-
Name
(R)-(+)-1-Phenylethylamine
- EINECS 223-423-4
- CAS No. 3886-69-9
- Article Data244
- CAS DataBase
- Density 0.956 g/cm3
- Solubility water: 40 g/L (20 °C)
- Melting Point -10 °C
- Formula C8H11N
- Boiling Point 183 °C at 760 mmHg
- Molecular Weight 121.182
- Flash Point 75.8 °C
- Transport Information UN 2735 8/PG 2
- Appearance Colorless to light yellow liqui
- Safety 26-28-36/37/39-45-27
- Risk Codes 21/22-34
-
Molecular Structure
-
Hazard Symbols
 C
C
- Synonyms D-(+)-1-Phenylethylamine;D-1-Phenylethylamine;D-a-Phenethylamine;Benzenemethanamine,a-methyl-, (R)-;(+)-(R)-1-Phenethylamine;(+)-a-Methylbenzenemethanamine;(+)-a-Phenethylamine;(1R)-1-Phenyl-1-ethanamine;(1R)-1-Phenylethylamine;(1R)-Phenylethylamine;(R)-(+)-1-Phenylethylamine;(R)-Phenethylamine;(R)-a-Aminoethylbenzene;(R)-a-Methylbenzenemethanamine;(R)-a-Phenethylamine;
- PSA 26.02000
- LogP 2.40660
Synthetic route
-
-
67-56-1
methanol

-
A
-
358629-51-3
(S)-methyl 2-(2-oxopyrrolidin-1-yl)-2-butanoate

-
B
-
3886-69-9
(R)-1-phenyl-ethyl-amine

| Conditions | Yield |
|---|---|
| Stage #1: (-)-(S)-alpha-ethyl-2-oxo-1-pyrrolidineacet-N-(+)-(R)-(1-phenylethyl)-amide With sulfonic acid type-ion exchange resin; water In toluene for 6h; not specified; Heating / reflux; Stage #2: methanol With sulfonic acid type-ion exchange resin In water; toluene at 60℃; for 3h; | A 100% B n/a |

| Conditions | Yield |
|---|---|
| With ammonia at 25℃; optical yield given as %ee; | 100% |

-
A
-
3886-69-9
(R)-1-phenyl-ethyl-amine

-
B
-
70918-54-6
(S)‐1,4‐benzodioxane‐2‐carboxylic acid

| Conditions | Yield |
|---|---|
| With hydrogenchloride In 1,4-dioxane at 90℃; | A 100% B 100% |

-
A
-
3886-69-9
(R)-1-phenyl-ethyl-amine

-
B
-
70918-53-5
(+)-(2R)-2,3-dihydro-1,4-benzodioxine-2-carboxylic acid

| Conditions | Yield |
|---|---|
| With hydrogenchloride In 1,4-dioxane at 90℃; | A 100% B 100% |
-
-
842121-02-2
C12H17NOS

-
-
3886-69-9
(R)-1-phenyl-ethyl-amine

| Conditions | Yield |
|---|---|
| Stage #1: C12H17NOS With [RhCl2(p-cymene)]2; potassium tert-butylate; (1S,2R)-1-amino-2-indanol In isopropyl alcohol at 40℃; for 2h; Molecular sieve; Stage #2: With hydrogenchloride In methanol optical yield given as %ee; stereoselective reaction; | 99% |

-
-
3886-69-9
(R)-1-phenyl-ethyl-amine

| Conditions | Yield |
|---|---|
| With aluminum (III) chloride; methoxybenzene In dichloromethane at 30℃; for 2h; | 98.4% |
| With aluminum (III) chloride; methoxybenzene In dichloromethane at 20℃; for 2h; Inert atmosphere; enantioselective reaction; | 95% |
-
-
933057-19-3
N-[(R)-(+)-1-phenylethyl]decanamide

-
-
3886-69-9
(R)-1-phenyl-ethyl-amine

| Conditions | Yield |
|---|---|
| With Candida antarctica lipase B In phosphate buffer at 70℃; for 48h; pH=7; | 98% |
-
-
36283-44-0
N-acetyl-1-phenylethylamine

-
-
3886-69-9
(R)-1-phenyl-ethyl-amine

| Conditions | Yield |
|---|---|
| Stage #1: N-acetyl-1-phenylethylamine With hydrogenchloride; methanol; water at 80℃; for 15h; Stage #2: With water; sodium hydroxide | 98% |

