-
Name
1,4-Butanediol
- EINECS 203-786-5
- CAS No. 110-63-4
- Article Data395
- CAS DataBase
- Density 1.006 g/cm3
- Solubility Miscible
- Melting Point 20 °C
- Formula C4H10O2
- Boiling Point 227.999 °C at 760 mmHg
- Molecular Weight 90.1222
- Flash Point 105.909 °C
- Transport Information
- Appearance viscous colourless liquid
- Safety 36
- Risk Codes 22
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms 1,4-Butyleneglycol;1,4-Dihydroxybutane;1,4-Tetramethylene glycol;DabcoDBO;Diol 14B;NSC 406696;Polycure D;Sucol B;Tetramethylene 1,4-diol;Tetramethylene glycol;Vibracure A 250;ZM 0025;
- PSA 40.46000
- LogP -0.24880
Synthetic route

| Conditions | Yield |
|---|---|
| With C56H70Cl3N10Ru2(1+)*F6P(1-); potassium tert-butylate; hydrogen In tetrahydrofuran; dodecane at 70℃; under 37503.8 Torr; for 16h; Inert atmosphere; Glovebox; Autoclave; | 100% |
| With C30H34Cl2N2P2Ru; potassium methanolate; hydrogen In tetrahydrofuran at 100℃; under 38002.6 - 76005.1 Torr; for 15h; Glovebox; Autoclave; | 91% |
| With ethanol; sodium |

| Conditions | Yield |
|---|---|
| With sodium aluminum tetrahydride In tetrahydrofuran at 0℃; for 0.0833333h; | 100% |
| With C31H33ClN2O3RuS; potassium tert-butylate; hydrogen In isopropyl alcohol at 60℃; under 37503.8 Torr; for 48h; Inert atmosphere; | 100% |
| With C39H39N6ORu(1+)*Br(1-); potassium methanolate; hydrogen In tetrahydrofuran at 100℃; under 37503.8 Torr; for 16h; Reagent/catalyst; | 100% |

| Conditions | Yield |
|---|---|
| With sodium aluminum tetrahydride In tetrahydrofuran for 24h; Ambient temperature; | 100% |
| With hydrogen In water at 155℃; under 165017 Torr; Reagent/catalyst; | 95% |
| With sodium tetrahydroborate; C36H30F6N10Ni4O10(2+)*2C2F3O2(1-); zinc(II) chloride In tetrahydrofuran at 45℃; for 12h; | 78% |
| With [Ru(1,1,1-tris(diphenylphosphinomethyl)ethane)(trimethylenemethane)]; hydrogen In 1,4-dioxane at 195℃; under 37503.8 Torr; for 24h; Autoclave; Inert atmosphere; | |
| With C38H54Cl2N2P2Ru; hydrogen; sodium hydride In toluene at 160 - 190℃; under 60006 Torr; for 23h; Autoclave; Sealed tube; | 98 %Spectr. |

| Conditions | Yield |
|---|---|
| With hydrogen In water | 100% |
| With hydrogen In water | 100% |
| With hydrogen In water | 100% |

| Conditions | Yield |
|---|---|
| With hydrogen cation In tetrahydrofuran shaken for 10 min at room temp.; centrifuged, decanted, soln. contains naphthalene, pptn. hydrolysed in THF: butanediol detd. by GLC in the organic layer and a pptn. (Yb(OH)3); | A 83% B 100% C 75% |


| Conditions | Yield |
|---|---|
| With hydrogen; copper-palladium; silica gel In ethanol at 25℃; under 760 Torr; Kinetics; | A n/a B 99% |
| With LaNi5 hydride In tetrahydrofuran; methanol at 0℃; for 6h; | A 10% B 67% |
-
-
1033736-93-4
4-hydroxybutyl (4-oxo-3-phenyl-4H-thiochromen-2-yl)methylcarbonate

-
A
-
110-63-4
Butane-1,4-diol

-
B
-
124-38-9
carbon dioxide

-
C
-
1033736-87-6
C16H10O3S

| Conditions | Yield |
|---|---|
| In d(4)-methanol at 20℃; for 1h; Conversion of starting material; light irradiation; | A 99% B n/a C 85% |

