Stage #2: With water In acetonitrile Heating; | A 81% B n/a |

-
-
71597-85-8
(p-hydroxyphenyl)boronic acid

-
-
51-28-5
2,4-Dinitrophenol

| Conditions | Yield |
|---|---|
| With bismuth (III) nitrate pentahydrate In toluene at 70 - 80℃; for 2h; Inert atmosphere; | 77% |
-
-
863033-41-4
2',3',5'-tri-O-acetyl-1-(2,4-dinitrobenzenesulfonyl)inosine

-
-
75-31-0
isopropylamine

-
B
-
51-28-5
2,4-Dinitrophenol

| Conditions | Yield |
|---|---|
| Stage #1: 2',3',5'-tri-O-acetyl-1-(2,4-dinitrobenzenesulfonyl)inosine; isopropylamine In acetonitrile at -30℃; Stage #2: With water In acetonitrile Heating; | A 76% B n/a |

-
-
99-65-0
meta-dinitrobenzene

-
-
62-53-3
aniline

-
A
-
51-28-5
2,4-Dinitrophenol

-
B
-
961-68-2
N-phenyl-2,4-dinitroaniline

| Conditions | Yield |
|---|---|
| With potassium permanganate; tetrabutyl ammonium fluoride In N,N-dimethyl-formamide at 20℃; for 1h; | A 22% B 75% |


-
A
-
51-28-5
2,4-Dinitrophenol

-
B
-
99-57-0
2-hydroxy-5-nitroaniline

-
C
-
3649-46-5
2-Phenyl-5,6,7,8-tetrahydro-imidazo<1,2-a>pyridin

| Conditions | Yield |
|---|---|
| In dimethyl sulfoxide at 25℃; for 5h; Inert atmosphere; Schlenk technique; Sealed tube; Irradiation; | A 33% B 22% C 75% |

-
-
67-56-1
methanol

-
-
70-34-8
2,4-Dinitrofluorobenzene

-
A
-
51-28-5
2,4-Dinitrophenol

-
B
-
119-27-7
2,4-dinitroanisole

| Conditions | Yield |
|---|---|
| With sodium hydroxide; cetyltrimethylammonim bromide In water at 26℃; Product distribution; other alcohols, var. concentrations alcohols; | A 26% B 74% |


| Conditions | Yield |
|---|---|
| With perchloric acid; montmorillonite K10 supported ammonium nitrate at 50℃; for 1.5h; | A 73% B 23% |
| durch Nitrieren; Trennung durch fraktionierte Faellung des Gemisches der Kaliumsalze mit BaCl2; | |
| With tetrachloromethane; nitrosylsulfuric acid at 30℃; | |
| With nitric acid |

| Conditions | Yield |
|---|---|
| With samarium; copper(l) iodide; potassium iodide In tetrahydrofuran at 20℃; Molecular sieve; diastereoselective reaction; | A n/a B 73% |


| Conditions | Yield |
|---|---|
| With thionyl chloride; bismuth subnitrate In dichloromethane at 20℃; for 2h; | A 72% B 14% |
-
-
863033-41-4
2',3',5'-tri-O-acetyl-1-(2,4-dinitrobenzenesulfonyl)inosine

-
-
75-04-7
ethylamine

-
B
-
51-28-5
2,4-Dinitrophenol

| Conditions | Yield |
|---|---|
| Stage #1: 2',3',5'-tri-O-acetyl-1-(2,4-dinitrobenzenesulfonyl)inosine; ethylamine In acetonitrile at -30℃; Stage #2: With water In acetonitrile Heating; | A 72% B n/a |


| Conditions | Yield |
|---|---|
| With benzyltriphenylphosphonium peroxodisulfate; potassium bromide In acetonitrile for 9.5h; Heating; | 100% |
| With N-benzyl-N,N-dimethyl anilinium peroxodisulfate; potassium bromide In acetonitrile for 9.5h; Reflux; regioselective reaction; | 91% |
| With poly(4-vinylpyridinium bromochromate) In acetonitrile at 20℃; for 2h; regioselective reaction; | 82% |
-
-
51-28-5
2,4-Dinitrophenol

-
-
56512-49-3
dabsyl chloride

-
-
146303-72-2
4-(4-Dimethylamino-phenylazo)-benzenesulfonic acid 2,4-dinitro-phenyl ester

| Conditions | Yield |
|---|---|
| With carbonate-bicarbonate buffer In acetone; acetonitrile 1.) 15 min, 2.) reflux; | 100% |
| With carbonate-bicarbonate buffer In acetone for 0.5h; Heating; |
-
-
51-28-5
2,4-Dinitrophenol

-
-
104293-02-9
1-ethoxy-2-(trimethylsilyl)vinyl acetate

-
-
4232-27-3
2,4-dinitrophenyl acetate

| Conditions | Yield |
|---|---|
| In dichloromethane at 40℃; for 22h; | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: 4BF4(1-)*C86H162N6O2(4+) With cobalt(II) acetate In ethanol at 20℃; for 3h; Stage #2: 2,4-Dinitrophenol With oxygen In dichloromethane for 3h; Stage #3: sodium 2,4-dinitrophenoxide With oxygen In dichloromethane Product distribution / selectivity; | 100% |

