-
Name
3-(4-Hydroxyphenyl)propionic acid
- EINECS 207-931-3
- CAS No. 501-97-3
- Article Data101
- CAS DataBase
- Density 1.26 g/cm3
- Solubility slightly soluble in water
- Melting Point 129-131 °C(lit.)
- Formula C9H10O3
- Boiling Point 352.4 °C at 760 mmHg
- Molecular Weight 166.177
- Flash Point 181.1 °C
- Transport Information
- Appearance Pale-yellow crystals
- Safety 26-36-37/39
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Hydrocinnamicacid, p-hydroxy- (7CI,8CI);2,3-Dihydro-p-coumaric acid;3-(4-Hydroxyphenyl)propanoic acid;3-(4'-Hydroxyphenyl)propionic acid;3-(p-Hydroxyphenyl)propanoic acid;3-(p-Hydroxyphenyl)propionic acid;4-(2-Carboxyethyl)phenol;4-Hydroxybenzenepropanoic acid;4-Hydroxydihydrocinnamic acid;Desaminotyrosine;Dihydro-p-coumaric acid;Hydro-p-coumaric acid;NSC 40949;NSC 65596;Phloretic acid;QuantaBlu;p-Hydroxyhydrocinnamic acid;p-Hydroxylbenzylacetic acid;p-Hydroxyphenylpropionic acid;b-(4-Hydroxyphenyl)propionic acid;b-(p-Hydroxyphenyl)propionic acid;
- PSA 57.53000
- LogP 1.40940
Synthetic route

| Conditions | Yield |
|---|---|
| With hydrogen; platinum(IV) oxide In methanol under 760 Torr; Ambient temperature; | 100% |
| With hydrogen; palladium on activated charcoal In methanol | 100% |
| With hydrogen; palladium on activated charcoal In ethyl acetate at 20℃; | 99% |

| Conditions | Yield |
|---|---|
| With 5% Pd(II)/C(eggshell); hydrogen In methanol; water for 3h; | 99% |
| Stage #1: para-coumaric acid With palladium diacetate; 4,4,5,5-tetramethyl-[1,3,2]-dioxaboralane In ethyl acetate at 25℃; for 12h; Schlenk technique; Inert atmosphere; Stage #2: With hydrogenchloride In water | 99% |
| With palladium diacetate; 4,4,5,5-tetramethyl-[1,3,2]-dioxaboralane In ethyl acetate at 25℃; for 12h; Sealed tube; Inert atmosphere; chemoselective reaction; | 99% |
-
-
84253-22-5
3-[4-(2,4-Dimethyl-benzyloxy)-phenyl]-propionic acid

-
-
501-97-3
4-hydroxyphenylpropionic acid

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal In ethanol under 3040 Torr; for 15h; | 98% |
-
-
31770-76-0
3-(4-hydroxyphenyl)propionic acid benzyl ester

-
-
501-97-3
4-hydroxyphenylpropionic acid

| Conditions | Yield |
|---|---|
| With aluminum oxide for 0.15h; microwave irradiation; | 92% |
-
-
20170-32-5
4-hydroxy-3,5-di-tert-butylphenylpropionic acid

-
-
501-97-3
4-hydroxyphenylpropionic acid

| Conditions | Yield |
|---|---|
| With hydrogen bromide for 2h; Heating; | 92% |
-
-
23838-70-2
3-(4-hydroxyphenyl)propanoic acid amide

-
-
501-97-3
4-hydroxyphenylpropionic acid

| Conditions | Yield |
|---|---|
| With water; sodium hydroxide at 80℃; for 4h; Green chemistry; | 91.5% |
-
-
6386-38-5
methyl 3-(4-hydroxy-3,5-di-tert-butyl)phenylpropanoate