-
-
110-16-7
maleic acid

-
A
-
109-99-9
tetrahydrofuran

-
B
-
96-48-0
4-butanolide

-
C
-
110-63-4
Butane-1,4-diol

-
D
-
110-15-6
succinic acid

-
E
-
64-19-7
acetic acid

| Conditions | Yield |
|---|---|
| With hydrogen; 0.5 percent Pd on Rutile TiO2 at 110℃; Product distribution / selectivity; | A 0.37% B 0.28% C 0.37% D 98.89% E 0.08% |

-
-
110-16-7
maleic acid

-
A
-
109-99-9
tetrahydrofuran

-
B
-
96-48-0
4-butanolide

-
C
-
67-56-1
methanol

-
D
-
110-63-4
Butane-1,4-diol

-
E
-
617-48-1
malic acid

-
F
-
110-15-6
succinic acid

-
G
-
64-19-7
acetic acid

-
H
-
71-36-3
butan-1-ol

| Conditions | Yield |
|---|---|
| With hydrogen; 0.5percent Pd on Rutile TiO2 at 110℃; Product distribution / selectivity; | A 0.45% B 0.06% C 0% D 0.21% E 0.36% F 98.73% G 0.04% H 0.08% |


| Conditions | Yield |
|---|---|
| With hydrogen; Ni catalyst as described in example 1 of U.S. Pat. No. 5,068,468 at 140℃; under 150015 Torr; for 336h; Conversion of starting material; | 98.3% |
| With hydrogen; Ni catalyst as described in example 1 of U.S. Pat. No. 5,068,468 In water at 140℃; under 150015 Torr; for 24 - 336h; Product distribution / selectivity; | 98.3% |
| With hydrogen In water at 100 - 135℃; under 60006 Torr; for 6h; Reagent/catalyst; Temperature; Pressure; | 90% |
-
-
110-16-7
maleic acid

-
A
-
109-99-9
tetrahydrofuran

-
B
-
96-48-0
4-butanolide

-
C
-
110-63-4
Butane-1,4-diol

-
D
-
591-81-1
4-hydroxybutanoic acid

-
E
-
110-15-6
succinic acid

-
F
-
64-19-7
acetic acid

-
G
-
71-36-3
butan-1-ol

| Conditions | Yield |
|---|---|
| With hydrogen; 0.5percent Pd on Rutile TiO2 at 110℃; Product distribution / selectivity; | A 0.77% B 0.38% C 0.24% D 0.05% E 98.28% F 0.02% G 0.26% |

-
-
122795-01-1
1,4-Bis-(tert-butyl-dimethyl-silanyloxy)-butane

-
-
110-63-4
Butane-1,4-diol

| Conditions | Yield |
|---|---|
| sulfated SnO2 In methanol at 20℃; for 0.166667h; | 98% |
| With sodium hydride In N,N,N,N,N,N-hexamethylphosphoric triamide at 25℃; for 12h; | 70% |
-
-
624-48-6
dimethyl cis-but-2-ene-1,4-dioate

-
A
-
109-99-9
tetrahydrofuran

-
B
-
13436-45-8
2-methoxytetrahydrofuran

-
C
-
96-48-0
4-butanolide

-
D
-
71-23-8
propan-1-ol

-
E
-
64001-06-5
2-(4'-hydroxybutoxy)-tetrahydrofuran

-
F
-
110-63-4
Butane-1,4-diol

-
G
-
71-36-3
butan-1-ol

| Conditions | Yield |
|---|---|
| With hydrogen; copper catalyst, T 4489, Sud-Chemie AG, Munich at 150 - 280℃; under 187519 Torr; Neat liquid(s) and gas(es)/vapour(s); | A 1% B n/a C 0.4% D n/a E n/a F 98% G 0.5% |