-
-
3886-69-9
(R)-1-phenyl-ethyl-amine

| Conditions | Yield |
|---|---|
| With hydrogenchloride; D-sorbitol; choline chloride In water at 25℃; for 3h; | 98% |

| Conditions | Yield |
|---|---|
| With Candida boidinii formate dehydrogenase; Geobacillus stearothermophilus ε‐deaminating L‐lysine dehydrogenase variant 22; nicotinamide adenine dinucleotide In aq. buffer at 50℃; for 48h; pH=8.5; Reagent/catalyst; Enzymatic reaction; | 97% |
| With glucose dehydrogenase; sodium hydroxide; D-Alanine; ATA-117 transaminase; NADH; 2,3,4,5,6-pentahydroxy-hexanal; pyridoxal 5'-phosphate; lactate dehydrogenase at 30℃; for 10h; pH=7.5; aq. phosphate buffer; Enzymatic reaction; optical yield given as %ee; | 96% |
| With Ru(2+)*2C2H3O2(1-)*C77H108O6P2; hydrogen; sodium acetate In 2,2,2-trifluoroethanol at 100℃; under 42754.3 Torr; for 24h; Autoclave; enantioselective reaction; | 96% |
-
-
212378-97-7
(R(S))-N-(1-phenylethylidene)-tert-butanesulfinamide

-
-
3886-69-9
(R)-1-phenyl-ethyl-amine

| Conditions | Yield |
|---|---|
| Stage #1: (R(S))-N-(1-phenylethylidene)-tert-butanesulfinamide With [ruthenium(II)(η6-1-methyl-4-isopropyl-benzene)(chloride)(μ-chloride)]2; potassium tert-butylate; 2-Amino-2-methyl-1-propanol In isopropyl alcohol at 50℃; for 2h; Molecular sieve; Stage #2: With hydrogenchloride In methanol optical yield given as %ee; diastereoselective reaction; | 97% |
| Stage #1: (R(S))-N-(1-phenylethylidene)-tert-butanesulfinamide With [RhCl2(p-cymene)]2; potassium tert-butylate; 2-Amino-2-methyl-1-propanol; isopropyl alcohol at 50℃; for 0.5h; Inert atmosphere; Microwave irradiation; Molecular sieve; Stage #2: With hydrogenchloride In methanol at 20℃; Catalytic behavior; Temperature; Inert atmosphere; enantioselective reaction; | 95% |
| Multi-step reaction with 2 steps 1: [RhCl2(p-cymene)]2; potassium tert-butylate; 2-Amino-2-methyl-1-propanol; isopropyl alcohol / 2 h / 50 °C / Inert atmosphere; Molecular sieve 2: hydrogenchloride / methanol / 20 °C View Scheme | |
| Multi-step reaction with 2 steps 1.1: 2-Amino-2-methyl-1-propanol; [ruthenium(II)(η6-1-methyl-4-isopropyl-benzene)(chloride)(μ-chloride)]2 / isopropyl alcohol / 0.25 h / 50 °C / Inert atmosphere; Molecular sieve 1.2: 2 h / 50 °C / Inert atmosphere; Molecular sieve 2.1: hydrogenchloride / methanol; diethyl ether / 3 h / 0 - 20 °C / Inert atmosphere View Scheme |

-
A
-
2627-86-3
(S)-1-phenyl-ethylamine

-
B
-
3886-69-9
(R)-1-phenyl-ethyl-amine

-
C
-
130973-54-5
(R)-(-)-o-(1-pivaloylaminoethyl)benzene sulfinic acid

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid In methanol Yields of byproduct given; | A n/a B n/a C 96% |


| Conditions | Yield |
|---|---|
| With nickel at 110℃; for 1h; Reagent/catalyst; Inert atmosphere; | 94% |
| With ωTA ATA-117 102907WW; Vibrio fluvialis Vf-ωTA 2100208MW; pyridoxal 5'-phosphate; pentan-3-one at 30℃; for 24h; pH=7; aq. phosphate buffer; Enzymatic reaction; | |
| With hydrogen In toluene at 70℃; for 35h; chemoselective reaction; |

-
-
3886-69-9
(R)-1-phenyl-ethyl-amine

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water pH=11; | 94% |

-
-
3886-69-9
(R)-1-phenyl-ethyl-amine

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water pH=11; | 93.3% |
-
-
162929-44-4
(R)-2-methoxy-N-(1-phenylethyl)acetamide

