-
Name
1-Amino-4-methylpiperazine
- EINECS 230-053-7
- CAS No. 6928-85-4
- Article Data22
- CAS DataBase
- Density 0.991 g/cm3
- Solubility miscible with water
- Melting Point
- Formula C5H13N3
- Boiling Point 172.811 °C at 760 mmHg
- Molecular Weight 115.178
- Flash Point 58.128 °C
- Transport Information
- Appearance clear colorless to light yellow liquid
- Safety 24/25-36-26
- Risk Codes 36/37/38-20/21/22
-
Molecular Structure
-
Hazard Symbols
 Xi,
Xi, Xn
Xn
- Synonyms Piperazine,1-amino-4-methyl- (6CI,7CI,8CI);(4-Methylpiperazin-1-yl)amine;1-Methyl-4-aminopiperazine;4-Methyl-1-aminopiperazine;N-Amino-N'-methylpiperazine;N1-Methyl-N4-aminopiperazine;NSC 80649;1-Piperazinamine,4-methyl-;
- PSA 32.50000
- LogP -0.31640
Synthetic route
-
-
16339-07-4
1-methyl-4-nitroso-piperazine

-
A
-
109-01-3
1-methyl-piperazine

-
B
-
6928-85-4
N-amino-N'-methylpiperazine

| Conditions | Yield |
|---|---|
| With carbon dioxide; water; ammonium chloride; zinc at 35℃; under 1500.15 Torr; for 1.5h; Reagent/catalyst; Temperature; Pressure; Autoclave; Green chemistry; | A n/a B 96% |
| With hydrogen In water at 50℃; under 37503.8 Torr; for 5h; Autoclave; Green chemistry; | A n/a B 38% |
-
-
16339-07-4
1-methyl-4-nitroso-piperazine

-
-
6928-85-4
N-amino-N'-methylpiperazine

| Conditions | Yield |
|---|---|
| With hydrogen In dichloromethane; water at 50℃; under 37503.8 Torr; for 5h; Solvent; Autoclave; Green chemistry; | 94% |
| With lithium aluminium tetrahydride In diethyl ether for 10h; Heating; | 88% |
| With acetic acid; zinc In water Cooling with ice; | 78% |

| Conditions | Yield |
|---|---|
| With hydrazine hydrate | 87% |

| Conditions | Yield |
|---|---|
| With hydrazine hydrate at 50℃; for 2h; | A 2% B 87% |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 96 percent / SOCl2 / CHCl3 / 2 h / 20 °C 2: 87 percent / aq. NH2NH2 View Scheme |
-
-
118306-22-2
4-chlorocarbonyl-4,9-dihydro-1-methyl-10H-thieno[3,4-b][1,5]benzodiazepin-10-one

-
A
-
6928-85-4
N-amino-N'-methylpiperazine
-
-
118306-21-1
4-(chlorocarbonyl)-4,9-dihydro-3-methyl-10H-thieno[3,4-b][1,5]benzodiazepin-10-one

-
A
-
6928-85-4
N-amino-N'-methylpiperazine
-
-
118306-23-3
4-(chlorocarbonyl)-4,9-dihydro-1,3-dimethyl-10H-thieno[3,4-b][1,5]benzodiazepin-10-one

-
A
-
6928-85-4
N-amino-N'-methylpiperazine
-
-
118306-24-4
3-chloro-4-(chlorocarbonyl)-4,9-dihydro-10H-thieno[3,4-b][1,5]benzodiazepin-10-one

-
A
-
6928-85-4
N-amino-N'-methylpiperazine
-
-
21223-19-8
CH3N(CH2CH2)2NNH2*BH3

-
-
7732-18-5
water

-
A
-
6928-85-4
N-amino-N'-methylpiperazine

-
B
-
1333-74-0
hydrogen

-
C
-
11113-50-1
boric acid

| Conditions | Yield |
|---|---|
| In water | |
| In water |


| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: hydrogenchloride; sodium nitrite / water; tetrahydrofuran / 4 h / 20 °C 2: acetic acid; zinc / water / Cooling with ice View Scheme | |
| Multi-step reaction with 2 steps 1: sodium nitrite; hydrogenchloride / water; tetrahydrofuran / 4 h / 0 - 25 °C 2: zinc; acetic acid / water / 5 h / 0 °C View Scheme | |
| Multi-step reaction with 2 steps 1: sodium nitrite; hydrogenchloride / water; tetrahydrofuran / 4 h / 25 °C 2: zinc; acetic acid / water / 5 h / 0 °C View Scheme | |
| Multi-step reaction with 2 steps 1.1: hydrogenchloride / water / 0.17 h / 40 °C 1.2: 1.5 h / 20 - 30 °C 2.1: hydrogen / water / 5 h / 50 °C / 37503.8 Torr / Autoclave; Green chemistry View Scheme |
-
-
6928-85-4
N-amino-N'-methylpiperazine

