-
Name
3,5-Dibromobenzaldehyde
- EINECS -0
- CAS No. 56990-02-4
- Article Data32
- CAS DataBase
- Density 1.977 g/cm3
- Solubility insoluble in water
- Melting Point 84-88 °C(lit.)
- Formula C7H4Br2O
- Boiling Point 287.221 °C at 760 mmHg
- Molecular Weight 263.916
- Flash Point 113.496 °C
- Transport Information UN 3261 8/PG 2
- Appearance White powder
- Safety 26-36/37/39-45
- Risk Codes 34
-
Molecular Structure
-
Hazard Symbols
 C
C
- Synonyms Benzaldehyde,3,5-dibromo-
- PSA 17.07000
- LogP 3.02410
Synthetic route
-
-
626-39-1
1,3,5-trisbromobenzene

-
-
68-12-2, 33513-42-7
N,N-dimethyl-formamide

-
-
56990-02-4
3,5-dibromobenzaldehyde

| Conditions | Yield |
|---|---|
| Stage #1: 1,3,5-trisbromobenzene With magnesium In tetrahydrofuran at 20℃; for 0.0833333h; Inert atmosphere; Large scale; Stage #2: With methylmagnesium chloride In tetrahydrofuran for 3.5h; Reflux; Inert atmosphere; Large scale; Stage #3: N,N-dimethyl-formamide In tetrahydrofuran at 0 - 10℃; for 2h; Temperature; Reagent/catalyst; Inert atmosphere; Large scale; | 95% |
| Stage #1: 1,3,5-trisbromobenzene With n-butyllithium In diethyl ether; hexane at -78℃; Stage #2: N,N-dimethyl-formamide In diethyl ether; hexane at -78 - 0℃; Further stages.; | 94% |
| Stage #1: 1,3,5-trisbromobenzene With n-butyllithium In diethyl ether at -78℃; Stage #2: N,N-dimethyl-formamide In diethyl ether for 1h; Stage #3: With hydrogenchloride In diethyl ether; water | 70% |
-
-
56908-88-4
3,5-dibromobenzyl bromide

-
-
56990-02-4
3,5-dibromobenzaldehyde

| Conditions | Yield |
|---|---|
| With 4-methylmorpholine N-oxide In tetrahydrofuran for 12h; Reflux; | 92% |
-
-
68-12-2, 33513-42-7
N,N-dimethyl-formamide

-
-
56990-02-4
3,5-dibromobenzaldehyde

| Conditions | Yield |
|---|---|
| In diethyl ether at -78℃; for 1h; | 86% |
-
-
1611-92-3
3,5-dibromotoluene

-
-
56990-02-4
3,5-dibromobenzaldehyde

| Conditions | Yield |
|---|---|
| With C15H11ClN4SZn; p-benzoquinone In tert-butyl alcohol for 8h; Reagent/catalyst; | 83% |
| With bromine at 180 - 200℃; Erwaermen des Reaktionsprodukts mit konz. Schwefelsaeure auf 70-80grad; | |
| Multi-step reaction with 2 steps 1: dibenzoyl peroxide; N-Bromosuccinimide / tetrachloromethane / 24 h / Reflux 2: 4-methylmorpholine N-oxide / tetrahydrofuran / 12 h / Reflux View Scheme |

-
-
56990-02-4
3,5-dibromobenzaldehyde

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water; N,N-dimethyl-formamide at 50℃; for 1h; | 82% |
| With hydrogenchloride; water at 50℃; for 1h; | 82% |

| Conditions | Yield |
|---|---|
| With 1,2,3,4-tetrahydroisoquinoline In acetonitrile at 20℃; Green chemistry; | 50% |
-
-
626-39-1
1,3,5-trisbromobenzene

-
-
56990-02-4
3,5-dibromobenzaldehyde

| Conditions | Yield |
|---|---|
| With magnesium In hexane; dichloromethane; ethyl acetate; N,N-dimethyl-formamide | 45% |
| With magnesium In tetrahydrofuran; hexane; dichloromethane; ethyl acetate; N,N-dimethyl-formamide |
-
-
19752-57-9
1,3-dibromo-5-iodobenzene

-
-
68-12-2, 33513-42-7
N,N-dimethyl-formamide

-
-
56990-02-4
3,5-dibromobenzaldehyde

| Conditions | Yield |
|---|---|
| Stage #1: 1,3-dibromo-5-iodobenzene With n-butyllithium In tetrahydrofuran at -78℃; for 0.5h; Stage #2: N,N-dimethyl-formamide In tetrahydrofuran at -78 - 20℃; | 28% |
-
-
42460-62-8
4-amino-3,5-dibromo-benzaldehyde