-
-
3886-69-9
(R)-1-phenyl-ethyl-amine

| Conditions | Yield |
|---|---|
| With sodium hydroxide; triethanolamine In water at 120℃; for 6h; optical yield given as %ee; | 93% |
-
-
247236-71-1
(RS)-2-methyl-N-((R)-1-phenylethyl)propane-2-sulfinamide

-
-
3886-69-9
(R)-1-phenyl-ethyl-amine

| Conditions | Yield |
|---|---|
| With hydrogenchloride In 1,4-dioxane; methanol at 25℃; for 0.5h; Inert atmosphere; | 92% |
| With hydrogenchloride In methanol; diethyl ether at 0 - 20℃; for 3h; Inert atmosphere; | 88% |
| Stage #1: (RS)-2-methyl-N-((R)-1-phenylethyl)propane-2-sulfinamide With thionyl chloride In methanol at 20℃; Inert atmosphere; Stage #2: With hydrogenchloride In water Inert atmosphere; Stage #3: With ammonia; ammonium chloride In water Inert atmosphere; optical yield given as %ee; enantioselective reaction; | |
| Stage #1: (RS)-2-methyl-N-((R)-1-phenylethyl)propane-2-sulfinamide With hydrogenchloride In methanol at 20℃; Stage #2: With ammonia; ammonium chloride In water |
-
-
28570-76-5
ethanone-1-phenyl-O-(phenylmethyl)oxime

-
A
-
2627-86-3
(S)-1-phenyl-ethylamine

-
B
-
3886-69-9
(R)-1-phenyl-ethyl-amine

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate; (S)-valinol; zirconium(IV) chloride In tetrahydrofuran Ambient temperature; Title compound not separated from byproducts; | A 91% B n/a |
-
-
368447-71-6
(2S)-2-phenyl-2-({[(1R)-1-phenylethyl]amino}oxy)ethanol

-
A
-
3886-69-9
(R)-1-phenyl-ethyl-amine

-
B
-
25779-13-9
(S)-1-phenyl-1,2-ethanediol

| Conditions | Yield |
|---|---|
| With hexacarbonyl molybdenum In water; acetonitrile at 85℃; for 1h; | A 90% B n/a |

-
A
-
3886-69-9
(R)-1-phenyl-ethyl-amine

-
B
-
91306-30-8
(R)-(+)-2-methyl-2-(3-oxobutyl)cyclohexanone

| Conditions | Yield |
|---|---|
| With acetic acid at 20℃; for 1h; Yields of byproduct given; | A n/a B 88% |
-
-
10277-86-8
(R)-1-phenylethylamine hydrochloride

-
-
3886-69-9
(R)-1-phenyl-ethyl-amine

| Conditions | Yield |
|---|---|
| With potassium carbonate In water at 70℃; for 1h; pH=9; | 88% |
| With sodium hydroxide In dichloromethane; water pH=10 - 11; |

-
-
3886-69-9
(R)-1-phenyl-ethyl-amine

| Conditions | Yield |
|---|---|
| With diethylamine; palladium diacetate; trisodium tris(3-sulfophenyl)phosphine In water; acetonitrile at 20℃; for 0.0833333h; | 85% |

-
-
3886-69-9
(R)-1-phenyl-ethyl-amine

| Conditions | Yield |
|---|---|
| With samarium diiodide In tetrahydrofuran for 4h; Ambient temperature; | 83% |
-
-
28570-76-5, 106770-94-9
(E)-ethanone-1-phenyl-O-(phenylmethyl)oxime

-
-
3886-69-9
(R)-1-phenyl-ethyl-amine

| Conditions | Yield |
|---|---|
| Stage #1: (E)-ethanone-1-phenyl-O-(phenylmethyl)oxime With C10H18BNO In tetrahydrofuran at 20℃; for 24h; Stage #2: With borane-THF In tetrahydrofuran for 24h; Reflux; optical yield given as %ee; enantioselective reaction; Further stages; | 82% |
| With dimethylsulfide borane complex; (R)-(+)-2-(1,3,2-dioxaborolan-2-yloxy)-1,2,2-triphenylethanamine In toluene at 50 - 110℃; for 15h; Product distribution / selectivity; | n/a |
| With dimethylsulfide borane complex; C11H14BNO3 In toluene at 50 - 110℃; for 15h; Product distribution / selectivity; | n/a |
| With borane-THF; N-isopropyl-N,2-dimethylpropan-2-amine; C22H22BNO3 In 1,4-dioxane at 20℃; for 48h; Product distribution / selectivity; | 100 %Chromat. |
-
-
2743-01-3, 126522-56-3
(R)-N-(4-methoxyphenyl)-(1-phenylethyl)amine