-
-
51-28-5
2,4-Dinitrophenol

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 20℃; for 2.5h; | 100% |
-
-
51-28-5
2,4-Dinitrophenol

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 20℃; for 2.5h; | 100% |
-
-
51-28-5
2,4-Dinitrophenol

| Conditions | Yield |
|---|---|
| In acetonitrile at 40℃; Inert atmosphere; | 100% |
-
-
51-28-5
2,4-Dinitrophenol

-
-
71-48-7, 917-69-1, 5931-89-5, 55881-15-7, 68931-68-0, 68931-69-1, 93029-27-7
cobalt(II) acetate

-
-
1011-73-0
sodium 2,4-dinitrophenoxide

| Conditions | Yield |
|---|---|
| Stage #1: C36H58N4O2(2+)*2BF4(1-); cobalt(II) acetate In ethanol at 20℃; for 2h; Glovebox; Inert atmosphere; Schlenk technique; Stage #2: 2,4-Dinitrophenol With oxygen In dichloromethane for 2h; Stage #3: sodium 2,4-dinitrophenoxide In dichloromethane at 20℃; | 100% |

-
-
51-28-5
2,4-Dinitrophenol

-
-
71-48-7, 917-69-1, 5931-89-5, 55881-15-7, 68931-68-0, 68931-69-1, 93029-27-7
cobalt(II) acetate

-
-
1011-73-0
sodium 2,4-dinitrophenoxide

| Conditions | Yield |
|---|---|
| Stage #1: C36H52N4O2(2+)*2BF4(1-); cobalt(II) acetate In ethanol at 20℃; for 2h; Glovebox; Inert atmosphere; Schlenk technique; Stage #2: 2,4-Dinitrophenol With oxygen In dichloromethane for 2h; Stage #3: sodium 2,4-dinitrophenoxide In dichloromethane at 20℃; | 100% |


| Conditions | Yield |
|---|---|
| With iodine; triethylamine; triphenylphosphine In dichloromethane at 0 - 20℃; for 0.333333h; | 99% |
| With dicyclohexyl-carbodiimide In dichloromethane at 25℃; for 12h; | 90% |

| Conditions | Yield |
|---|---|
| With N-butyl-4-methylpyridinium bromide at 170℃; for 0.5h; Inert atmosphere; Ionic liquid; Green chemistry; chemoselective reaction; | 99% |

| Conditions | Yield |
|---|---|
| With hydrazine hydrate at 90℃; for 18h; | 98% |
| With hydrazine hydrate In water at 110℃; Sealed tube; Green chemistry; | 95% |
| With hydrazine hydrate In isopropyl alcohol at 110℃; for 0.25h; Catalytic behavior; Sealed tube; chemoselective reaction; | 94% |
-
-
75-91-2
tert.-butylhydroperoxide

-
-
51-28-5
2,4-Dinitrophenol

-
-
5395-43-7
tri(p-tolyl)antimony

-
-
108-88-3
toluene

| Conditions | Yield |
|---|---|
| Stage #1: tert.-butylhydroperoxide; 2,4-Dinitrophenol; tri(p-tolyl)antimony In diethyl ether at 20℃; for 24h; Stage #2: toluene In octane | 98% |


| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate; pyrographite In tetrahydrofuran; water at 50 - 60℃; for 5h; | 97% |
| With sodium tetrahydroborate In ethanol; water at 45℃; for 0.0833333h; | 96% |
| Stage #1: 2,4-Dinitrophenol With palladium on activated charcoal In methanol at 20℃; for 0.0833333h; Autoclave; Inert atmosphere; Stage #2: With hydrogen In methanol at 65℃; under 3600.36 - 6375.64 Torr; for 1.5h; Pressure; Temperature; Autoclave; | 96.16% |
-
-
51-28-5
2,4-Dinitrophenol

-
-
4903-36-0
N-phenyl-benzimidoyl chloride

-
-
107569-59-5
2,4-dinitrophenyl N-phenylbenzimidate

| Conditions | Yield |
|---|---|
| diethylamine In diethyl ether | 96% |
| triethylamine In 1,4-dioxane |

| Conditions | Yield |
|---|---|
| With sodium ethanolate at 18 - 20℃; for 0.5h; | 96% |
-
-
51-28-5
2,4-Dinitrophenol

-
-
31562-43-3
tert-butylsulfinyl chloride

-
-
112881-95-5
2-Methyl-propane-2-sulfinic acid 2,4-dinitro-phenyl ester

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane for 16h; Ambient temperature; | 95% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In neat (no solvent) at 90℃; for 6h; Green chemistry; | 95% |

| Conditions | Yield |
|---|---|
| With lithium hydroxide In tetrahydrofuran at 70℃; for 0.5h; | 94% |
Related products
Raw Materials
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View














 T,
T, N,
N, Xi,
Xi, F
F