-
Name
2-BUTYL-1-OCTANOL
- EINECS 223-470-0
- CAS No. 3913-02-8
- Article Data35
- CAS DataBase
- Density 0.833 g/mL at 25 °C(lit.)
-
Solubility
- Hazard Symbols UN NO.
- Synonyms 2-Butyl-1-octanol;2-Butyloctanol; 2-Butyloctyl alcohol; 5-(Hydroxymethyl)undecane; Guerbet C12;Guerbet dodecanol; Isofol 12; Jarcol I 12; NSC 2414
- PSA 20.23000
- LogP 3.75550
Synthetic route
-
-
27610-92-0
2-butyloctanoic acid

-
-
3913-02-8
2-(n-butyl)octanol

| Conditions | Yield |
|---|---|
| With samarium diiodide; water; triethylamine In tetrahydrofuran at 20℃; for 18h; Inert atmosphere; | 94% |
| With samarium diiodide; water; triethylamine In tetrahydrofuran at 20℃; Inert atmosphere; | 99 %Spectr. |
| With samarium diiodide; tributyl-amine; water In tetrahydrofuran at 23℃; Kinetics; Concentration; Inert atmosphere; chemoselective reaction; |

| Conditions | Yield |
|---|---|
| With zinc(II) oxide; sodium hydroxide at 210℃; for 3h; Temperature; Inert atmosphere; | 86% |
| With dichloro(pentamethylcyclopentadienyl) iridium; potassium tert-butylate; 1,7-Octadiene In para-xylene at 120℃; for 4h; Guerbet reaction; | 77% |
| With dicarbonyl(η4-3,4-bis(4-methoxyphenyl)-2,5-diphenylcyclopenta-2,4-dienone)(iodine)ruthenium[1,3 -dimethylimidazolium]; p-benzoquinone; sodium hydroxide at 150℃; for 4h; Reagent/catalyst; Schlenk technique; Inert atmosphere; | 30.6% |
-
-
53692-86-7
methyl 2-butyloctanoate

-
-
3913-02-8
2-(n-butyl)octanol

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride In tetrahydrofuran at 20℃; for 2h; Cooling with ice; | 83% |
| With isopropyl alcohol In hexane at 0 - 20℃; for 0.583333h; Bouveault-Blanc Reduction; Inert atmosphere; | 73% |
| With methanol; thulium(II) iodide In tetrahydrofuran at 23℃; Inert atmosphere; Schlenk technique; | 63 %Spectr. |
| With samarium diiodide; water; triethylamine In tetrahydrofuran Inert atmosphere; chemoselective reaction; |
-
-
112-30-1
1-Decanol

-
-
111-27-3
hexan-1-ol

-
A
-
3913-02-8
2-(n-butyl)octanol

-
B
-
2425-77-6
2-hexyldecan-1-ol

-
C
-
21078-85-3
5-hydroxymethyl-pentadecane

-
D
-
5333-42-6
2-octyldodecan-1-ol

| Conditions | Yield |
|---|---|
| With potassium hydroxide at 255℃; for 6h; Reagent/catalyst; Dean-Stark; Inert atmosphere; | A 8.4% B n/a C n/a D 39.1% |

-
-
111-87-5
octanol

-
-
111-27-3
hexan-1-ol

-
A
-
17658-63-8
2-hexadecyleicosyl alcohol

-
B
-
3913-02-8
2-(n-butyl)octanol

-
C
-
19780-79-1
2-hexyl-1-octanol

-
D
-
21078-81-9, 123518-89-8
2-butyl-1-decanol

| Conditions | Yield |
|---|---|
| With potassium hydroxide at 225℃; for 6h; Dean-Stark; Inert atmosphere; | A 22.32% B 19.2% C n/a D n/a |