-
A
-
6311-60-0
3,5-dibromonitrobenzene

-
B
-
56990-02-4
3,5-dibromobenzaldehyde

| Conditions | Yield |
|---|---|
| With potassium disulphite; nitric acid Erwaermen der mit Eiswasser verd. Diazoniumsalz-Loesung mit Alkohol in Gegenwart von Kupfersulfat; |

| Conditions | Yield |
|---|---|
| With chromium(VI) oxide; sulfuric acid; acetic acid anschl. Erhitzen mit wss. H2SO4; |

-
-
56990-02-4
3,5-dibromobenzaldehyde

| Conditions | Yield |
|---|---|
| With ethanol |
-
-
42460-62-8
4-amino-3,5-dibromo-benzaldehyde

-
-
7697-37-2
nitric acid

-
A
-
6311-60-0
3,5-dibromonitrobenzene

-
B
-
56990-02-4
3,5-dibromobenzaldehyde

| Conditions | Yield |
|---|---|
| at 0℃; anschliessend Erwaermen mit waessrig-alkoholischer Kupfersulfat-Loesung.Diazotization; |

-
-
145691-59-4
(3,5-dibromophenyl)methanol

-
-
56990-02-4
3,5-dibromobenzaldehyde

| Conditions | Yield |
|---|---|
| With pyridinium chlorochromate In dichloromethane at 40℃; |
-
-
6311-60-0
3,5-dibromonitrobenzene

-
-
56990-02-4
3,5-dibromobenzaldehyde

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: 89 percent / SnCl2*2H2O / ethanol; tetrahydrofuran / 20 h / 20 °C 2.1: H2SO4; NaNO2 / 2 h / 0 °C 2.2: 70 percent / KI / 0.25 h / 80 °C 3.1: n-BuLi / tetrahydrofuran / 0.5 h / -78 °C 3.2: 28 percent / tetrahydrofuran / -78 - 20 °C View Scheme |
-
-
626-40-4
3,5-dibromoaniline

-
-
56990-02-4
3,5-dibromobenzaldehyde

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: H2SO4; NaNO2 / 2 h / 0 °C 1.2: 70 percent / KI / 0.25 h / 80 °C 2.1: n-BuLi / tetrahydrofuran / 0.5 h / -78 °C 2.2: 28 percent / tetrahydrofuran / -78 - 20 °C View Scheme |
-
-
827-94-1
2,6-dibromo-4-nitroaniline

-
-
56990-02-4
3,5-dibromobenzaldehyde

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: 80 percent / H2SO4; NaNO2 / ethanol / 36 h / 90 °C 2.1: 89 percent / SnCl2*2H2O / ethanol; tetrahydrofuran / 20 h / 20 °C 3.1: H2SO4; NaNO2 / 2 h / 0 °C 3.2: 70 percent / KI / 0.25 h / 80 °C 4.1: n-BuLi / tetrahydrofuran / 0.5 h / -78 °C 4.2: 28 percent / tetrahydrofuran / -78 - 20 °C View Scheme |
-
-
100-01-6
4-nitro-aniline

-
-
56990-02-4
3,5-dibromobenzaldehyde

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: 97 percent / Br2 / acetic acid / 4 h / 65 °C 2.1: 80 percent / H2SO4; NaNO2 / ethanol / 36 h / 90 °C 3.1: 89 percent / SnCl2*2H2O / ethanol; tetrahydrofuran / 20 h / 20 °C 4.1: H2SO4; NaNO2 / 2 h / 0 °C 4.2: 70 percent / KI / 0.25 h / 80 °C 5.1: n-BuLi / tetrahydrofuran / 0.5 h / -78 °C 5.2: 28 percent / tetrahydrofuran / -78 - 20 °C View Scheme |
-
-
618-58-6
3,5-dibromobenzoic acid

-
-
56990-02-4
3,5-dibromobenzaldehyde

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: H3B*S(CH3)2 / tetrahydrofuran / 70 °C 2: PCC / CH2Cl2 / 40 °C View Scheme |
-
-
56990-02-4
3,5-dibromobenzaldehyde

