-
Name
2-N-HEXYLCYCLOPENTANONE
- EINECS 235-970-6
- CAS No. 13074-65-2
- Article Data19
- CAS DataBase
- Density 0.891 g/cm3
- Solubility at 25 deg C (mg/L): 63.27 Insoluble in water; soluble in alcohol.
- Melting Point 234.9 °C at 760 mmHg
- Formula C11H20 O
- Boiling Point 92 ºC at 4 mm Hg
- Molecular Weight 168.279
- Flash Point 88.6 °C
- Transport Information
- Appearance light yellow liquid
- Safety Low toxicity by ingestion and skin contact. When heated to decomposition it emits acrid smoke and irritating vapors.
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms 2-Hexyl-1-cyclopentanone;2-Hexylcyclopentanone;2-n-Hexylcyclopentanone;Jasmatone;
- PSA 17.07000
- LogP 3.32600
Synthetic route

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide at 150 - 160℃; under 12000.9 - 13501.1 Torr; | 68% |
| With di-tert-butyl peroxide at 130℃; for 6.5h; | 43.5% |
| With 7-azaindoline; chlorobis(cyclooctene)rhodium(I) dimer; tert-Octylamine; toluene-4-sulfonic acid; [1,3-bis(2,4,6-trimethylphenyl)imidazol]-2-ylidene In water at 130℃; for 48h; Catalytic behavior; regioselective reaction; | |
| With di-tert-butyl peroxide at 140 - 160℃; Autoclave; |
-
-
17373-89-6
2-hexylidenecyclopentanone

-
-
13074-65-2
2-hexylcyclopentanone

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal | |
| Ir(CO)(PPh3)2Cl at 240℃; |

| Conditions | Yield |
|---|---|
| Yield given. Multistep reaction; |
-
-
58567-72-9
(2-Hexyl-cyclopent-1-enylsulfanyl)-benzene

-
-
13074-65-2
2-hexylcyclopentanone

| Conditions | Yield |
|---|---|
| With water; titanium tetrachloride; acetic acid |
-
-
39834-30-5
1-Trimethylsiloxy-2-n-hexylcyclopenten

-
-
13074-65-2
2-hexylcyclopentanone

| Conditions | Yield |
|---|---|
| (hydrolysis); | |
| With hydrogenchloride |
-
-
111-25-1
1-bromo-hexane

-
-
14090-60-9
2-cyclopentylidene-1,1-dimethylhydrazine

-
-
13074-65-2
2-hexylcyclopentanone

| Conditions | Yield |
|---|---|
| With potassium dihydrogenphosphate; n-butyllithium; phosphonic Acid 1.) THF, -30 deg C, 1 h, 2.) 25 deg C, 2 h, 3.) 25 deg C, overnight; Yield given. Multistep reaction; |

-
-
13074-65-2
2-hexylcyclopentanone

| Conditions | Yield |
|---|---|
| With ethanol; nickel Hydrogenation; |

-
-
13074-65-2
2-hexylcyclopentanone

| Conditions | Yield |
|---|---|
| With ethanol; nickel Hydrogenation; |

-
-
13074-65-2
2-hexylcyclopentanone

| Conditions | Yield |
|---|---|
| Hydrogenation; |
-
-
120-92-3
cyclopentanone

-
-
13074-65-2
2-hexylcyclopentanone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 2: 1.) n-BuLi, 3.) KH2OP4, H3PO3 / 1.) THF, -30 deg C, 1 h, 2.) 25 deg C, 2 h, 3.) 25 deg C, overnight View Scheme |
-
-
66-25-1
hexanal

-
-
13074-65-2
2-hexylcyclopentanone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: (i) aq. KOH, (ii) oxalic acid 2: H2 / Pd-C View Scheme | |
| Multi-step reaction with 2 steps 1: (i) benzene, (ii) aq. HCl 2: Ir(CO)(PPh3)2Cl / 240 °C View Scheme |
-
-
39834-28-1
2-Hexyl-2-methyl-1-oxa-spiro[2.2]pentane

-
-
13074-65-2
2-hexylcyclopentanone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: (i) LiNEt2, (ii) /BRN= 1209232/ 2: 330 °C 3: aq. HCl View Scheme |
-
-
39834-29-2
2-(1-Trimethylsiloxycyclopropyl)oct-1-en