-
-
3886-69-9
(R)-1-phenyl-ethyl-amine

| Conditions | Yield |
|---|---|
| Stage #1: (R)-N-(4-methoxyphenyl)-(1-phenylethyl)amine With ammonium cerium (IV) nitrate In methanol; water at 0℃; for 6h; Stage #2: With sodium hydroxide In water | 81.3% |

-
-
3886-69-9
(R)-1-phenyl-ethyl-amine

| Conditions | Yield |
|---|---|
| With thiophenol; zinc(II) chloride In dichloromethane at 20℃; for 6h; | 81% |
-
-
1233200-21-9
(Z)-acetophenone O-benzyloxime

-
-
3886-69-9
(R)-1-phenyl-ethyl-amine

| Conditions | Yield |
|---|---|
| With borane-THF; C19H23BNO3(1-)*H(1+) In tetrahydrofuran; 1,4-dioxane at 0℃; Temperature; Solvent; Reagent/catalyst; Time; Inert atmosphere; enantioselective reaction; | 76% |
-
-
3886-69-9
(R)-1-phenyl-ethyl-amine

-
-
541-41-3
chloroformic acid ethyl ester

-
-
14185-43-4
(R)-(1-phenylethyl)-carbamic acid ethyl ester

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at -30 - 20℃; | 100% |
| With triethylamine In diethyl ether at 0 - 20℃; for 2.5h; | 95% |
| Stage #1: (R)-1-phenyl-ethyl-amine With triethylamine In dichloromethane at 20℃; Inert atmosphere; Stage #2: chloroformic acid ethyl ester at -30 - 20℃; for 8h; Inert atmosphere; | 90.5% |
| With sodium carbonate | |
| With sodium hydroxide |

| Conditions | Yield |
|---|---|
| at 55℃; for 2h; | 100% |
| In chloroform at 0℃; | 95% |
| In benzene 1.) 90 deg C, 1 h, 2.) r.t., 12 h; | 86% |
-
-
924-44-7
glyoxylic acid ethyl ester

-
-
3886-69-9
(R)-1-phenyl-ethyl-amine

-
-
37662-05-8
((R)-1-phenyl-ethylimino)-acetic acid ethyl ester

| Conditions | Yield |
|---|---|
| In toluene for 0.333333h; Heating; | 100% |
| In toluene Heating; | 100% |
| In toluene at 15 - 25℃; | 95% |
-
-
3886-69-9
(R)-1-phenyl-ethyl-amine

-
-
109-94-4
formic acid ethyl ester

-
-
6948-01-2, 19145-06-3, 31502-34-8, 42412-77-1
(R)-(+)-α-methylbenzyl formamide

| Conditions | Yield |
|---|---|
| for 8h; Reflux; | 100% |
| With amberlyst 15 In tetrahydrofuran for 21h; Reflux; Inert atmosphere; | 99% |
| With Novozyme 435 CALB In tetrahydrofuran at 20℃; Green chemistry; Enzymatic reaction; | 92% |
-
-
28979-90-0
8-oxo-(E)-non-2-enoic acid methyl ester

-
-
3886-69-9
(R)-1-phenyl-ethyl-amine

-
-
130721-15-2
(E)-8-[(E)-(R)-1-Phenyl-ethylimino]-non-2-enoic acid methyl ester

| Conditions | Yield |
|---|---|
| With molecular sieve In cyclohexane at 40℃; for 1h; | 100% |
-
-
1189-64-6
(E)-9-Oxo-2-decanoic acid methyl ester

-
-
3886-69-9
(R)-1-phenyl-ethyl-amine

-
-
130721-16-3
(E)-9-[(E)-(R)-1-Phenyl-ethylimino]-dec-2-enoic acid methyl ester

| Conditions | Yield |
|---|---|
| With molecular sieve In cyclohexane at 40℃; for 1h; | 100% |
-
-
3886-69-9
(R)-1-phenyl-ethyl-amine