-
-
501-97-3
4-hydroxyphenylpropionic acid

| Conditions | Yield |
|---|---|
| With hydrogen bromide Heating; | 91% |

| Conditions | Yield |
|---|---|
| With hydrogen; platinum | 89% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; aluminum (III) chloride at 180 - 200℃; under 5250.53 - 7500.75 Torr; Temperature; | 87.5% |

| Conditions | Yield |
|---|---|
| With copper(I) oxide; 1D-1-O-Methyl-muco-inostol; sodium hydroxide In water at 100℃; for 6h; | 79% |
| With copper(I) oxide; 1-D-O-Methyl-chiro-inositol; sodium hydroxide In water at 100℃; for 6h; | 79% |

| Conditions | Yield |
|---|---|
| In water Alkaline conditions; | 70.2% |

-
-
20238-83-9
dihydro-p-coumaryl aldehyde

-
-
13530-68-2
dichromic acid

-
-
501-97-3
4-hydroxyphenylpropionic acid

| Conditions | Yield |
|---|---|
| In sulfuric acid; ethyl acetate; acetone | 63.1% |


| Conditions | Yield |
|---|---|
| With boron tribromide In dichloromethane at 20℃; for 2.5h; | 59% |
| With aluminum tri-bromide; toluene | |
| With hydrogen bromide |

| Conditions | Yield |
|---|---|
| With sodium hydroxide; baker's yeast for 48h; | 45% |
| With tyrosine transaminase |
-
-
60-82-2
3-(4-Hydroxy-phenyl)-1-(2,4,6-trihydroxy-phenyl)-propan-1-on

-
A
-
10210-17-0
3-(4-hydroxyphenyl)propan-1-ol

-
B
-
501-97-3
4-hydroxyphenylpropionic acid

-
C
-
108-46-3
recorcinol

| Conditions | Yield |
|---|---|
| With sodium hydroxide; sodium tetrahydroborate In water at 100℃; Product distribution; | A 22% B 8% C 38% |


| Conditions | Yield |
|---|---|
| With sulfur Erwaermen des Reaktionsprodukts mit aethanol. Natronlauge; |
-
-
17362-17-3
3-(p-hydroxyphenyl)propiononitrile

-
-
501-97-3
4-hydroxyphenylpropionic acid

| Conditions | Yield |
|---|---|
| With potassium hydroxide |
-
-
60-82-2
3-(4-Hydroxy-phenyl)-1-(2,4,6-trihydroxy-phenyl)-propan-1-on

-
A
-
108-73-6
3,5-dihydroxyphenol

-
B
-
501-97-3
4-hydroxyphenylpropionic acid

| Conditions | Yield |
|---|---|
| With potassium hydroxide |

| Conditions | Yield |
|---|---|
| Diazotization; |
-
-
22767-72-2
ethyl 3-(4-methoxyphenyl)propanoate

-
-
501-97-3
4-hydroxyphenylpropionic acid

| Conditions | Yield |
|---|---|
| With hydrogen iodide |

-
-
501-97-3
4-hydroxyphenylpropionic acid

| Conditions | Yield |
|---|---|
| With bacterium proteus vulgaris at 37℃; | |
| bei der Einw. eines Bacteriums aus der Gruppe der Pyocyaneen; |

| Conditions | Yield |
|---|---|
| With sodium acetate; acetic anhydride anschliessendes Hydrieren an Raney-Nickel in wss. NaOH; | |
| Multi-step reaction with 3 steps 1: 90 percent / KI, Na2CO3 / acetone / 14 h / Heating 2: 75 percent / pyridine, piperidine / 1 h 3: 89 percent / H2 / Pt View Scheme | |
| Multi-step reaction with 2 steps 1: 3-amino propanoic acid / pyridine / 0.17 h / 70 °C / Microwave irradiation 2: palladium 10% on activated carbon; hydrogen / ethanol / 0.5 h View Scheme |

| Conditions | Yield |
|---|---|
| With 1,4-dioxane; ammonium polysulfide at 160℃; Behandeln des Reaktionsprodukts mit wss. Salzsaeure; |

| Conditions | Yield |
|---|---|
| With sulfuric acid bei der elektrolytischen Oxydation; |
-
-
106009-02-3
punarnavoside

-
A
-
501-97-3
4-hydroxyphenylpropionic acid

-
B
-
122738-91-4
1-glucopyrano-2-benzyl-4,5-dihydroxybenzene

| Conditions | Yield |
|---|---|
| With potassium hydroxide for 4h; Heating; |

-
-
501-97-3
4-hydroxyphenylpropionic acid

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal In methanol for 1.33333h; | 40 mg |
-
-
540-38-5
4-Iodophenol