-
-
145691-59-4
(3,5-dibromophenyl)methanol

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In ethanol; dichloromethane at 20℃; for 3h; | 100% |
| With sodium tetrahydroborate In methanol at 0 - 20℃; | 95% |
| With sodium tetrahydroborate In ethanol; dichloromethane at 20℃; for 3h; | 93% |
-
-
56990-02-4
3,5-dibromobenzaldehyde

-
-
107-21-1
ethylene glycol

-
-
773094-77-2
2-(3,5-dibromophenyl)-1,3-dioxolane

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In toluene for 1h; Heating / reflux; | 100% |
| With toluene-4-sulfonic acid In benzene Heating; | 98% |
| With toluene-4-sulfonic acid In toluene at 110℃; for 4h; Inert atmosphere; | 83.6% |
| With toluene-4-sulfonic acid In benzene for 5h; Heating; | |
| With toluene-4-sulfonic acid In toluene for 5h; Reflux; Dean-Stark; | 6.27 g |
-
-
56990-02-4
3,5-dibromobenzaldehyde

-
-
126-30-7
2,2-Dimethyl-1,3-propanediol

-
-
213622-10-7
2-(3,5-Dibromo-phenyl)-5,5-dimethyl-[1,3]dioxane

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In tetrahydrofuran for 8h; Reflux; | 100% |
| With toluene-4-sulfonic acid In benzene for 48h; Reflux; Inert atmosphere; | 97% |
| With toluene-4-sulfonic acid In benzene Dean-Stark; | 97% |
| With toluene-4-sulfonic acid In tetrahydrofuran for 8h; Reflux; |
-
-
56990-02-4
3,5-dibromobenzaldehyde

-
-
106-49-0
p-toluidine

| Conditions | Yield |
|---|---|
| In neat (no solvent) at 20℃; Green chemistry; | 99% |
| In dichloromethane at 27℃; for 0.0833333h; |
-
-
92-91-1
biphenyl-4-acetaldehyde

-
-
56990-02-4
3,5-dibromobenzaldehyde

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol; water at 20℃; for 2h; | 99% |
| In ethanol; water at 20℃; for 2h; | 98% |
| With sodium t-butanolate In ethanol at 25℃; Inert atmosphere; | 98.49% |

-
-
225931-80-6
bis[(trimethylsilyl)methyl](1,5-cyclooctadiene)palladium(II)

-
-
56990-02-4
3,5-dibromobenzaldehyde

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 20℃; for 1h; | 99% |


| Conditions | Yield |
|---|---|
| In toluene at 20℃; for 3h; | 99% |
-
-
56990-02-4
3,5-dibromobenzaldehyde

-
-
13331-27-6
m-nitrobenzene boronic acid

| Conditions | Yield |
|---|---|
| With tetrakis(triphenylphosphine) palladium(0); potassium carbonate In ethanol; water; toluene at 90℃; for 24h; Schlenk technique; Inert atmosphere; | 99% |
| With tetrakis(triphenylphosphine) palladium(0); potassium carbonate In ethanol; water; toluene at 90℃; for 24h; Inert atmosphere; | 80% |
-
-
56990-02-4
3,5-dibromobenzaldehyde

-
-
98-80-6
phenylboronic acid

-
-
220955-80-6
[1,1′:3′,1″-terphenyl]-5′-carbaldehyde

| Conditions | Yield |
|---|---|
| With sodium carbonate; tetrakis(triphenylphosphine) palladium(0) In tetrahydrofuran; water; toluene at 105℃; for 120h; Suzuki-Miyaura cross-coupling reaction; | 98% |
| With tetrakis(triphenylphosphine) palladium(0); sodium carbonate In 1,2-dimethoxyethane; water at 90℃; Suzuki Coupling; Inert atmosphere; | 98% |
| With palladium diacetate; sodium carbonate; triphenylphosphine In ethanol; toluene at 100℃; for 24h; Suzuki Coupling; Inert atmosphere; Sealed tube; | 93% |
-
-
56990-02-4
3,5-dibromobenzaldehyde

-
-
536-74-3
phenylacetylene

| Conditions | Yield |
|---|---|
| Stage #1: phenylacetylene With (-)-N-methylephedrine; triethylamine; zinc trifluoromethanesulfonate In toluene for 0.25h; Stage #2: 3,5-dibromobenzaldehyde In toluene Further stages.; | 98% |

| Conditions | Yield |
|---|---|
| In ethanol; water at 20℃; for 2h; | 98% |

| Conditions | Yield |
|---|---|
| With sodium hydrogensulfite In ethanol; water for 48h; Reflux; | 98% |
-
-
22665-67-4
2-methoxyisobutanol