-
-
96293-28-6
7-oxo-2(E)-octenoic acid methyl ester

-
-
130739-49-0
(E)-7-[(E)-(R)-1-Phenyl-ethylimino]-oct-2-enoic acid methyl ester

| Conditions | Yield |
|---|---|
| With molecular sieve In cyclohexane at 40℃; for 1h; | 100% |
-
-
3886-69-9
(R)-1-phenyl-ethyl-amine

-
-
127987-42-2
(E)-7-(2-Oxo-cyclohexyl)-hept-2-enoic acid methyl ester

-
-
127987-43-3, 127987-44-4
(E)-7-{2-[(E)-(R)-1-Phenyl-ethylimino]-cyclohexyl}-hept-2-enoic acid methyl ester

| Conditions | Yield |
|---|---|
| In cyclohexane for 3h; Heating; | 100% |
-
-
3886-69-9
(R)-1-phenyl-ethyl-amine

| Conditions | Yield |
|---|---|
| 100% |
-
-
3886-69-9
(R)-1-phenyl-ethyl-amine

| Conditions | Yield |
|---|---|
| 100% |
-
-
3886-69-9
(R)-1-phenyl-ethyl-amine

-
-
100-52-7
benzaldehyde

-
-
56941-77-6
(R)-1-phenylethyl(benzylidene)amine

| Conditions | Yield |
|---|---|
| With magnesium sulfate In dichloromethane at 20℃; for 12h; | 100% |
| In benzene for 2h; Condensation; Heating; | 96% |
| With magnesium sulfate In tetrahydrofuran for 3.5h; | 83% |
-
-
108-30-5
succinic acid anhydride

-
-
3886-69-9
(R)-1-phenyl-ethyl-amine

-
-
94054-97-4
1-[(1R)-1-phenylethyl]pyrrolidine-2,5-dione

| Conditions | Yield |
|---|---|
| Stage #1: succinic acid anhydride; (R)-1-phenyl-ethyl-amine In toluene for 18h; Reflux; Stage #2: In acetic anhydride for 2h; Reflux; | 100% |
| Stage #1: succinic acid anhydride; (R)-1-phenyl-ethyl-amine In tetrahydrofuran Heating; Stage #2: With acetic anhydride In tetrahydrofuran for 2h; Heating; | 60% |
| With acetic anhydride 1.) THF, reflux, 2 h, 2.) reflux, 2 h; Yield given. Multistep reaction; |
-
-
924-44-7
glyoxylic acid ethyl ester

-
-
3886-69-9
(R)-1-phenyl-ethyl-amine

-
-
851389-20-3
<(R)-(1-phenylethyl)imino>acetic acid ethyl ester

| Conditions | Yield |
|---|---|
| In toluene for 1.5h; Heating; | 100% |
| With molecular sieve In dichloromethane at 0℃; |
-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
3886-69-9
(R)-1-phenyl-ethyl-amine

-
-
184888-43-5
(R)-(1-phenylethyl)carbamic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With indium(III) bromide at 30 - 35℃; for 0.0333333h; | 100% |
| With perchloric acid at 30 - 35℃; for 0.0166667h; | 100% |
| With sulfonic-acid-functionalized silica In dichloromethane at 20℃; for 0.0833333h; | 99% |
-
-
383-63-1
ethyl trifluoroacetate,

-
-
3886-69-9
(R)-1-phenyl-ethyl-amine

-
-
2354-91-8, 28332-81-2, 39995-50-1, 39995-51-2
N-<(R)-(+)-α-phenylethyl>-2,2,2-trifluoroacetamide

| Conditions | Yield |
|---|---|
| With sodium hydride In diethyl ether at 0 - 20℃; Condensation; | 100% |
| at 25℃; for 20h; Sealed tube; | 100% |
| at 20℃; for 0.05h; Acylation; | 89% |
-
-
3886-69-9
(R)-1-phenyl-ethyl-amine

-
-
183266-61-7
1-(trifluoroacetyl)benzotriazole

-
A
-
95-14-7
1,2,3-Benzotriazole

-
B
-
2354-91-8, 28332-81-2, 39995-50-1, 39995-51-2
N-<(R)-(+)-α-phenylethyl>-2,2,2-trifluoroacetamide

| Conditions | Yield |
|---|---|
| In tetrahydrofuran for 4h; Ambient temperature; | A n/a B 100% |