-
-
59586-11-7
3-(trichlorostannyl)propanoic acid

-
-
501-97-3
4-hydroxyphenylpropionic acid

| Conditions | Yield |
|---|---|
| With potassium hydroxide; Pd(dpm)4Cl2 1.) H2O, 2.) H2O, 100 deg C, 3 h; Yield given. Multistep reaction; |
-
-
199444-18-3
3-(4-Hydroxy-phenyl)-propionic acid pentachlorophenyl ester

-
A
-
87-86-5
Pentachlorophenol

-
B
-
501-97-3
4-hydroxyphenylpropionic acid

| Conditions | Yield |
|---|---|
| With potassium hydroxide In 1,4-dioxane at 25℃; Rate constant; 0.1 M ionic strength; |
-
-
7664-93-9
sulfuric acid

-
-
501-52-0
3-Phenylpropionic acid

-
A
-
501-97-3
4-hydroxyphenylpropionic acid

-
B
-
106-34-3
quinhydrone

-
C
-
106-51-4
p-benzoquinone

| Conditions | Yield |
|---|---|
| bei der elektrolytischen Oxydation; |

-
-
67-56-1
methanol

-
-
501-97-3
4-hydroxyphenylpropionic acid

-
-
5597-50-2
3-(4-hydroxyphenyl)propionic acid methyl ester

| Conditions | Yield |
|---|---|
| With hydrogenchloride In 1,4-dioxane at 20℃; for 2h; | 100% |
| With sulfuric acid for 2h; Inert atmosphere; Reflux; | 100% |
| With thionyl chloride at 0 - 20℃; | 100% |
-
-
64-17-5
ethanol

-
-
501-97-3
4-hydroxyphenylpropionic acid

-
-
23795-02-0
3-(4-hydroxy-phenyl)-propionic acid ethyl ester

| Conditions | Yield |
|---|---|
| With chloro-trimethyl-silane at 25℃; for 24h; | 100% |
| With thionyl chloride at 20℃; | 99% |
| With sulfuric acid at 20℃; for 4h; | 99% |
-
-
71-23-8
propan-1-ol

-
-
501-97-3
4-hydroxyphenylpropionic acid

-
-
83281-52-1
3-(4-hydroxyphenyl)propionic acid propyl ester

| Conditions | Yield |
|---|---|
| With chloro-trimethyl-silane at 25℃; for 24h; | 100% |
| With 3 A molecular sieve; sulfuric acid for 72h; Heating; | |
| With hydrogenchloride Heating; | |
| With chloro-trimethyl-silane at 25℃; for 24h; |

| Conditions | Yield |
|---|---|
| With chloro-trimethyl-silane at 25℃; for 24h; | 100% |
| With hydrogenchloride Heating; | |
| With chloro-trimethyl-silane at 25℃; for 24h; |

| Conditions | Yield |
|---|---|
| With triethylamine hydrochloride; triethylamine In tetrahydrofuran; dichloromethane | 100% |
-
-
501-97-3
4-hydroxyphenylpropionic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol; water | 100% |
-
-
501-97-3
4-hydroxyphenylpropionic acid

-
-
100-39-0
benzyl bromide

-
-
50463-48-4
3-(4-benzyloxyphenyl)propanoic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide; water; tetra(n-butyl)ammonium hydrogensulfate In tetrahydrofuran at 20 - 80℃; | 99% |
| With sodium carbonate; tetra(n-butyl)ammonium hydrogensulfate In tetrahydrofuran; water at 20 - 80℃; | 99% |
| Stage #1: 4-hydroxyphenylpropionic acid; benzyl bromide With potassium carbonate In acetone Reflux; Stage #2: With sodium hydroxide In methanol; water Reflux; | 98% |
-
-
501-97-3
4-hydroxyphenylpropionic acid