-
-
13074-65-2
2-hexylcyclopentanone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 0 h / 330 °C 2: (hydrolysis) View Scheme | |
| Multi-step reaction with 2 steps 1: 330 °C 2: aq. HCl View Scheme |
-
-
58567-67-2
[1-(1-Methylene-heptyl)-cyclopropylsulfanyl]-benzene

-
-
13074-65-2
2-hexylcyclopentanone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 350 °C 2: TiCl4, AcOH, H2O View Scheme |
-
-
63365-75-3
2-(1-Phenylsulfanyl-cyclopropyl)-octan-2-ol

-
-
13074-65-2
2-hexylcyclopentanone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: SOCl2, Py 2: 350 °C 3: TiCl4, AcOH, H2O View Scheme |
-
-
111-13-7
hexyl-methyl-ketone

-
-
13074-65-2
2-hexylcyclopentanone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: (i) KOH, (ii) Et2NLi, (iii) /BRN= 1209232/ 2: 0 h / 330 °C 3: (hydrolysis) View Scheme |

| Conditions | Yield |
|---|---|
| With hydrogen at 140℃; under 26252.6 Torr; for 6.66667h; Autoclave; | 75 %Chromat. |
-
-
13074-65-2
2-hexylcyclopentanone

-
A
-
108861-13-8
(6S)-6-hexyltetrahydropyran-2-one

-
B
-
214335-64-5
(R)-2-n-hexylcyclopentanone

| Conditions | Yield |
|---|---|
| With YP-Gal; cyclomaltooctaose; engineered bakers' yeast cell culture at 30℃; for 60h; Baeyer-Villiger oxidation; | A 88% B 78% |
-
-
75-77-4
chloro-trimethyl-silane

-
-
13074-65-2
2-hexylcyclopentanone

-
-
39834-30-5
1-Trimethylsiloxy-2-n-hexylcyclopenten

| Conditions | Yield |
|---|---|
| With pyridine; sodium iodide In acetonitrile | 87% |
| With pyridine; sodium iodide In acetonitrile |
-
-
13074-65-2
2-hexylcyclopentanone

-
-
710-04-3
6-hexyltetrahydro-2H-pyran-2-one

| Conditions | Yield |
|---|---|
| With MoOBr3; dihydrogen peroxide; acetic anhydride; acetic acid at 50 - 70℃; for 6h; | 86% |
| With urea hydrogen peroxide adduct; acetic acid at 60℃; under 750.075 Torr; for 6h; Baeyer-Villiger oxidation; | 78% |
| With Candida antarctica lipase; dihydrogen peroxide; n-tetradecanoic acid In toluene for 144h; | 73% |
-
-
13074-65-2
2-hexylcyclopentanone

-
A
-
95-41-0
2-(n-hexyl)-2-cyclopenten-1-one

-
B
-
83593-56-0
2-chloro-2-hexylcyclopentanone

| Conditions | Yield |
|---|---|
| With sulfuryl dichloride In tetrachloromethane for 1h; temp.: RT to 40 deg C; | A n/a B 78% |
-
-
13074-65-2
2-hexylcyclopentanone

-
-
107-21-1
ethylene glycol

-
-
87735-16-8
6-hexyl-1,4-dioxaspiro[4.4]nonane

| Conditions | Yield |
|---|---|
| With GdPO4*(MoO2)0.5PMo12O40 In toluene Reflux; Dean-Stark; | 61.8% |
| With P0.17Mo2.4CoBr0.27O6.8 In 5,5-dimethyl-1,3-cyclohexadiene at 130℃; for 6h; | 57% |
-
-
13074-65-2
2-hexylcyclopentanone

-
A
-
95-41-0
2-(n-hexyl)-2-cyclopenten-1-one

-
B
-
103517-11-9
(E)-2-n-hexylidenecyclopentanone

| Conditions | Yield |
|---|---|
| With CuCl2*2H2O In ethanol; water Heating; 2:1 molar ratio of salt to ketone; | A 56% B n/a |
| With CuCl2*2H2O In ethanol; water Heating; | A 56% B n/a |
-
-
13074-65-2
2-hexylcyclopentanone

-
-
120-14-9
3,4-dimethoxy-benzaldehyde

-
-
102600-88-4
5-hexyl-2-veratrylidene-cyclopentanone

| Conditions | Yield |
|---|---|
| With potassium hydroxide |
-
-
13074-65-2
2-hexylcyclopentanone

