-
Name
3-Amino-2,2-dimethylpropionamide
- EINECS 1806241-263-5
- CAS No. 324763-51-1
- Article Data12
- CAS DataBase
- Density 1.004 g/cm3
- Solubility
- Melting Point 74-75 °C
- Formula C5H12N2O
- Boiling Point 270.7 °C at 760 mmHg
- Molecular Weight 116.163
- Flash Point 117.5 °C
- Transport Information
- Appearance
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms 3-Amino-2,2-dimethylpropanamide;
- PSA 69.11000
- LogP 0.85720
Synthetic route
-
-
4300-97-4
3-Chloropivaloyl chloride

-
-
324763-51-1
aminopivalinamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: ammonia / dichloromethane / 2.75 h / 20 °C / Cooling with ice 2: sodium methylate; ammonia / methanol / 50 h / 60 °C / Autoclave View Scheme | |
| Multi-step reaction with 4 steps 1.1: ammonia / dichloromethane / 2.75 h / 20 °C / Cooling with ice 2.1: sodium hydroxide / methanol / 70 h / 50 - 53 °C / Sealed tube 2.2: 0.17 h / 0 - 20 °C 3.1: hydrogenchloride / 20 h / 20 °C 4.1: ammonia / methanol / 48 h / 60 °C / 3750.38 Torr / Autoclave View Scheme |
-
-
2843-17-6
3-bromo-2,2-dimethylpropionic acid

-
-
324763-51-1
aminopivalinamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: ammonia / 1,4-dioxane / 16 h / 60 °C / 2250.23 Torr / Autoclave 2: hydrogenchloride / 20 h / 20 °C 3: ammonia / methanol / 48 h / 60 °C / 3750.38 Torr / Autoclave View Scheme |
-
-
324763-51-1
aminopivalinamide

-
-
324763-46-4
(3S,5S)-5-{(1S,3S)-1-azido-3-{[4-methoxy-3-(3-methoxypropoxy)phenyl]methyl}-4-methylpentyl}-dihydro-3-(1-methylethyl)furan-2(3H)-one

| Conditions | Yield |
|---|---|
| With 2-hydroxypyridin; triethylamine at 90℃; for 16h; After distillation of a part of Et3N over a period of 0.5 h further 8.5 h heating at 90 °ree;C; | 100% |
-
-
324763-51-1
aminopivalinamide

-
-
1309922-72-2
(3S,5R)-5-((1S,3S)-1-azido-3-(4-methoxy-3-(methoxypropoxy)benzyl)-4-methylpentyl)-3-isopropyl-dihydrofuran-2(3H)-one

-
-
1607844-94-9
(2S,4R,5S,7S)-N-(3-amino-2,2-dimethyl-3-oxopropyl)-5-azido-4-hydroxy-2-isopropyl-7-(4-methoxy-3-(methoxypropoxy)benzyl)-8-methylnonanamide

| Conditions | Yield |
|---|---|
| With propionic acid at 110℃; for 2h; | 95% |
-
-
866030-35-5
tert-butyl {(1S,3S)-1-((2S,4S)-4-isopropyl-5-oxotetrahydrofuran-2-yl)-3-[4-methoxy-3-(3-methoxypropoxy)benzyl]-4-methylpentyl}carbamate

-
-
324763-51-1
aminopivalinamide

-
-
173338-07-3
tert-butyl (3S,5S,6S,8S)-8-(3-amino-2,2-dimethyl-3-oxopropylcarbamoyl)-6-hydroxy-3-(4-methoxy-3-(3-methoxypropoxy)benzyl)-2,9-dimethyldecan-5-ylcarbamate

| Conditions | Yield |
|---|---|
| With 2-Ethylhexanoic acid In n-heptane at 60℃; Reagent/catalyst; Temperature; Solvent; Time; | 94% |
| With 2-Ethylhexanoic acid In n-heptane at 60℃; for 40h; Solvent; Reagent/catalyst; | 93% |
| With 2-Ethylhexanoic acid In n-heptane at 70℃; for 8h; Inert atmosphere; | 92% |
-
-
324763-51-1
aminopivalinamide


| Conditions | Yield |
|---|---|
| 2-Ethylhexanoic acid In tetrahydrofuran at 100℃; for 6h; Product distribution / selectivity; | 93% |
-
-
324763-51-1
aminopivalinamide

-
-
324763-46-4
(3S,5S)-5-{(1S,3S)-1-azido-3-{[4-methoxy-3-(3-methoxypropoxy)phenyl]methyl}-4-methylpentyl}-dihydro-3-(1-methylethyl)furan-2(3H)-one

| Conditions | Yield |
|---|---|
| With propionic acid at 120℃; for 1.5h; | 87% |
-
-
324763-51-1
aminopivalinamide

-
-
1236549-00-0
{(1S,3S)-1-((2S,4S)-4-isopropyl-5-oxo-furanidin-2-yl)-3-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-4-methyl-pentyl}-carbamic acid benzyl ester