-
-
10210-17-0
3-(4-hydroxyphenyl)propan-1-ol

| Conditions | Yield |
|---|---|
| With borane-THF; Trimethyl borate In tetrahydrofuran at 0℃; for 4h; Inert atmosphere; | 99% |
| With sodium tetrahydroborate; Trimethyl borate; dimethyl sulfate In tetrahydrofuran at 20℃; for 1.5h; | 98% |
| With dimethylsulfide borane complex In tetrahydrofuran at 0 - 20℃; | 98% |
-
-
501-97-3
4-hydroxyphenylpropionic acid

-
-
100-39-0
benzyl bromide

-
-
31770-76-0
3-(4-hydroxyphenyl)propionic acid benzyl ester

| Conditions | Yield |
|---|---|
| With potassium hydrogencarbonate In acetone for 18h; Reflux; | 99% |
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 12h; | 94% |
| With potassium hydrogencarbonate In N,N-dimethyl-formamide at 40℃; | 91% |
| With potassium carbonate In N,N-dimethyl-formamide for 15h; | 88% |
| With potassium hydrogencarbonate In N,N-dimethyl-formamide at 20℃; for 12h; | 765 mg |
-
-
501-97-3
4-hydroxyphenylpropionic acid

-
-
77-78-1
dimethyl sulfate

-
-
5597-50-2
3-(4-hydroxyphenyl)propionic acid methyl ester

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone Heating; | 99% |
-
-
501-97-3
4-hydroxyphenylpropionic acid

-
-
77-78-1
dimethyl sulfate

-
-
15823-04-8
methyl 3-(4-methoxyphenyl)propionate

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone Substitution; Heating; | 99% |
| With potassium carbonate In acetone for 48h; Reflux; | 95% |

| Conditions | Yield |
|---|---|
| With borane-THF; Trimethyl borate In tetrahydrofuran at 0℃; for 4h; Inert atmosphere; | 99% |
-
-
501-97-3
4-hydroxyphenylpropionic acid

-
-
100-39-0
benzyl bromide

-
-
58608-97-2
3-(4-benzyloxyphenyl)propionic acid benzyl ester

| Conditions | Yield |
|---|---|
| With sodium hydride; tetra-(n-butyl)ammonium iodide In tetrahydrofuran; N,N,N,N,N,N-hexamethylphosphoric triamide for 15h; Heating; | 98% |
| Stage #1: 4-hydroxyphenylpropionic acid With potassium carbonate In N,N-dimethyl-formamide at 20℃; Stage #2: benzyl bromide In N,N-dimethyl-formamide at 0 - 80℃; | 97% |
| With potassium carbonate In N,N-dimethyl-formamide at 20 - 80℃; for 4h; | 91% |
-
-
501-97-3
4-hydroxyphenylpropionic acid

-
-
67-63-0
isopropyl alcohol

-
-
116144-68-4
isopropyl 3-(4-hydroxyphenyl)propanoate

| Conditions | Yield |
|---|---|
| With hydrogenchloride at 22℃; for 12h; Esterification; | 98% |
| With hydrogenchloride at 20℃; for 16h; | 62% |
| With hydrogenchloride Heating; |
-
-
501-97-3
4-hydroxyphenylpropionic acid

-
-
23735-43-5
(S)-1-O-tosyl-2,3-O-isopropylideneglycerol

-
-
144256-11-1
3-(4-hydroxyphenyl)propionic acid [(4S)-2,2-dimethyl-1,3-dioxolan-4-yl]methyl ester

| Conditions | Yield |
|---|---|
| With potassium hydroxide In dimethyl sulfoxide at 100℃; for 1h; | 98% |

| Conditions | Yield |
|---|---|
| With Agaricus bisporus; oxygen In aq. phosphate buffer; dichloromethane at 25℃; for 24h; pH=7; Reagent/catalyst; | 98% |
| Stage #1: 4-hydroxyphenylpropionic acid With Agaricus bisporus tyrosinase immobilized on polydiallyldimethyl ammonium chloride functionalized oxidized multi-walled carbonanotubes; oxygen In aq. phosphate buffer; dichloromethane at 25℃; for 24h; pH=7.0; Enzymatic reaction; Stage #2: With sodium dithionite In tetrahydrofuran; water at 25℃; for 0.0833333h; Reagent/catalyst; | 98% |
| With Agaricus bisporus tyrosinase; oxygen; ascorbic acid In water at 20℃; for 24h; pH=7; Na-phosphate buffer; Enzymatic reaction; | 88% |
| With sodium dihydrogenphosphate at 25℃; for 0.25h; pH=7; Glovebox; | 14% |
| With mushroom tyrosinase In N,N-dimethyl-formamide at 25℃; pH=7; Kinetics; aq. phosphate buffer; Enzymatic reaction; |
-
-
501-97-3
4-hydroxyphenylpropionic acid