-
-
1121-60-4
pyridine-2-carbaldehyde

-
-
3886-69-9
(R)-1-phenyl-ethyl-amine

-
-
38010-70-7
(R,E)-N-(1-phenylethyl)-1-(pyridin-2-yl)methanimine

| Conditions | Yield |
|---|---|
| In toluene Reflux; Inert atmosphere; Dean-Stark; | 100% |
| With magnesium sulfate In diethyl ether for 4h; Ambient temperature; | 90% |
| With aluminum oxide | |
| In diethyl ether for 1h; Molecular sieve; Inert atmosphere; |
-
-
3886-69-9
(R)-1-phenyl-ethyl-amine

-
-
79-04-9
chloroacetyl chloride

-
-
36293-00-2
(R)-N-(α-methylbenzyl)chloroacetamide

| Conditions | Yield |
|---|---|
| In dichloromethane | 100% |
| With potassium carbonate In acetonitrile | 98% |
| With triethylamine In dichloromethane at 0 - 20℃; for 6h; | 86.07% |
-
-
75-15-0
carbon disulfide

-
-
3886-69-9
(R)-1-phenyl-ethyl-amine

-
-
67375-00-2
(R,R)-N,N'-bis[α-methylbenzyl]thiourea

| Conditions | Yield |
|---|---|
| aluminum oxide; zinc(II) oxide at 100℃; for 2h; Condensation; | 100% |
| at 47℃; | 97% |
| In ethanol Inert atmosphere; Reflux; | 95% |
| MCM-41-TBD; 1,3,4,6,7,8-hexahydro-2H-pyrimido[1,2-a]pyrimidine at 90℃; for 15h; | 91% |
| In ethanol at 88℃; for 24h; | 74% |
-
-
3886-69-9
(R)-1-phenyl-ethyl-amine

-
-
82799-91-5
5,5'-(2,2'-bipyridine)diacid chloride

| Conditions | Yield |
|---|---|
| Condensation; | 100% |
-
-
3886-69-9
(R)-1-phenyl-ethyl-amine

-
-
234436-57-8
(SS)-(+)-2-(p-Tolylsulfinyl)prop-2-en-1-ol

| Conditions | Yield |
|---|---|
| In dichloromethane at 0 - 20℃; | 100% |

| Conditions | Yield |
|---|---|
| at 20℃; for 6h; | 100% |
-
-
3886-69-9
(R)-1-phenyl-ethyl-amine

-
-
134989-37-0
ethyl 4-allyloxy-3-oxobutanoate

-
-
565170-41-4
(Z)-4-Allyloxy-3-((R)-1-phenyl-ethylamino)-but-2-enoic acid ethyl ester

| Conditions | Yield |
|---|---|
| With acetic acid In tetrahydrofuran at 60℃; | 100% |
-
-
74015-70-6, 13679-85-1
4,5-Dihydro-2-methylthiophen-3-(2H)-on

-
-
3886-69-9
(R)-1-phenyl-ethyl-amine

-
-
946134-08-3
(R)-(2-methyl-dihydrothiophen-3-ylidene)(1-phenylethyl)amine

| Conditions | Yield |
|---|---|
| With aluminum oxide; 5A molecular sieve; silica gel In cyclohexane at 20℃; for 144h; | 100% |
| With aluminum oxide; silica gel In cyclohexane at 20℃; for 100h; |
-
-
3886-69-9
(R)-1-phenyl-ethyl-amine

-
-
141836-47-7
4-(((tert-butyldimethylsilyl)oxy)methyl)cyclohexane carbaldehyde

-
-
672314-40-8
(R)-N-[(4'-tert-butyldimethylsilyloxymethyl-cyclohexyl)methylidene]-1-phenylethanamine

| Conditions | Yield |
|---|---|
| With magnesium sulfate In dichloromethane at 20℃; | 100% |
| With magnesium sulfate In dichloromethane |

| Conditions | Yield |
|---|---|
| In butan-1-ol for 0.166667h; Zincke's reaction; microwave irradiation; | 100% |
-
-
872-85-5
pyridine-4-carbaldehyde

-
-
3886-69-9
(R)-1-phenyl-ethyl-amine

-
-
1000387-94-9
((R)-1-Phenyl-ethyl)-[1-pyridin-4-yl-meth-(E)-ylidene]-amine

| Conditions | Yield |
|---|---|
| With magnesium sulfate In dichloromethane at 20℃; for 12h; | 100% |
| With boron trifluoride diethyl etherate at 20℃; for 0.0166667h; |