| Conditions | Yield |
|---|---|
| With C24H38Cl2N3PRu; hydrogen; sodium methylate In isopropyl alcohol at 25℃; under 38002.6 Torr; for 6h; Autoclave; | 97% |
| With C13H34BFeNOP2; hydrogen In tetrahydrofuran at 100℃; under 22502.3 Torr; for 18h; Autoclave; Inert atmosphere; | 97% |
| With C24H38Cl2N3PRu; hydrogen; sodium methylate In isopropyl alcohol at 25℃; under 37503.8 Torr; for 6h; | 97% |

| Conditions | Yield |
|---|---|
| With C36H54IrN2P2(1+)*C24H20B(1-); hydrogen; sodium hydride In toluene at 180℃; under 7500.75 - 45004.5 Torr; for 18h; Reagent/catalyst; Temperature; Pressure; Autoclave; Sealed tube; | A 5% B 95% |
| Stage #1: succinic acid In 1,4-dioxane at 500℃; for 4h; Stage #2: With hydrogen In 1,4-dioxane at 200℃; under 60006 Torr; for 5h; Catalytic behavior; Reagent/catalyst; | A n/a B 64.7% |
| With hydrogen In water at 130℃; under 37503.8 Torr; for 12h; Pressure; Reagent/catalyst; Temperature; Autoclave; | A 34% B 23% |

| Conditions | Yield |
|---|---|
| With perrhenic acid anhydride; hydrogen In 1,4-dioxane at 209.84℃; under 187519 Torr; for 4h; Catalytic behavior; Autoclave; Overall yield = 100 %; | A 94% B 6% |
| With hydrogen In water at 130℃; under 37503.8 Torr; for 12h; Pressure; Reagent/catalyst; Temperature; Autoclave; | A 92% B 5% |
| With hydrogen In water at 99.84℃; under 45004.5 Torr; |

| Conditions | Yield |
|---|---|
| With C25H19BrMnN2O2P; potassium tert-butylate; hydrogen In tetrahydrofuran at 130℃; under 22502.3 Torr; for 48h; Inert atmosphere; Glovebox; Autoclave; Green chemistry; | A 85% B 93% |
| With [Ru(PtBuNNHtBu)H(CO)Cl]; potassium tert-butylate; hydrogen In 1,4-dioxane at 135℃; under 30003 Torr; for 40h; Autoclave; |
-
-
117785-64-5
1-tert-butyldimethylsilyloxy-4-triethylsilyloxybutane

-
-
110-63-4
Butane-1,4-diol

| Conditions | Yield |
|---|---|
| With H-Y zeolite In methanol for 4h; Ambient temperature; | 92% |
-
-
821-11-4, 6117-80-2, 110-64-5
1,4-butenediol

-
-
5747-23-9
2-phenyl-1,3,2-benzodioxaborole

-
A
-
110-63-4
Butane-1,4-diol

-
B
-
108-95-2
phenol

| Conditions | Yield |
|---|---|
| With oxygen; hydrazine hydrate In acetonitrile at 32℃; under 760.051 Torr; for 4h; Schlenk technique; | A 92% B 90% |

-
-
110-16-7
maleic acid

-
A
-
109-99-9
tetrahydrofuran

-
B
-
96-48-0
4-butanolide

-
C
-
110-63-4
Butane-1,4-diol

-
D
-
591-81-1
4-hydroxybutanoic acid

-
E
-
617-48-1
malic acid

-
F
-
110-15-6
succinic acid

-
G
-
64-19-7
acetic acid

| Conditions | Yield |
|---|---|
| With hydrogen; 0.5percent Pd/2.0percent Re on Rutile TiO2 at 110℃; Product distribution / selectivity; | A 1.27% B 4.78% C 1.55% D 1.24% E 0.48% F 90.6% G 0.08% |