-
-
66-25-1
hexanal

-
-
100-51-6
benzyl alcohol

-
A
-
3913-02-8
2-(n-butyl)octanol

-
B
-
7500-73-4
(±)-2-benzyl-1-hexanol

| Conditions | Yield |
|---|---|
| With potassium hydroxide |


| Conditions | Yield |
|---|---|
| With potassium hydroxide | |
| With calcium carbide at 110℃; | |
| Multi-step reaction with 3 steps 1: L-lysine / chloroform-d1 / 20 °C / Inert atmosphere 2: glucose dehydrogenase; NADPH; D-glucose; ene reductase from gluconobacter oxydans / methanol; aq. phosphate buffer / 24 h / 20 °C / pH 7 / Inert atmosphere; Enzymatic reaction 3: alcohol dehydrogenase rhodococcus sp.; glucose dehydrogenase; NADPH; D-glucose / methanol; aq. phosphate buffer / 24 h / 20 °C / pH 7 / Inert atmosphere; Enzymatic reaction View Scheme |

-
-
3913-02-8
2-(n-butyl)octanol

| Conditions | Yield |
|---|---|
| With N,N,N,N,N,N-hexamethylphosphoric triamide; tert-butyl alcohol |

| Conditions | Yield |
|---|---|
| With zirconocene dichloride; oxygen; diisobutylaluminum chloride 1.) 40 deg C, 4 h, 2.) 4 h; Yield given. Multistep reaction; |
-
-
19779-06-7
sodium hexanolate

-
-
66-25-1
hexanal

-
-
111-27-3
hexan-1-ol

-
A
-
3913-02-8
2-(n-butyl)octanol

-
B
-
142-62-1
hexanoic acid


| Conditions | Yield |
|---|---|
| at 270℃; under 14710.2 Torr; |


| Conditions | Yield |
|---|---|
| at 270℃; under 14710.2 Torr; |


| Conditions | Yield |
|---|---|
| at 230 - 290℃; unter Druck; |

| Conditions | Yield |
|---|---|
| at 205℃; under 1471.02 Torr; |

-
-
71-36-3
butan-1-ol

-
-
111-27-3
hexan-1-ol

-
A
-
104-76-7
2-Ethylhexyl alcohol

-
B
-
3913-02-8
2-(n-butyl)octanol


-
-
3913-02-8
2-(n-butyl)octanol

| Conditions | Yield |
|---|---|
| With ethanol; sodium |


| Conditions | Yield |
|---|---|
| With sodium hydroxide anschliessendes Erhitzen in Gegenwart von Zinkstaub+Zinkoxyd auf 205grad unter ca. 2 at Druck unter Abtrennen des gebildeten Wassers; |
-
-
88015-70-7
2-butyl-1-octanal

-
-
3913-02-8
2-(n-butyl)octanol

| Conditions | Yield |
|---|---|
| With glucose dehydrogenase; D-glucose; alcohol dehydrogenase rhodococcus sp.; NADPH In methanol; aq. phosphate buffer at 20℃; for 24h; pH=7; Solvent; Inert atmosphere; Enzymatic reaction; |
-
-
64935-38-2, 99915-14-7, 13019-16-4
2-(n-butyl)-2-octenal

-
-
3913-02-8
2-(n-butyl)octanol

| Conditions | Yield |
|---|---|
| Stage #1: 2-butyl-oct-2-en-1-al With methanol; 5%-palladium/activated carbon at 50℃; under 7500.75 Torr; Large scale; Stage #2: With sodium tetrahydroborate at 50℃; for 1h; Temperature; Large scale; | 4.5 kg |
-
-
592-41-6
1-hexene

-
-
75-24-1
trimethylaluminum

-
A
-
624-22-6
2-methylhexan-1-ol

-
B
-
3913-02-8
2-(n-butyl)octanol

| Conditions | Yield |
|---|---|
| Stage #1: 1-hexene; trimethylaluminum With (η5–neomenthylindenyl)2ZrCl2 In dichloromethane at 20℃; for 72h; Inert atmosphere; Stage #2: With oxygen In dichloromethane at 0℃; for 26h; |