-
-
56990-02-4
3,5-dibromobenzaldehyde

-
-
213622-10-7
2-(3,5-Dibromo-phenyl)-5,5-dimethyl-[1,3]dioxane

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In benzene Heating; | 97% |
-
-
56990-02-4
3,5-dibromobenzaldehyde

-
-
79887-10-8
1-ethynyl-4-(n-pentyl)benzene

-
-
496043-84-6
3,5-bis-(4-pentyl-phenylethynyl)-benzaldehyde

| Conditions | Yield |
|---|---|
| With 4 A molecular sieve; triethylamine; triphenylphosphine; bis-triphenylphosphine-palladium(II) chloride; copper(l) iodide In N,N-dimethyl-formamide at 60℃; for 7h; Sonogashira coupling; | 97% |
-
-
56990-02-4
3,5-dibromobenzaldehyde

-
-
32316-92-0
naphthalene-2-boronic acid

| Conditions | Yield |
|---|---|
| With tetrakis(triphenylphosphine) palladium(0); potassium carbonate In tetrahydrofuran for 19h; Inert atmosphere; | 97% |
| With tetrakis(triphenylphosphine) palladium(0); sodium carbonate In water; toluene at 120℃; Inert atmosphere; | 95% |

| Conditions | Yield |
|---|---|
| With 1,4-diazabicyclo[2.2.2]octane hydroacetate In neat (no solvent) at 25℃; for 0.666667h; Green chemistry; | 97% |

-
-
56990-02-4
3,5-dibromobenzaldehyde

-
-
7480-35-5, 13286-59-4, 74165-73-4, 126456-43-7, 136030-00-7, 140632-19-5, 140632-20-8
(1S,2R)-1-amino-2-indanol

| Conditions | Yield |
|---|---|
| Stage #1: 3,5-dibromobenzaldehyde; (1S,2R)-1-amino-2-indanol In methanol; dimethyl sulfoxide at 20℃; for 2h; Petasis Reaction; Schlenk technique; Stage #2: pinacol homoallenylboronate In methanol; dimethyl sulfoxide at 20℃; for 48h; Petasis Reaction; Schlenk technique; regioselective reaction; | 97% |


| Conditions | Yield |
|---|---|
| With bis-triphenylphosphine-palladium(II) chloride; triethylamine; triphenylphosphine; copper(l) iodide In tetrahydrofuran at 65℃; Sonogashira reaction; | 96% |
-
-
56990-02-4
3,5-dibromobenzaldehyde

-
-
1066-54-2
trimethylsilylacetylene

-
-
153390-73-9
3,5‐bis((trimethylsilyl)ethynyl)benzaldehyde

| Conditions | Yield |
|---|---|
| With copper(l) iodide; bis(benzonitrile)palladium(II) dichloride; diisopropylamine; triphenylphosphine In 1,4-dioxane at 20℃; Inert atmosphere; | 95% |
| With copper(l) iodide; bis(benzonitrile)palladium(II) dichloride; diisopropylamine; triphenylphosphine In 1,4-dioxane at 20℃; | 95% |
| With bis-triphenylphosphine-palladium(II) chloride; copper(l) iodide; triethylamine In tetrahydrofuran Sonogashira Cross-Coupling; | 95% |
-
-
56990-02-4
3,5-dibromobenzaldehyde

-
-
256386-08-0
5-dibromomethyl-1,3-dibromobenzene

| Conditions | Yield |
|---|---|
| With boron tribromide In dichloromethane at 20℃; for 24h; Inert atmosphere; | 95% |
| With boron tribromide In dichloromethane at 20℃; for 24h; | 80% |
| With boron tribromide In dichloromethane at 20℃; Substitution; |

| Conditions | Yield |
|---|---|
| Stage #1: carbon tetrabromide With triphenylphosphine; zinc In dichloromethane at 20℃; for 24h; Stage #2: 3,5-dibromobenzaldehyde In dichloromethane at 0 - 20℃; Further stages.; | 95% |
-
-
56990-02-4
3,5-dibromobenzaldehyde

-
-
21204-67-1
methyl (triphenylphosphoranylidene)acetate

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 7h; Inert atmosphere; | 95% |

| Conditions | Yield |
|---|---|
| With catena-poly[di-μ-iodido-bis[(1,2-bis(4-chlorophenylthio)propane)copper(I)]] In neat (no solvent) at 80℃; for 4h; | 95% |