-
A
-
110-63-4
Butane-1,4-diol

-
B
-
175849-51-1
4-((triisopropylsilyl)oxy)butan-1-ol

| Conditions | Yield |
|---|---|
| With iron(III) chloride In methanol at 23℃; for 4h; | A 4% B 90% |

| Conditions | Yield |
|---|---|
| With water; hydrogen In 1,4-dioxane at 139.84℃; under 60006 Torr; for 4h; Reagent/catalyst; Solvent; | 90% |
| With cerium(IV) oxide; hydrogen In 1,4-dioxane at 139.84℃; under 60006 Torr; for 24h; |

| Conditions | Yield |
|---|---|
| With titanium(III)-tris-(tetrahydridoborate) In dichloromethane at -20℃; for 6h; | 89% |
| With chloro-trimethyl-silane; Benzyltriethylammonium borohydride; oxygen In dichloromethane at 0℃; for 8h; other enol ethers; | 73% |
| With chloro-trimethyl-silane; Benzyltriethylammonium borohydride; oxygen In dichloromethane at 0℃; for 8h; | 73% |
| With hydrogen In 1,4-dioxane; water at 139.84℃; under 60006 Torr; for 4h; | 24% |
| With water at 5℃; |
-
-
51326-51-3
4-(tetrahydropyran-2-yloxy)butan-1-ol

-
-
110-63-4
Butane-1,4-diol

| Conditions | Yield |
|---|---|
| With ammonium nitrate; Montmorillonite-K10 for 0.0416667h; deprotection; microwave irradiation; | 89% |
| With lithium bromide In methanol for 6h; Substitution; Heating; | 80% |
-
-
110-15-6
succinic acid

-
A
-
109-99-9
tetrahydrofuran

-
B
-
96-48-0
4-butanolide

-
C
-
110-63-4
Butane-1,4-diol

-
D
-
71-36-3
butan-1-ol

| Conditions | Yield |
|---|---|
| With hydrogen In 1,4-dioxane at 139.84℃; under 60006 Torr; for 96h; Catalytic behavior; Reagent/catalyst; Time; Temperature; Autoclave; Overall yield = 100 %; | A 0.2% B 3.1% C 89% D 7.6% |
| With hydrogen; 1.0percent Pd/ 3.0percent Re on Rutile TiO2 at 164 - 185℃; for 21 - 237h; Product distribution / selectivity; | A 2.95% B 0% C 81.5% D 3.35% |
| With hydrogen; 0percent Pd/5.0percent Re on Rutile TiO2 at 170 - 185℃; for 90 - 825h; Product distribution / selectivity; | A 3.38% B 0% C 64.14% D 2.86% |

-
-
110-15-6
succinic acid

-
A
-
109-99-9
tetrahydrofuran

-
B
-
96-48-0
4-butanolide

-
C
-
110-63-4
Butane-1,4-diol

-
D
-
107-92-6
butyric acid

-
E
-
106-97-8
n-butane

-
F
-
71-36-3
butan-1-ol

| Conditions | Yield |
|---|---|
| With hydrogen In 1,4-dioxane at 139.84℃; under 60006 Torr; for 24h; Catalytic behavior; Reagent/catalyst; Time; Autoclave; Overall yield = > 99 %; | A 0.2% B 3.1% C 89% D n/a E n/a F 7.6% |

-
-
110-16-7
maleic acid

-
A
-
109-99-9
tetrahydrofuran

-
B
-
96-48-0
4-butanolide

-
C
-
110-63-4
Butane-1,4-diol

-
D
-
617-48-1
malic acid

-
E
-
110-15-6
succinic acid

-
F
-
64-19-7
acetic acid

-
G
-
71-36-3
butan-1-ol

| Conditions | Yield |
|---|---|
| With hydrogen; 0.5percent Pd on Rutile TiO2 at 110℃; for 96 - 238h; Product distribution / selectivity; | A 0.6% B 0.04% C 0.62% D 0.19% E 88.49% F 0.12% G 0.11% |


| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate; C36H30F6N10Ni4O10(2+)*2C2F3O2(1-); zinc(II) chloride In tetrahydrofuran at 45℃; for 12h; | 87% |

| Conditions | Yield |
|---|---|
| With hydrogen In water at 130℃; under 37503.8 Torr; for 18h; Pressure; Reagent/catalyst; Autoclave; | A 87% B 13% |

| Conditions | Yield |
|---|---|
| With methanesulfonic acid; diisopropyl sulfite In toluene at 50℃; under 95 Torr; | 100% |
| With pyridine; thionyl chloride In benzene for 4h; Ambient temperature; | 70% |
| With thionyl chloride In dichloromethane Heating; | 67% |
-
-
110-63-4
Butane-1,4-diol