-
-
592-41-6
1-hexene

-
-
75-24-1
trimethylaluminum

-
A
-
3913-02-8
2-(n-butyl)octanol

-
B
-
75854-77-2
1-deuterio-2-methylhexane

| Conditions | Yield |
|---|---|
| Stage #1: 1-hexene; trimethylaluminum With (η5–neomenthylindenyl)2ZrCl2 In dichloromethane at 20℃; for 72h; Inert atmosphere; Stage #2: With hydrogen chloride In dichloromethane; water-d2 at 0℃; |


| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: sodium methylate / N,N-dimethyl-formamide / 0.5 h / 80 °C / Large scale 1.2: 2 h / 120 °C / Large scale 2.1: sodium methylate / N,N-dimethyl-formamide / 0.5 h / 80 °C / Large scale 2.2: 2 h / 120 °C / Large scale 3.1: lithium chloride / N,N-dimethyl-formamide; water / 24 h / 160 °C / Large scale 4.1: lithium aluminium tetrahydride / tetrahydrofuran / 2 h / 20 °C / Cooling with ice View Scheme |
-
-
122459-92-1
Hexylmalonsaeure-dimethylester

-
-
3913-02-8
2-(n-butyl)octanol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: sodium methylate / N,N-dimethyl-formamide / 0.5 h / 80 °C / Large scale 1.2: 2 h / 120 °C / Large scale 2.1: lithium chloride / N,N-dimethyl-formamide; water / 24 h / 160 °C / Large scale 3.1: lithium aluminium tetrahydride / tetrahydrofuran / 2 h / 20 °C / Cooling with ice View Scheme |

-
-
3913-02-8
2-(n-butyl)octanol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: lithium chloride / N,N-dimethyl-formamide; water / 24 h / 160 °C / Large scale 2: lithium aluminium tetrahydride / tetrahydrofuran / 2 h / 20 °C / Cooling with ice View Scheme |
-
-
64-17-5
ethanol

-
-
111-27-3
hexan-1-ol

-
A
-
111-87-5
octanol

-
B
-
104-76-7
2-Ethylhexyl alcohol

-
C
-
20592-10-3
2-ethyloctane-1-ol

-
D
-
112-30-1
1-Decanol

-
E
-
3913-02-8
2-(n-butyl)octanol

| Conditions | Yield |
|---|---|
| With bis[dichloro(pentamethylcyclopentadienyl)iridium(III)]; potassium tert-butylate; 1,7-Octadiene In tetrahydrofuran at 100℃; for 24h; Inert atmosphere; Overall yield = 19 percent; Overall yield = 25 mg; |

-
-
3913-02-8
2-(n-butyl)octanol

-
-
1219732-61-2
4-dodecyloxy-4'-hydroxybenzil

-
-
1219732-56-5
4-dodecyloxy-4'-(2-butyloctyloxy)benzil

| Conditions | Yield |
|---|---|
| With triphenylphosphine; diethylazodicarboxylate In tetrahydrofuran; toluene at 0 - 20℃; for 2h; Mitsunobu reaction; | 100% |
-
-
3913-02-8
2-(n-butyl)octanol

| Conditions | Yield |
|---|---|
| With dmap; oxo[hexa(trifluoroacetato)]tetrazinc In toluene at 110℃; Inert atmosphere; Molecular sieve; | 99.8% |
| With dmap; oxo[hexa(trifluoroacetato)]tetrazinc In toluene at 100℃; Inert atmosphere; Molecular sieve; Schlenk technique; Sealed tube; | 81.6% |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 0 - 20℃; for 24h; Inert atmosphere; | 99% |
-
-
3913-02-8
2-(n-butyl)octanol

-
-
85531-02-8
5-(bromomethyl)undecane

| Conditions | Yield |
|---|---|
| With bromine; triphenylphosphine In dichloromethane at 0 - 20℃; Inert atmosphere; | 95.3% |
| With 1H-imidazole; carbon tetrabromide; triphenylphosphine In dichloromethane at 20℃; for 3h; Cooling with ice; | 90% |
| With bromine; triphenylphosphine In dichloromethane at 0 - 20℃; Inert atmosphere; Schlenk technique; | 88.1% |
-
-
3913-02-8
2-(n-butyl)octanol