| Conditions | Yield |
|---|---|
| With dmap; O‐(1H‐benzotriazol‐1‐yl)‐N,N,N′,N′‐tetramethyluronium tetrafluoroborate In N,N-dimethyl-formamide for 8h; | 98% |

| Conditions | Yield |
|---|---|
| With ammonium cerium (IV) nitrate In neat (no solvent) at 160 - 165℃; for 2h; Microwave irradiation; Green chemistry; | 98% |
-
-
501-97-3
4-hydroxyphenylpropionic acid

-
-
108-24-7
acetic anhydride

-
-
7249-16-3
3-(4'-acetoxyphenyl)propionic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water at 0 - 20℃; | 97.1% |
| Stage #1: 4-hydroxyphenylpropionic acid; acetic anhydride With potassium hydroxide In water for 16h; Stage #2: With hydrogenchloride In water | 96% |
| With sodium hydroxide In water at 0℃; | 80% |

| Conditions | Yield |
|---|---|
| With phenylbis(2,2,2-trifluoroethoxy)-λ3-iodane; lithium perchlorate In 2,2,2-trifluoroethanol at 20℃; for 0.5h; | 97% |
| With phenylbis(2,2,2-trifluoroethoxy)-λ3-iodane In 2,2,2-trifluoroethanol at 20℃; for 0.166667h; Flow reactor; | 93% |
| With μ-Oxo-I,I'-bis(trifluoroacetato-O)-I,I'-diphenyldiiodine(III) In acetonitrile at 0℃; for 0.25h; | 90% |
-
-
3490-06-0
N-methylhomoveratrylamine

-
-
501-97-3
4-hydroxyphenylpropionic acid

-
-
637358-42-0
N-[2-(3,4-dimethoxyphenyl)ethyl]-3-(4-hydroxyphenyl)-N-methylpropanamide

| Conditions | Yield |
|---|---|
| With O-(benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium tetrafluoroborate; N-ethyl-N,N-diisopropylamine In DMF (N,N-dimethyl-formamide) at 0 - 20℃; | 97% |
-
-
501-97-3
4-hydroxyphenylpropionic acid

-
-
74-88-4
methyl iodide

-
-
5597-50-2
3-(4-hydroxyphenyl)propionic acid methyl ester

| Conditions | Yield |
|---|---|
| With potassium hydrogencarbonate In N,N-dimethyl-formamide at 40℃; | 96% |
| Stage #1: 4-hydroxyphenylpropionic acid With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 0.5h; Stage #2: methyl iodide In N,N-dimethyl-formamide at 0 - 20℃; for 3h; | 95.9% |
| With potassium hydroxide In dimethyl sulfoxide at 40℃; |

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 16h; | 96% |
-
-
19235-89-3
4-chloro-pyridine-2-carbonitrile

-
-
501-97-3
4-hydroxyphenylpropionic acid

-
-
900254-54-8
3-{4-[(2-cyanopyridin-4-yl)oxy]phenyl}propanoic acid

| Conditions | Yield |
|---|---|
| With caesium carbonate In N,N-dimethyl-formamide at 50℃; for 72h; | 95% |

| Conditions | Yield |
|---|---|
| With formaldehyd In toluene at 110℃; for 16h; | 95% |
-
-
51857-17-1
tert-butyl N-(6-aminohexyl)carbamate

-
-
501-97-3
4-hydroxyphenylpropionic acid

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine In dichloromethane at 20℃; for 1h; | 95% |
-
-
501-97-3
4-hydroxyphenylpropionic acid