-
-
97-97-2
chloroacetaldehyde dimethyl acetal

-
-
54237-96-6
2-Chloromethyl-1,3-dioxepane

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid Heating; | 100% |
| With Dowex 50(H+) at 120℃; for 1h; | 75% |
| Substitution; | 38% |
| With toluene-4-sulfonic acid at 115℃; for 5h; |

| Conditions | Yield |
|---|---|
| With sulfuric acid In toluene Heating; | 100% |
-
-
110-63-4
Butane-1,4-diol

-
-
75898-24-7
1,2 bis (tricosa 10,12 diynoyl)-sn-3-glycerophosphocholine, DC8,9PC

-
-
150891-85-3
DC8,9 phosphatidylhydroxybutanol

| Conditions | Yield |
|---|---|
| With acetate buffer In isopropyl alcohol at 37℃; for 10h; phospholipase D; | 100% |
-
-
110-63-4
Butane-1,4-diol

-
-
18162-48-6
tert-butyldimethylsilyl chloride

-
-
87184-99-4
4-(tert-butyldimethylsiloxy)-1-butanol

| Conditions | Yield |
|---|---|
| With sodium hydride In tetrahydrofuran at 0℃; for 1h; | 100% |
| Stage #1: Butane-1,4-diol With sodium hydride In tetrahydrofuran; mineral oil at 0℃; for 0.666667h; Inert atmosphere; Stage #2: tert-butyldimethylsilyl chloride In tetrahydrofuran; mineral oil at 0℃; for 1.75h; Inert atmosphere; | 100% |
| Stage #1: Butane-1,4-diol With sodium hydride In tetrahydrofuran; mineral oil at 0℃; for 0.5h; Stage #2: tert-butyldimethylsilyl chloride In tetrahydrofuran; mineral oil at 0℃; for 1h; | 100% |
-
-
110-63-4
Butane-1,4-diol

-
-
58479-61-1
tert-butylchlorodiphenylsilane

-
-
130372-07-5
4-(tret-butyldiphenylsilyloxy)butan-1-ol

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In dichloromethane; N,N-dimethyl-formamide at 0 - 25℃; | 100% |
| With N-ethyl-N,N-diisopropylamine In dichloromethane at 20℃; for 2h; Inert atmosphere; | 100% |
| With N-ethyl-N,N-diisopropylamine In dichloromethane at 18℃; for 20h; Inert atmosphere; | 100% |
-
-
110-63-4
Butane-1,4-diol

-
-
19009-39-3
N,N-diisopropylcarbamoyl chloride

| Conditions | Yield |
|---|---|
| Stage #1: Butane-1,4-diol With sodium hydride In tetrahydrofuran at 0℃; for 1h; Stage #2: N,N-diisopropylcarbamoyl chloride In tetrahydrofuran Heating; Further stages.; | 100% |
| Stage #1: Butane-1,4-diol With sodium hydride In tetrahydrofuran; mineral oil at 0℃; for 1h; Stage #2: N,N-diisopropylcarbamoyl chloride In tetrahydrofuran Reflux; | 91% |
-
-
110-63-4
Butane-1,4-diol

-
-
163462-32-6
[Al2(OCH(CH3)2)2(CH3COCHCOCH3)2(O(CH2)4O)]2

| Conditions | Yield |
|---|---|
| In benzene byproducts: i-PrOH; moisture free; refluxing; solvent removal; elem. anal.; | 100% |

| Conditions | Yield |
|---|---|
| at 20℃; for 0.25h; Neat (no solvent); | 100% |

| Conditions | Yield |
|---|---|
| With dilithium tetra(tert-butyl)zincate In toluene at 0℃; for 1h; Inert atmosphere; | 100% |
| With steapsin lipase In hexane at 55℃; for 24h; Enzymatic reaction; | 99 %Chromat. |
-
-
110-63-4
Butane-1,4-diol