-
-
95-41-0
2-(n-hexyl)-2-cyclopenten-1-one

| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide; aniline 1) CCl4, reflux, 3 h, 2) rt, 15 h; Yield given. Multistep reaction; | |
| Multi-step reaction with 2 steps 1: 78 percent / sulfuryl chloride / CCl4 / 1 h / temp.: RT to 40 deg C 2: 94 percent Chromat. / 0.5 h / 60 °C / 15 Torr View Scheme |
-
-
13074-65-2
2-hexylcyclopentanone

-
A
-
108861-12-7
(R)-6-hexyl-tetrahydro-2H-pyran-2-one

-
B
-
108861-13-8
(6S)-6-hexyltetrahydropyran-2-one

| Conditions | Yield |
|---|---|
| In water at 30℃; for 1.5h; pH 7.1; NADPH-dependent biotransformation by partially purified monooxygenase from (+)-camphor grown Ps. putida NCIMB 10007; further monooxygenases; Yield given. Yields of byproduct given. Title compound not separated from byproducts; | |
| In water at 30℃; for 1.5h; pH 7.1; Yield given. Yields of byproduct given. Title compound not separated from byproducts; |


| Conditions | Yield |
|---|---|
| at 0 - 10℃; Erwaermen des Reaktionsprodukts mit 5prozent ig. wss. Schwefelsaeure auf dem Dampfbad; |

-
-
13074-65-2
2-hexylcyclopentanone

-
-
229638-73-7
5-Hexyl-bicyclo[3.1.0]hexan-1-ol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: NaI; pyridine / acetonitrile 2: Et2Zn / hexane 3: ClSiMe3; methanol / 0.25 h / 20 °C View Scheme | |
| Multi-step reaction with 3 steps 1: 87 percent / NaI, pyridine / acetonitrile 2: 90 percent / Et2Zn / hexane / 0.25 h / 25 °C 3: MeOH, Me3SiCl / 0.25 h / 25 °C View Scheme |
-
-
13074-65-2
2-hexylcyclopentanone

-
-
211234-63-8
(5-Hexyl-bicyclo[3.1.0]hex-1-yloxy)-trimethyl-silane

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: NaI; pyridine / acetonitrile 2: Et2Zn / hexane View Scheme | |
| Multi-step reaction with 2 steps 1: 87 percent / NaI, pyridine / acetonitrile 2: 90 percent / Et2Zn / hexane / 0.25 h / 25 °C View Scheme |
-
-
13074-65-2
2-hexylcyclopentanone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 87 percent / NaI, pyridine / acetonitrile 2: 90 percent / Et2Zn / hexane / 0.25 h / 25 °C 3: MeOH, Me3SiCl / 0.25 h / 25 °C 4: DMAP / CH2Cl2 / 2 h / 25 °C 5: NaOH / methanol / 4 h / 60 °C View Scheme | |
| Multi-step reaction with 3 steps 1: 87 percent / NaI, pyridine / acetonitrile 2: 90 percent / Et2Zn / hexane / 0.25 h / 25 °C 3: NaOH / methanol / 4 h / 60 °C View Scheme |
-
-
13074-65-2
2-hexylcyclopentanone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 87 percent / NaI, pyridine / acetonitrile 2: 90 percent / Et2Zn / hexane / 0.25 h / 25 °C 3: MeOH, Me3SiCl / 0.25 h / 25 °C 4: DMAP / CH2Cl2 / 2 h / 25 °C 5: NaOH / methanol / 4 h / 60 °C View Scheme | |
| Multi-step reaction with 3 steps 1: 87 percent / NaI, pyridine / acetonitrile 2: 90 percent / Et2Zn / hexane / 0.25 h / 25 °C 3: NaOH / methanol / 4 h / 60 °C View Scheme |
-
-
13074-65-2
2-hexylcyclopentanone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 87 percent / NaI, pyridine / acetonitrile 2: 90 percent / Et2Zn / hexane / 0.25 h / 25 °C 3: MeOH, Me3SiCl / 0.25 h / 25 °C 4: DMAP / CH2Cl2 / 2 h / 25 °C 5: 1.) Lipozyme, t-BuOMe, 1-propanol, 2.) Et3N / 1.) 36 deg C, 8 h, 2.) 25 deg C, 18 h View Scheme |
-
-
13074-65-2
2-hexylcyclopentanone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 87 percent / NaI, pyridine / acetonitrile 2: 90 percent / Et2Zn / hexane / 0.25 h / 25 °C 3: MeOH, Me3SiCl / 0.25 h / 25 °C 4: DMAP / CH2Cl2 / 2 h / 25 °C 5: 1.) Lipozyme, t-BuOMe, 1-propanol, 2.) Et3N / 1.) 36 deg C, 8 h, 2.) 25 deg C, 18 h View Scheme |
-
-
13074-65-2
2-hexylcyclopentanone