-
-
1256345-37-5
5-(iodomethyl)undecane

| Conditions | Yield |
|---|---|
| With 1H-imidazole; iodine; triphenylphosphine In dichloromethane at 0 - 20℃; for 2h; | 95% |
| With 1H-imidazole; iodine; triphenylphosphine In dichloromethane at 0 - 20℃; for 2h; | 95% |
-
-
3913-02-8
2-(n-butyl)octanol

-
-
93778-52-0
2-decyltetradecanoic acid

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid at 80 - 83℃; for 24h; | 93.4% |

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 5 - 20℃; | 93% |
-
-
824-72-6
P,P-dichlorophenylphosphine oxide

-
-
3913-02-8
2-(n-butyl)octanol

-
-
141024-65-9
2-butyl-1-octyl phenylphosphonic acid

| Conditions | Yield |
|---|---|
| Stage #1: P,P-dichlorophenylphosphine oxide; 2-(n-butyl)octanol With pyridine at 5 - 10℃; for 1.5 - 8.5h; Stage #2: With hydrogenchloride; water pH=1; | 90% |
-
-
3913-02-8
2-(n-butyl)octanol

-
-
33288-79-8
4,4'-dihydroxybenzil

-
-
1219732-57-6
4,4'-di(2-butyloctyloxy)benzil

| Conditions | Yield |
|---|---|
| With triphenylphosphine; diethylazodicarboxylate In tetrahydrofuran; toluene at 0 - 20℃; for 2h; Mitsunobu reaction; | 90% |
-
-
3913-02-8
2-(n-butyl)octanol

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In water at 200℃; Inert atmosphere; | 90% |
-
-
24287-95-4
2-bromothiophene-3-carboxylic acid

-
-
3913-02-8
2-(n-butyl)octanol

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 20℃; for 48h; | 90% |

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide | 85% |

| Conditions | Yield |
|---|---|
| With sulfuric acid In toluene at 120 - 130℃; for 4h; Dean-Stark; | 83% |
-
-
69778-83-2
4-methoxy-3-pyrrolin-2-one

-
-
3913-02-8
2-(n-butyl)octanol

-
-
914275-86-8
4-(2-butyloctyloxy)-1,5-dihydropyrrol-2-one

| Conditions | Yield |
|---|---|
| With methanesulfonic acid at 80℃; for 24h; | 82% |

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In toluene for 18h; Dean-Stark; | 79% |
-
-
3913-02-8
2-(n-butyl)octanol

| Conditions | Yield |
|---|---|
| With Ti(acac)2 In toluene at 110℃; for 10h; Inert atmosphere; | 79% |
-
-
3913-02-8
2-(n-butyl)octanol

-
-
56962-08-4
4,5-dichlorophthalic acid

-
-
1030871-33-0
bis(2-butyloctyl)-4,5-dichlorophthalate

| Conditions | Yield |
|---|---|
| With sulfuric acid In toluene Heating; | 78% |
-
-
3913-02-8
2-(n-butyl)octanol

-
-
58334-40-0
2,3,9,10,16,17,23,24-(29H,31H)-Phthalocyanineoctacarboxylic acid

-
-
944909-73-3
2,3,9,10,16,17,23,24-octakis(2-butyloctyl-1-oxycarbonyl)-(29H,31H)-phthalocyanine