-
-
100-07-2
4-methoxy-benzoyl chloride

-
-
119649-45-5
4-(p-methoxybenzyloxy)butan-1-ol

| Conditions | Yield |
|---|---|
| Stage #1: Butane-1,4-diol With sodium hydride In tetrahydrofuran at 0℃; for 0.166667h; Stage #2: 4-methoxy-benzoyl chloride With tetra-(n-butyl)ammonium iodide In tetrahydrofuran at 0 - 20℃; for 17.5h; | 100% |

| Conditions | Yield |
|---|---|
| With sulfonic group functionalized polyacrylonitrile preoxidated nanofiber mat In cyclohexane at 150℃; for 2h; Dean-Stark; | 99.7% |
| With phosphorus modified SO4(2-)/TiO2 In cyclohexane for 2h; Dean-Stark; Reflux; | 98% |
| With cyclohexane for 2h; Dean-Stark; | 92% |

| Conditions | Yield |
|---|---|
| Trichlorbutylstannan at 80 - 84℃; for 9h; | 99% |
| Trichlorbutylstannan at 80 - 84℃; for 19h; Mechanism; different molar ratios, different times; | 99% |
| zirconium(IV) sulfate at 200℃; under 760.051 Torr; Product distribution / selectivity; Gas phase; | 99.5% |

| Conditions | Yield |
|---|---|
| With cyclohexane for 4h; Dean-Stark; | 99% |
| With sulfonic group functionalized polyacrylonitrile preoxidated nanofiber mat In cyclohexane at 150℃; for 2h; Dean-Stark; | 99.5% |
| With phosphorus modified SO4(2-)/TiO2 In cyclohexane for 2h; Dean-Stark; Reflux; | 98% |
| With [SOClMIm]Cl at 40℃; for 15h; | |
| With melamine formaldehyde resin supported ionic liquid and cuprous catalyst In cyclohexane for 2h; Dean-Stark; Reflux; | 80.24 %Chromat. |

| Conditions | Yield |
|---|---|
| With hydrogen; Cu-based catalyst at 210℃; Product distribution; Further Variations:; Temperatures; reaction in vapour phase, fixed bed reactor, coupled dehydrogenation reactions of title comp. and INO 160; | A 96.5% B 99.4% |


| Conditions | Yield |
|---|---|
| With calcium hydroxide for 2h; Autoclave; Cooling with ice; Inert atmosphere; Green chemistry; | 99.3% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide for 3h; Ambient temperature; | 99% |
| With potassium hydroxide at 20℃; Inert atmosphere; | 99% |
| Stage #1: Butane-1,4-diol With sodium hydride In tetrahydrofuran; mineral oil at 20℃; for 0.833333h; Stage #2: benzyl bromide In tetrahydrofuran; mineral oil at 20℃; for 14h; | 97% |

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine Inert atmosphere; | 99% |
| With N-ethyl-N,N-diisopropylamine In acetonitrile at 0 - 20℃; | 92% |
| With triethylamine In dichloromethane at 0 - 20℃; for 6h; Inert atmosphere; | 90% |
-
-
110-63-4
Butane-1,4-diol

-
-
13154-24-0
triisopropylsilyl chloride

-
-
175849-51-1
4-((triisopropylsilyl)oxy)butan-1-ol

| Conditions | Yield |
|---|---|
| With sodium hydride In tetrahydrofuran 1.) room temperature, 45 min, 2.) 0 deg C o room temperature, 30 min; | 99% |
| Stage #1: Butane-1,4-diol With sodium hydride In tetrahydrofuran at 0 - 20℃; for 0.75h; Inert atmosphere; Stage #2: triisopropylsilyl chloride In tetrahydrofuran Inert atmosphere; | 97% |
| Stage #1: Butane-1,4-diol With sodium hydride In tetrahydrofuran; mineral oil at 20℃; for 1h; Inert atmosphere; Cooling with ice; Stage #2: triisopropylsilyl chloride In tetrahydrofuran at 20℃; for 2h; Inert atmosphere; | 95.6% |
-
-
110-63-4
Butane-1,4-diol