-
-
75-26-3
isopropyl bromide

-
-
301224-94-2
4-(1-methylethoxy)benzenepropanoic acid (1-methyl)ethyl ester

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 60℃; for 24h; | 94% |
| With sodium hydroxide; potassium carbonate In N,N-dimethyl-formamide | 94% |
3-(4-Hydroxyphenyl)propionic acid Specification
The IUPAC name of 3-(4-Hydroxyphenyl)propionic acid is 3-(4-Hydroxyphenyl)propanoic acid. With the CAS registry number 501-97-3, it is also named as Dihydro-p-coumaric acid. The product's categories are Aromatic Propionic Acids; Organic acids; Aromatics. Besides, it is pale-yellow crystals, which should be stored in sealed, ventilated and dry place at 2-8 °C. In addition, this chemical is slightly soluble in water.
The other characteristics of this product can be summarized as: (1)EINECS: 207-931-3; (2)ACD/LogP: 1.10; (3)# of Rule of 5 Violations: 0; (4)ACD/LogD (pH 5.5): 0.27; (5)ACD/LogD (pH 7.4): -1.53; (6)ACD/BCF (pH 5.5): 1; (7)ACD/BCF (pH 7.4): 1; (8)ACD/KOC (pH 5.5): 14.04; (9)ACD/KOC (pH 7.4): 1; (10)H bond acceptors: 3; (11)H bond donors: 2; (12)Freely Rotating Bonds: 4; (13)Index of Refraction: 1.58; (14)Molar Refractivity: 43.88 cm3; (15)Molar Volume: 131.8 cm3; (16)Surface Tension: 55.9 dyne/cm; (17)Density: 1.26 g/cm3; (18)Flash Point: 181.1 °C; (19)Melting point: 126-130 °C; (20)Enthalpy of Vaporization: 63.02 kJ/mol; (21)Boiling Point: 352.4 °C at 760 mmHg; (22)Vapour Pressure: 1.43E-05 mmHg at 25 °C.
Preparation of 3-(4-Hydroxyphenyl)propionic acid: this chemical can be prepared by 3t-(4-Hydroxy-phenyl)-acrylic acid.
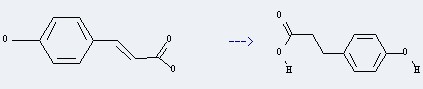
This reaction needs H2, PtO2 and Methanol at ambient temperature. The reaction pressure is 760 Kpa. The yield is 100 %.
Uses of 3-(4-Hydroxyphenyl)propionic acid: this chemical can be used as Antioxidant and pharmaceutical intermediates. Additionally, it can react with Methanol to get 3-(4-Hydroxy-phenyl)-propionic acid methyl ester.

This reaction needs 4N HCl. The yield is 100 %.
When you are using this chemical, please be cautious about it as the following: 3-(4-Hydroxyphenyl)propionic acid is irritating to eyes, respiratory system and skin. You should wear suitable protective clothing, gloves and eye/face protection when use it. Moreover, in case of contact with eyes, please rinse immediately with plenty of water and seek medical advice.
People can use the following data to convert to the molecule structure.
(1)SMILES:O=C(O)CCc1ccc(O)cc1
(2)InChI:InChI=1/C9H10O3/c10-8-4-1-7(2-5-8)3-6-9(11)12/h1-2,4-5,10H,3,6H2,(H,11,12)
(3)InChIKey:NMHMNPHRMNGLLB-UHFFFAOYAT
(4)Std. InChI:InChI=1S/C9H10O3/c10-8-4-1-7(2-5-8)3-6-9(11)12/h1-2,4-5,10H,3,6H2,(H,11,12)
(5)Std. InChIKey:NMHMNPHRMNGLLB-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intravenous | 1809mg/kg (1809mg/kg) | SENSE ORGANS AND SPECIAL SENSES: OTHER: EYE BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD LUNGS, THORAX, OR RESPIRATION: DYSPNEA | Oyo Yakuri. Pharmacometrics. Vol. 12, Pg. 265, 1976. |
Related Products
- 3-(4-Hydroxyphenyl)propionic acid
- 5019-82-9
- 501-98-4
- 5020-41-7
- 5020-72-4
- 502132-86-7
- 5021-33-0
- 502133-49-5
- 50-21-5
- 502161-03-7
- 5022-29-7
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View