-
-
1079-66-9
chloro-diphenylphosphine

-
-
849371-46-6
1-diphenylphosphinoamino-4-methylpiperazine

| Conditions | Yield |
|---|---|
| With triethylamine In diethyl ether at 20℃; for 1h; | 100% |
| With triethylamine In tetrahydrofuran for 5h; | 85% |
-
-
6928-85-4
N-amino-N'-methylpiperazine

| Conditions | Yield |
|---|---|
| With trifluoroborane diethyl ether In 1,4-dioxane | 100% |
-
-
6928-85-4
N-amino-N'-methylpiperazine

-
-
879486-81-4
N-(4-methylpiperazin-1-yl)-N'-[4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-phenyl]-urea

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 6h; | 100% |
-
-
6928-85-4
N-amino-N'-methylpiperazine

-
-
1407543-46-7
(Z)-5-(benzo[d][1,3]dioxol-5-ylmethylene)-4-(methylthio)thiazol-2(5H)-one

-
-
1408060-02-5
(4Z,5Z)-5-(benzo[d][1,3]dioxol-5-ylmethylene)-4-((4-methylpiperazin-1-yl)imino)thiazolidin-2-one

| Conditions | Yield |
|---|---|
| In ethanol at 85℃; | 100% |
-
-
6928-85-4
N-amino-N'-methylpiperazine

| Conditions | Yield |
|---|---|
| With hydrazine In tetrahydrofuran for 4h; Condensation; Heating; | 98% |
-
-
6928-85-4
N-amino-N'-methylpiperazine

-
-
97480-49-4
difluoro(diethoxyphosphinyl)acetyl chloride

-
-
1217898-90-2
diethyl 1,1-difluoro-2-(4-methylpiperazin-1-ylamino)-2-oxoethylphosphonate

| Conditions | Yield |
|---|---|
| With pyridine; dmap In tetrahydrofuran at 0 - 20℃; Inert atmosphere; | 98% |

| Conditions | Yield |
|---|---|
| With acetic acid In butan-1-ol at 70℃; for 2h; Temperature; Solvent; Large scale; | 97.87% |
| In water; N,N-dimethyl-formamide at 75℃; for 0.333333h; Temperature; Time; | 92% |
| In N,N-dimethyl-formamide at 80℃; for 0.333333h; Concentration; |
-
-
6928-85-4
N-amino-N'-methylpiperazine

-
-
35967-24-9
(3,4,5-trimethoxyphenyl)isothiocyanate

| Conditions | Yield |
|---|---|
| In ethanol for 1h; Reflux; | 97% |
-
-
6928-85-4
N-amino-N'-methylpiperazine

-
-
49627-27-2
sulindac sulfide

| Conditions | Yield |
|---|---|
| With O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate; triethylamine In acetonitrile at 20℃; Inert atmosphere; | 96% |
-
-
6928-85-4
N-amino-N'-methylpiperazine

-
-
75-15-0
carbon disulfide

-
-
31599-79-8
2-(chloromethyl)-3-methylnaphthalene-1,4-dione

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 20℃; | 95.9% |


| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide; trifluoroacetic acid for 5h; | 95% |

| Conditions | Yield |
|---|---|
| With 5-(di-tert-butylphosphino)-1′, 3′, 5′-triphenyl-1′H-[1,4′]bipyrazole; bis[chloro(1,2,3-trihapto-allylbenzene)palladium(II)]; sodium t-butanolate at 110℃; for 12h; Buchwald-Hartwig Coupling; | 95% |
| With potassium tert-butylate; tris(dibenzylideneacetone)dipalladium (0) In 1,4-dioxane at 120℃; for 5h; | 88% |
| With C38H49N2P*C9H10ClPd In 1,4-dioxane at 20℃; for 7h; Inert atmosphere; Schlenk technique; | 77% |

| Conditions | Yield |
|---|---|
| In hexane hexane soln. of Me2Cd added to hexane; then 1-amino-4-methylpiperazine added; mixt. stirred; elem. anal.; | 94% |
-
-
6928-85-4
N-amino-N'-methylpiperazine

| Conditions | Yield |
|---|---|
| With sodium sulfate In dichloromethane at 20℃; for 12h; | 94% |
-
-
6928-85-4
N-amino-N'-methylpiperazine