-
-
38261-81-3
(S)-2-methylbutyl tosylate

-
-
104773-60-6
(S)-4-(2-methylbutoxy)butan-1-ol

| Conditions | Yield |
|---|---|
| Stage #1: Butane-1,4-diol With sodium In tetrahydrofuran at 20℃; Stage #2: (S)-2-methylbutyl tosylate In tetrahydrofuran at 70 - 80℃; | 99% |
| Stage #1: Butane-1,4-diol With sodium In tetrahydrofuran at 20℃; Stage #2: (S)-2-methylbutyl tosylate In tetrahydrofuran at 70 - 80℃; | 78% |
-
-
110-63-4
Butane-1,4-diol

-
-
498-21-5, 636-60-2
2-methylbutanedioic acid

| Conditions | Yield |
|---|---|
| With scandium tris(trifluoromethanesulfonate) at 35℃; under 0.3 - 3 Torr; for 110h; | 99% |
1,4-Butanediol History
Other early preparations were by reduction of succinaldehyde or succinic esters and by saponification of the diacetate prepared from 1,4-dihalobutanes. Catalytic hydrogenation of butynediol, now the principal commercial route, was first described in 1910.
1,4-Butanediol Analytical Methods
1,4-Butanediol Specification
The IUPAC name of this chemical is butane-1,4-diol. With the CAS registry number 110-63-4 and EINECS registry number 203-786-5, it is also named as 1,4-Butanediol. In addition, the molecular formula is C4H10O2 and the molecular weight is 90.121. It is odorless colorless liquid or solid (depending upon temperature).This colorless viscous liquid is derived from butane by placement of alcohol groups at each end of the chain. It is one of four stable isomers of butanediol. What's more, it is incompatible with strong oxidizing agents, mineral acids, acid chlorides, acid anhydrides. It should be stored in a cool, ventilated and dry place. The storage place must stay away from oxidant and water.
Physical properties about this chemical are: (1)# of Rule of 5 Violations: 0 ; (2)ACD/BCF (pH 5.5): 1; (3)ACD/BCF (pH 7.4): 1; (4)ACD/KOC (pH 5.5): 9.112; (5)ACD/KOC (pH 7.4): 9.112; (6)#H bond acceptors: 2; (7)#H bond donors: 2; (8)#Freely Rotating Bonds: 5; (9)Polar Surface Area: 40.46 Å2; (10)Index of Refraction: 1.441; (11)Molar Refractivity: 23.65 cm3; (12)Molar Volume: 89.574 cm3; (13)Polarizability: 9.376 10-24cm3; (14)Surface Tension: 39.637 dyne/cm; (15)Density: 1.006 g/cm3; (16)Flash Point: 105.909 °C; (17)Enthalpy of Vaporization: 54.011 kJ/mol; (18)Boiling Point: 227.999 °C at 760 mmHg; (19)Vapour Pressure: 0.015 mmHg at 25°C.
Preparation of 1,4-Butanediol: it can be prepared by 1,4-dichlorobutene. Through the hydrolysis and hydrogenation, you can get 1,4-Butanediol.