-
-
56990-02-4
3,5-dibromobenzaldehyde

-
-
74258-81-4
3-(3,4,5-trimethoxyphenyl)-1H-1,2,4-triazol-5-amine

-
-
123-54-6
acetylacetone

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In N,N-dimethyl-formamide at 90℃; for 12h; | 95% |


| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol; water at 20℃; for 3h; | 94% |
-
-
56990-02-4
3,5-dibromobenzaldehyde

-
-
504-02-9
1,3-cylohexanedione

-
-
74258-81-4
3-(3,4,5-trimethoxyphenyl)-1H-1,2,4-triazol-5-amine

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In N,N-dimethyl-formamide at 90℃; for 12h; | 94% |

-
-
56990-02-4
3,5-dibromobenzaldehyde

| Conditions | Yield |
|---|---|
| With isonicotinate tert-butyl ester; bis(pinacol)diborane for 6h; Reflux; | 94% |

| Conditions | Yield |
|---|---|
| With copper(l) iodide; triethylamine; bis-triphenylphosphine-palladium(II) chloride In tetrahydrofuran at 80℃; for 48h; Sonogashira coupling; | 93% |
-
-
56990-02-4
3,5-dibromobenzaldehyde

-
-
363622-48-4
1,3-dibromo-5-[(diethylphosphonyl)methyl]benzene

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In tetrahydrofuran at 20℃; for 3h; Wittig reaction; Inert atmosphere; | 93% |
| With potassium tert-butylate In tetrahydrofuran at 20℃; for 3h; Inert atmosphere; | 81% |
3,5-Dibromobenzaldehyde Chemical Properties
IUPAC Name: 3,5-Dibromobenzaldehyde
The MF of 3,5-Dibromobenzaldehyde (56990-02-4) is C7H4Br2O.
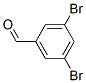
The MW of 3,5-Dibromobenzaldehyde (56990-02-4) is 263.91.
Synonyms of 3,5-Dibromobenzaldehyde (56990-02-4): 3,5-Dibrombenzolcarbaldehyd ; Benzaldehyde, 3,5-dibromo- ; 3,5-Dibromobenzaldehyde ; TL8001017
Product Categories: Aromatic Aldehydes & Derivatives;Benzaldehyde;Adehydes, Acetals & Ketones;Bromine Compounds;Building Blocks for Dendrimers
Index of Refraction: 1.645
Apperance: White powder
Density: 1.977 g/ml
Flash Point: 113.5 °C
Boiling Point: 287.2 °C
Melting Point: 89-93 °C
Water Solubility: insoluble
Sensitive: Air Sensitive
BRN: 2573432
3,5-Dibromobenzaldehyde Uses
3,5-Dibromobenzaldehyde (56990-02-4) is used as pharmaceutical intermediates.
3,5-Dibromobenzaldehyde Safety Profile
Safety information of 3,5-Dibromobenzaldehyde (56990-02-4):
Hazard Codes ![]() C
C
Risk Statements
34 Causes burns
Safety Statements
26 In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
36/37/39
45 If swallowed, seek medical advice immediately and show this container or label
RIDADR UN 3261 8/PG 2
WGK Germany 3
Related Products
- 3,10-Diaminotricyclo(5.2.1.0(sup 2,6))decane
- 3,10-Dinitrophenanthrene
- 3-((10-ETHYL-11-(p-HYDROXYPHENYL)DIBENZ-(B,F)OXEPIN-3-YL)OXY)-1,2-PROPANEDIOL HYDRATE (4:1)
- 3-(1,1,2,2-Tetrafluoroethoxy)aniline
- 3-(1,1,2,2-Tetrafluoroethoxy)benzaldehyde
- 3-(1,1,2,2-Tetrafluoroethoxy)bromobenzene
- 3-(1,1,2,2-Tetrafluoroethoxy)toluene
- 3-[1,1'-Biphenyl]-4-yl-1,2,3,4-tetrahydro-1-naphthol
- 3,11-Dichloro-6,11-dihydro-6-methyldibenzo[c,f][1,2]thiazepine 5,5-dioxide
- 3-[(1,1-Dimethyl-2-hydroxyethyl)amino]-2-hydroxypropanesulfonicacid
- 5699-00-3
- 569-90-4
- 5699-40-1
- 56995-20-1
- 5699-54-7
- 56996-62-4
- 5699-79-6
- 5700-03-8
- 57000-78-9
- 57-00-1
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View