-
-
42141-25-3
2-thiocyanato-cyclohex-1-enecarbaldehyde

| Conditions | Yield |
|---|---|
| Stage #1: N-amino-N'-methylpiperazine; 2-thiocyanato-cyclohex-1-enecarbaldehyde With acetic acid for 0.25h; Stage #2: With perchloric acid; acetic acid for 1h; | 93% |
-
-
6928-85-4
N-amino-N'-methylpiperazine

| Conditions | Yield |
|---|---|
| In ethanol for 8h; Reflux; | 93% |
-
-
6928-85-4
N-amino-N'-methylpiperazine

-
-
21375-88-2
1-bromo-2-(phenylethenyl)benzene

-
-
1264670-27-0
1-(4-methyl-piperazin-1-yl)-2-phenyl-1H-indole

| Conditions | Yield |
|---|---|
| With bis[chloro(1,2,3-trihapto-allylbenzene)palladium(II)]; OTips-DalPhos; potassium tert-butylate In toluene at 90℃; for 12h; Inert atmosphere; Sealed vial; | 92% |
| With bis[chloro(1,2,3-trihapto-allylbenzene)palladium(II)]; OTips-DalPhos; potassium tert-butylate In toluene at 90℃; for 12h; Buchwald-Hartwig Coupling; Inert atmosphere; Sealed tube; | 92% |
| With caesium carbonate; palladium dichloride; tri tert-butylphosphoniumtetrafluoroborate In N,N-dimethyl-formamide at 110℃; for 3h; Inert atmosphere; | 74% |
-
-
6928-85-4
N-amino-N'-methylpiperazine

-
-
75-15-0
carbon disulfide

-
-
359869-12-8
2-(1-chloromethyl-2,6-dioxo-piperidine-3-yl)-1,3-dihydro-2H-isoindole-1,3-dione

-
-
1083189-99-4
[3-(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)-2,6-dioxopiperidin-1-yl]methyl 4-methylpiperazin-1-yldithiocarbamate

| Conditions | Yield |
|---|---|
| In acetonitrile at 20℃; for 48h; | 91% |


| Conditions | Yield |
|---|---|
| With dimethyl sulfoxide In tetrahydrofuran for 0.416667h; | 90% |
-
-
6928-85-4
N-amino-N'-methylpiperazine

-
-
36881-02-4
2-(4-chlorophenyl)-2-methyl-1,3-dioxolane

| Conditions | Yield |
|---|---|
| With potassium tert-butylate; tris(dibenzylideneacetone)dipalladium (0) In 1,4-dioxane at 120℃; for 2.5h; | 90% |

| Conditions | Yield |
|---|---|
| With potassium tert-butylate; tris(dibenzylideneacetone)dipalladium (0) In 1,4-dioxane at 120℃; for 5.5h; | 90% |

| Conditions | Yield |
|---|---|
| With triethylamine In acetone for 8h; | 90% |
-
-
6928-85-4
N-amino-N'-methylpiperazine

| Conditions | Yield |
|---|---|
| With acetic acid In methanol for 12h; Reflux; | 90% |

| Conditions | Yield |
|---|---|
| In ethanol at 20℃; for 5h; | 90% |
| at 50℃; | 53% |
-
-
6928-85-4
N-amino-N'-methylpiperazine

-
-
20335-60-8
1-formyl-5-nitroisoquinoline

| Conditions | Yield |
|---|---|
| In ethanol for 3h; Heating; | 89% |

| Conditions | Yield |
|---|---|
| With titanium dioxide nanoparticles at 80℃; for 7h; Neat (no solvent); | 89% |

-
-
6928-85-4
N-amino-N'-methylpiperazine

-
-
162758-35-2
rimonabant acid

-
-
953758-69-5
5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-N-(4-methylpiperazin-1-yl)-1H-pyrazole-3-carboxamide