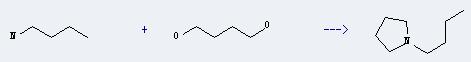
Uses of 1,4-Butanediol: it can be used in the production of THF, γ-butyllactone and butanediol polyp-phthalate. It also can be used as chain extender and raw materials of polyester to produce polyurethane elastomer and soft polyurethane foam plastics. It has good absorbency and increased flexibility, can make gelatin softener and SAP. In addition, it can prepare derivative of N-methyl pyrrole, N-vinyl pyrrole and other pyrrole, also can be used in preparation of vitamin B6, pesticides and herbicides. What's more, it can react with butylamine to get 1-butyl-pyrrolidine. This reaction will need catalyst RuCl3. The reaction time is 5 hours at reaction temperature of 220 °C. The yield is about 36%.
When you are using this chemical, please be cautious about it as the following:
It is harmful if swallowed. You should wear suitable protective clothing during using it.
You can still convert the following datas into molecular structure:
(1)SMILES: C(CCO)CO
(2)InChI: InChI=1/C4H10O2/c5-3-1-2-4-6/h5-6H,1-4H2
(3)InChIKey: WERYXYBDKMZEQL-UHFFFAOYAD
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| guinea pig | LD50 | oral | 1200mg/kg (1200mg/kg) | BEHAVIORAL: ALTERED SLEEP TIME (INCLUDING CHANGE IN RIGHTING REFLEX) BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BLOOD: OTHER CHANGES | Hygiene and Sanitation Vol. 33(1-3), Pg. 41, 1968. |
| man | LDLo | rectal | 429mg/kg (429mg/kg) | Pharmazie. Vol. 3, Pg. 110, 1948. | |
| mouse | LD50 | intraperitoneal | 1650mg/kg (1650mg/kg) | Toxicology and Applied Pharmacology. Vol. 49, Pg. 385, 1979. | |
| mouse | LD50 | oral | 2062mg/kg (2062mg/kg) | BEHAVIORAL: ALTERED SLEEP TIME (INCLUDING CHANGE IN RIGHTING REFLEX) BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BLOOD: OTHER CHANGES | Hygiene and Sanitation Vol. 33(1-3), Pg. 41, 1968. |
| rabbit | LD50 | oral | 2531mg/kg (2531mg/kg) | BEHAVIORAL: ALTERED SLEEP TIME (INCLUDING CHANGE IN RIGHTING REFLEX) BLOOD: OTHER CHANGES BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) | Hygiene and Sanitation Vol. 33(1-3), Pg. 41, 1968. |
| rat | LCLo | inhalation | 15gm/m3/4H (15000mg/m3) | SENSE ORGANS AND SPECIAL SENSES: OTHER CHANGES: OLFACTION LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES | Inhalation Toxicology. Vol. 3, Pg. 379, 1991. |
| rat | LD50 | intraperitoneal | 1070mg/kg (1070mg/kg) | Journal of Pharmacy and Pharmacology. Vol. 26, Pg. 597, 1974. | |
| rat | LD50 | oral | 1525mg/kg (1525mg/kg) | BEHAVIORAL: ALTERED SLEEP TIME (INCLUDING CHANGE IN RIGHTING REFLEX) BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BLOOD: OTHER CHANGES | Hygiene and Sanitation Vol. 33(1-3), Pg. 41, 1968. |
| women | LDLo | unreported | 300mg/kg (300mg/kg) | BEHAVIORAL: ALTERED SLEEP TIME (INCLUDING CHANGE IN RIGHTING REFLEX) | Pharmazie. Vol. 3, Pg. 110, 1948. |
Related Products
- 10,10'-Bis([1,1'-biphenyl]-4-yl)-9,9'-bianthracene
- 10,10'-Dibromo-9,9'-bianthryl
- 10,10-Dimethylanthrone
- 10,10-Oxybisphenoxarsine
- 10-[1,1'-Biphenyl]-4-yl-2-(1-methylethyl)-9-oxo-9H-thioxanthenium hexafluorophosphate
- 10,11-Dehydroimipramine
- 10,11-Dihydro-11-oxodibenzo[b,f][1,4]thiazepine
- 10,11-Dihydro-2-(4-methyl-1-piperazinyl)-11-(2-athiazolyl)-pyridazino(3,4-b)(1,4)benzoxazepine
- 10,11-DIHYDRO-2-(4-METHYL-1-PIPERAZINYL)-11-(3,4-XYLYL)PYRIDAZINO(3,4b)(1,4)-BENZOXAZEPINE MALEATE
- 10,11-Dihydro-5-(3-dimethylamino-2-methylpropyl)-5h-dibenz (b,f)azepine
- 110637-52-0
- 110637-53-1
- 110638-01-2
- 110638-68-1
- 110642-44-9
- 110-64-5
- 110-65-6
- 110659-07-9
- 110661-91-1
- 110-66-7
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View