-
-
3886-69-9
(R)-1-phenyl-ethyl-amine

| Conditions | Yield |
|---|---|
| In chloroform-d1 at 24℃; | 100% |

-
-
3886-69-9
(R)-1-phenyl-ethyl-amine

| Conditions | Yield |
|---|---|
| In chloroform-d1 at 24℃; | 100% |
-
-
3886-69-9
(R)-1-phenyl-ethyl-amine

-
-
344907-95-5
ethyl (E,E)-4-oxo-2-styrylbut-2-enoate

| Conditions | Yield |
|---|---|
| In chloroform-d1 at 24℃; | 100% |
(R)-(+)-1-Phenylethylamine Chemical Properties
IUPAC Name: 1-phenylethanamine
Empirical Formula: C8H11N
Molecular Weight: 121.1796g/mol
EINECS: 223-423-4
Structure of Benzenemethanamine, a-methyl-, (aR)- (CAS NO.3886-69-9):
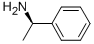
Index of Refraction: 1.533
Molar Refractivity: 39.34 cm3
Molar Volume: 126.6 cm3
Polarizability: 15.59×10-24cm3
Surface Tension: 36.5 dyne/cm
Density: 0.956 g/cm3
Flash Point: 75.8 °C
Enthalpy of Vaporization: 41.93 kJ/mol
Melting Point: -10 °C
Boiling Point: 183 °C at 760 mmHg
Vapour Pressure: 0.788 mmHg at 25°C
Water Solubility: 40 g/L (20 ºC)
Sensitive: Air Sensitive
Product Categories: Benzene derivatives;chiral;Amines (Chiral);Analytical Chemistry;Asymmetric Synthesis;Chiral Building Blocks;e.e. / Absolute Configuration Determination (NMR);Enantiomer Excess & Absolute Configuration Determination;for Resolution of Acids;Optical Resolution;Synthetic Organic Chemistry;Chiral Compound;Amines;Aromatics;Chiral Reagents
Canonical SMILES: CC(C1=CC=CC=C1)N
InChI: InChI=1S/C8H11N/c1-7(9)8-5-3-2-4-6-8/h2-7H,9H2,1H3
InChIKey: RQEUFEKYXDPUSK-UHFFFAOYSA-N
(R)-(+)-1-Phenylethylamine Toxicity Data With Reference
RTECS#: CAS# 3886-69-9: None listed
LD50/LC50: RTECS: Not available. Other: CAS 3886-69-9: supplier info (prod 15182) Oral, rat LD50: 950 mg/kg. Skin, rabbit LD50: 730 mg/kg. Inh, rat: no acute danger.
Carcinogenicity: (R)-(+)-1-Phenylethylamine - Not listed as a carcinogen by ACGIH, IARC, NTP, or CA Prop 65.
Other: The toxicological properties have not been fully investigated. Mutagenicity: Ames-test: negative.
(R)-(+)-1-Phenylethylamine Safety Profile
Hazard Codes:  C
C
Risk Statements: 21/22-34
R21/22:Harmful in contact with skin and if swallowed.
R34:Causes burns.
Safety Statements: 26-28-36/37/39-45-27
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S28:After contact with skin, wash immediately with plenty of soap-suds.
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection.
S45:In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
S27:Take off immediately all contaminated clothing.
RIDADR: UN 2735 8/PG 2
WGK Germany: 3
F: 3-10-23-34
HazardClass: 8
PackingGroup: III
HS Code: 29214980
(R)-(+)-1-Phenylethylamine Specification
Benzenemethanamine, a-methyl-, (aR)- , its cas register number is 3886-69-9. It also can be called (+/-)-alpha-Methylbenzylamine ; (R,S)-(+/-)-1-Phenylethylamine ; alpha.-Methylbenzylamine ; alpha.-Phenylethylamine ; 1-Phenylethanamin ; 1-Phenylethanamine ; (R)-alpha-Methylbenzenemethanamine .
Benzenemethanamine, a-methyl-, (aR)- (CAS NO.3886-69-9) is a colorless to light yellow liquid.
Related Products
- (R)-(+)-1-Phenylethylamine
- 3886-70-2
- 3886-90-6
- 38869-46-4
- 38869-47-5
- 38869-82-8
- 38870-72-3
- 38870-89-2
- 3887-13-6
- 38872-49-0
- 38873-82-4
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View