-
-
211234-74-1
Chloro-acetic acid 5-hexyl-bicyclo[3.1.0]hex-1-yl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 87 percent / NaI, pyridine / acetonitrile 2: 90 percent / Et2Zn / hexane / 0.25 h / 25 °C 3: MeOH, Me3SiCl / 0.25 h / 25 °C 4: DMAP / CH2Cl2 / 2 h / 25 °C View Scheme |
-
-
13074-65-2
2-hexylcyclopentanone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 87 percent / NaI, pyridine / acetonitrile 2: 90 percent / Et2Zn / hexane / 0.25 h / 25 °C 3: MeOH, Me3SiCl / 0.25 h / 25 °C 4: 1.) DMAP, 2.) Lipozyme, t-BuOMe, 1-propanol / 1.) CHCl2, 25 deg C, 2 h, 2.) 36 deg C, 8 h View Scheme |
-
-
13074-65-2
2-hexylcyclopentanone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 87 percent / NaI, pyridine / acetonitrile 2: 90 percent / Et2Zn / hexane / 0.25 h / 25 °C 3: MeOH, Me3SiCl / 0.25 h / 25 °C 4: 1.) DMAP, 2.) Lipozyme, t-BuOMe, 1-propanol / 1.) CHCl2, 25 deg C, 2 h, 2.) 36 deg C, 8 h View Scheme |
2-Hexylcyclopentanone Chemical Properties
Molecular Structure of 2-Hexylcyclopentanone (CAS NO.13074-65-2):
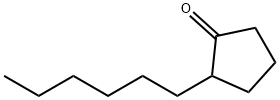
EINECS: 235-970-6
IUPAC Name: 2-Hexylcyclopentan-1-one
Molecular Formula: C11H20O
Molecular Weight: 168.275900 g/mol
XLogP3-AA: 3.5
H-Bond Donor: 0
H-Bond Acceptor: 1
Canonical SMILES: CCCCCCC1CCCC1=O
InChI: InChI=1S/C11H20O/c1-2-3-4-5-7-10-8-6-9-11(10)12/h10H,2-9H2,1H3
InChIKey: JTHVYOIHZNYRCC-UHFFFAOYSA-N
Index of Refraction: 1.453
Molar Refractivity: 51.04 cm3
Molar Volume: 188.8 cm3
Surface Tension: 30.6 dyne/cm
Density: 0.891 g/cm3
Flash Point: 88.6 °C
Enthalpy of Vaporization: 47.16 kJ/mol
Boiling Point: 234.9 °C at 760 mmHg
Vapour Pressure: 0.0517 mmHg at 25 °C
Water Solubility: mg/L at 25 °C
Refractive Index: 1.4495
BRN: 2041950
2-Hexylcyclopentanone Toxicity Data With Reference
| 1. | orl-rat LD50:>5 g/kg | FCTOD7 Food and Chemical Toxicology. 26 (1988),349. | ||
| 2. | skn-rbt LDLo:5 g/kg | FCTOD7 Food and Chemical Toxicology. 26 (1988),349. |
2-Hexylcyclopentanone Consensus Reports
Reported in EPA TSCA Inventory.
2-Hexylcyclopentanone Safety Profile
Safety Information of 2-Hexylcyclopentanone (CAS NO.13074-65-2):
RTECS: GY4972000
Low toxicity by ingestion and skin contact. When heated to decomposition it emits acrid smoke and irritating vapors.
2-Hexylcyclopentanone Specification
2-Hexylcyclopentanone with CAS Registry Number of 13074-65-2 is also known as 2-n-Hexylcyclopentanone ; 4-07-00-00099 (Beilstein Handbook Reference) ; 2-Hexylcyclopentan-1-one ; Cyclopentanone, 2-hexyl- .
Related Products
- 2-Hexylcyclopentanone
- 130747-13-6
- 130752-32-8
- 130753-13-8
- 13076-17-0
- 130761-99-8
- 13076-29-4
- 13078-04-1
- 13078-12-1
- 13078-15-4
- 13078-21-2
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View