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In toluene for 48h; Heating; | 78% |
| With toluene-4-sulfonic acid In toluene Heating; |
2-Butyl-1-octanol Chemical Properties
IUPAC Name: 2-Butyloctan-1-ol
Molecular Formula: C12H26O
Molecular Weight: 186.34g/mol
EINECS: 223-470-0
Density: 0.829 g/cm3
Melting Point: < -30 °C
Boiling Point: 245.5 °C at 760 mmHg
Flash Point: 100.1 °C
Appearance: clear liquid
Stability: stable, incompatible with strong oxidizing agents, combustible
Transport Information: 160kgs
Freely Rotating Bonds: 10
Polar Surface Area: 9.23 Å2
Index of Refraction: 1.439
Molar Refractivity: 59.13 cm3
Molar Volume: 224.4 cm3
Polarizability: 23.44 ×10-24 cm3
Surface Tension: 29.6 dyne/cm
Enthalpy of Vaporization: 56.07 kJ/mol
Vapour Pressure: 0.00476 mmHg at 25°C
2-Butyl-1-octanol , its Cas Register Number is 3913-02-8.The chemical synonyms of 2-Butyl-1-octanol (CAS NO.3913-02-8) are 2-Butyl-1-octano ; 2-Butyloctyl alcohol ; 2-Butyloctylalcohol ; 5-(Hydroxymethyl)undecane ; Isododecyl alcoho l ; Michel XO-150-12 ; 2-Butyl-1-octanol ; 2-Butyloctan-1-ol .Product categories of 2-Butyl-1-octanol (CAS NO.3913-02-8) are Alcohols ; C9 to C30 ; Oxygen Compounds . The molecular structure of 2-Butyl-1-octanol (CAS NO.3913-02-8) is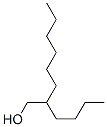 .
.
2-Butyl-1-octanol Uses
It is used in organic synthesis.
2-Butyl-1-octanol Production
It is derived from natural fats and oils, are high molecular straight chain primary alcohols.
2-Butyl-1-octanol Toxicity Data With Reference
| 1. | skn-rbt 10 mg/24H open MLD | AMIHBC AMA Archives of Industrial Hygiene and Occupational Medicine. 4 (1951),119. | ||
| 2. | eye-rbt 500 mg open | AMIHBC AMA Archives of Industrial Hygiene and Occupational Medicine. 4 (1951),119. | ||
| 3. | orl-rat LD50:13 g/kg | + | AMIHBC AMA Archives of Industrial Hygiene and Occupational Medicine. 4 (1951),119. |
2-Butyl-1-octanol Consensus Reports
Reported in EPA TSCA Inventory.
2-Butyl-1-octanol Safety Profile
Mildly toxic by ingestion. A skin and eye irritant. See also ALCOHOL, DENATURED; ALCOHOLS, C6-12; ALCOHOLS, C9-11; ALCOHOLS, C12-13, ETHOXYLATED; ALCOHOLS, C12-15, ETHOXYLATED; ALCOHOLS, C12-16, ETHOXYLATED; ALCOHOLS, C14-15, ETHOXYLATED; ALCOHOLS, C16-18, ETHOXYLATED; ALCOHOLS, C8-10, ETHOXYLATED PROPOXYLATED; ALCOHOLS, C12-15, ETHOXYLATED PROPOXYLATED; ALCOHOLS, N.O.S.. Combustible when exposed to heat or flame. Incompatible with oxidizing materials. To fight fire, use CO2, dry chemical. When heated to decomposition it emits acrid and irritating fumes.
2-Butyl-1-octanol Specification
Their deriver include lauryl (C12) , MyrIstyl (C14) , Cetyl ( or palmityl: C16) , Stearyl (C18) , Oleyl (C18, unsaturated) , and Linoleyl (C18, polyunsaturated) alcohols . There are synthetic fatty alcohols equivalent physically and chemically to natural alcohols obtained from oleochemical sources such as coconut and palm kernel oil. Fatty alcohols are emulsifiers and emollients to make skin smoother and prevent moisture loss. As chemical intermediates, the primary use of fatty alcohols are as raw material for the production of fatty sulfate salts and alcohol ethoxylates for foaming and cleaning purposes in the field of detergent industry. Chemical reactions of primary alcohols include esterifications, ethoxylation, sulfation, oxidation and many other reactions.
Large amount of fatty alcohols are used as special solvents, fillers in plasticizer and insulating materials for the building industry. Fatty alcohols are used as ingredients in the industries of agricultural, foodstuff, metal processing, cosmetics, lube additive, pharmaceutical, rubber, textile, perfume and flavouring as well as synthetic detergent.
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View