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine In dichloromethane at 0 - 20℃; for 6h; Inert atmosphere; | 89% |

| Conditions | Yield |
|---|---|
| In ethanol for 1h; Reflux; | 89% |
1-Amino-4-methylpiperazine Specification
1. Introduction of 1-Amino-4-methylpiperazine
The 1-Amino-4-methylpiperazine, with the CAS registry number 6928-85-4, is also known as 1-Piperazinamine, 4-methyl-. It belongs to the product categories of Piperaizine; Piperazine Derivates; Building Blocks; Heterocyclic Building Blocks; Piperazines. This chemical's molecular formula is C5H13N3 and molecular weight is 115.18. What's more, its systematic name is called 4-Methyl-1-piperazinamine. It should be stored in a cool, dry and well-ventilated place.
2. Properties of 1-Amino-4-methylpiperazine
Physical properties about 1-Amino-4-methylpiperazine are: (1)ACD/LogP: -1.301; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): -4.36; (4)ACD/LogD (pH 7.4): -3.43; (5)ACD/BCF (pH 5.5): 1.00; (6)ACD/BCF (pH 7.4): 1.00; (7)ACD/KOC (pH 5.5): 1.00; (8)ACD/KOC (pH 7.4): 1.00; (9)#H bond acceptors: 3; (10)#H bond donors: 2; (11)#Freely Rotating Bonds: 1; (12)Polar Surface Area: 32.5 Å2; (13)Index of Refraction: 1.495; (14)Molar Refractivity: 33.841 cm3; (15)Molar Volume: 116.143 cm3; (16)Polarizability: 13.416×10-24 cm3; (17)Surface Tension: 35.26 dyne/cm; (18)Density: 0.992 g/cm3; (19)Flash Point: 58.128 °C; (20)Enthalpy of Vaporization: 40.918 kJ/mol; (21)Boiling Point: 172.811 °C at 760 mmHg; (22)Vapour Pressure: 1.31 mmHg at 25 °C
3. Structure Descriptors of 1-Amino-4-methylpiperazine
You can still convert the following datas into molecular structure:
(1) SMILES: NN1CCN(C)CC1
(2) InChI: InChI=1S/C5H13N3/c1-7-2-4-8(6)5-3-7/h2-6H2,1H3
(3) InChIKey: RJWLLQWLBMJCFD-UHFFFAOYSA-N
4. Safety Information of 1-Amino-4-methylpiperazine
Hazard Statement(s) H315: Causes skin irritation.
H319: Causes serious eye irritation.
H335: May cause respiratory irritation.
Precautionary Statement(s) P302 + P352: IF ON SKIN: Wash with plenty of soap and water.
P304 + P340: IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
P305 + P351 + P338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing.
5. Preparation of 1-Amino-4-methylpiperazine
1-Amino-4-methylpiperazine can be prepared by 1-methyl-4-nitroso-piperazine. The reaction occurs with reagent LiAlH4 and other condition of heating for 10 hours. The yield is 88 %.

6. Uses of 1-Amino-4-methylpiperazine
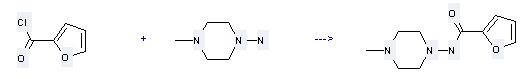
1-Amino-4-methylpiperazine is used as an intermediate of pharmaceutical and for synthesis of antibiotics of rifampicin; (2) it is used to produce other chemicals. For example, it can react with furan-2-carbonyl chloride to get furan-2-carboxylic acid (4-methyl-piperazin-1-yl)-amide. The reaction occurs with solvent benzene and other condition of heating for 1 hour. The yield is 88 %.
7. Other details of 1-Amino-4-methylpiperazine
When you are dealing with this chemical, you should be very careful. This chemical is inflammation to the skin, eyes and respiratory system. It may cause damage to health by inhalation, in contacting with skin and if swallowed. Therefore, you should wear suitable protective clothing and avoid contacting with skin, eyes. In case of contacting with eyes, you should rinse immediately with plenty of water and seek medical advice.
Related Products
- 1-Amino-4-methylpiperazine
- 6929-04-0
- 6929-08-4
- 6929-24-4
- 69300-15-8
- 693-02-7
- 693-03-8
- 69304-37-6
- 69304-47-8
- 693-04-9
- 693-05-0
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View