-
-
1236549-06-6
(1S,2S,4S)-4-(2-carbamoyl-2-methylpropyl-carbamoyl)-2-hydroxy-1-{(S)-2-[4-methoxy-3-(3-methoxypropoxy)-benzyl]-3-methylbutyl}-5-methylhexyl-carbamic acid benzyl ester

| Conditions | Yield |
|---|---|
| With 2-Ethylhexanoic acid In di-isopropyl ether at 60℃; Solvent; Reagent/catalyst; | 86% |
| With 2-Ethylhexanoic acid In di-isopropyl ether at 60℃; Reagent/catalyst; Solvent; | 86% |
| With 2-hydroxypyridin; triethylamine In toluene for 16h; Reflux; Inert atmosphere; | 82% |

| Conditions | Yield |
|---|---|
| With 2-Ethylhexanoic acid at 120℃; for 0.833333h; | 84% |
-
-
142-08-5
2-hydroxypyridin

-
-
324763-51-1
aminopivalinamide

-
-
324763-46-4
(3S,5S)-5-{(1S,3S)-1-azido-3-{[4-methoxy-3-(3-methoxypropoxy)phenyl]methyl}-4-methylpentyl}-dihydro-3-(1-methylethyl)furan-2(3H)-one

-
-
324763-47-5
(2S,4S,5S,7S)-N-(3-amino-2,2-dimethyl-3-oxopropyl)-5-azido-4-hydroxy-2-isopropyl-7-(4-methoxy-3-(methoxypropoxy)benzyl)-8-methylnonanamide

| Conditions | Yield |
|---|---|
| With triethylamine | 79% |

-
-
324763-51-1
aminopivalinamide


| Conditions | Yield |
|---|---|
| With triethylamine In toluene at 80 - 90℃; for 20h; | 78.5% |
-
-
324763-51-1
aminopivalinamide

-
-
1000047-42-6
N-{(S)-2-[2,2-dimethyl-4-(2-methylphenyl)-5-oxopiperazin-1-yl]-1-[(2S,4S)-4-isopropyl-5-oxotetrahydrofuran-2-yl]ethyl}-2-nitrobenzenesulfonamide

-
-
1000047-43-7
(2S,4S,5S)-6-[2,2-dimethyl-4-(2-methylphenyl)-5-oxopiperazin-1-yl]-4-hydroxy-2-isopropyl-5-(2-nitrobenzenesulfonylamino)hexanoic acid (2-carbamoyl-2-methylpropyl)amide

| Conditions | Yield |
|---|---|
| With 2-hydroxypyridin; triethylamine at 80℃; for 15h; | 78% |
-
-
324763-51-1
aminopivalinamide

-
-
324763-46-4
(3S,5S)-5-{(1S,3S)-1-azido-3-{[4-methoxy-3-(3-methoxypropoxy)phenyl]methyl}-4-methylpentyl}-dihydro-3-(1-methylethyl)furan-2(3H)-one

-
-
324763-47-5
(2S,4S,5S,7S)-N-(3-amino-2,2-dimethyl-3-oxopropyl)-5-azido-4-hydroxy-2-isopropyl-7-(4-methoxy-3-(methoxypropoxy)benzyl)-8-methylnonanamide

| Conditions | Yield |
|---|---|
| With 2-hydroxypyridin; triethylamine at 80℃; | 76% |
| With 2-hydroxypyridin; triethylamine | 59% |
-
-
324763-51-1
aminopivalinamide


| Conditions | Yield |
|---|---|
| With 2-hydroxypyridin; triethylamine at 80℃; for 2h; | 73% |
-
-
25307-82-8
methyl 3-amino-2,2-dimethylpropionate

-
-
324763-51-1
aminopivalinamide

| Conditions | Yield |
|---|---|
| With ammonia In methanol at 60℃; under 3750.38 Torr; for 48h; Autoclave; | 100% |
-
-
7505-93-3
2-cyano-2-methylpropanamide

-
-
324763-51-1
aminopivalinamide

| Conditions | Yield |
|---|---|
| Stage #1: 2-cyano-2-methylpropanamide With ammonia; Raney nickel In methanol at 25 - 35℃; Autoclave; Stage #2: With hydrogen In methanol at 60 - 65℃; under 5149.01 - 5884.58 Torr; for 10h; | 87.5% |
| With ammonia; hydrogen; Raney nickle In methanol at 60 - 70℃; under 3750.38 Torr; for 10h; Autoclave; | 78% |
| Multi-step reaction with 2 steps 1.1: LAH / tetrahydrofuran 1.2: Et3N 2.1: H2 / Pd()OH)2/C View Scheme | |
| With ammonia; hydrogen; raney nickel In methanol at 40 - 45℃; under 2942.29 Torr; for 14h; | |
| With ammonium hydroxide; 5% rhodium on activated aluminium oxide; hydrogen In ethanol; water under 3102.97 Torr; for 72h; |
-
-
666844-61-7
(2-carbamoyl-2-methylpropyl)carbamic acid benzyl ester

-
-
324763-51-1
aminopivalinamide

| Conditions | Yield |
|---|---|
| With hydrogen; palladium-carbon In ethanol at 20℃; for 2h; | 85% |
| With hydrogen; palladium hydroxide - carbon | |
| With hydrogen; palladium 10% on activated carbon In methanol at 20℃; for 2h; |
-
-
41461-75-0
chloro-pivalic acid amide

-
-
324763-51-1
aminopivalinamide

| Conditions | Yield |
|---|---|
| With ammonia; sodium methylate In methanol at 60℃; for 50h; Autoclave; | 62% |
| Multi-step reaction with 3 steps 1.1: sodium hydroxide / methanol / 70 h / 50 - 53 °C / Sealed tube 1.2: 0.17 h / 0 - 20 °C 2.1: hydrogenchloride / 20 h / 20 °C 3.1: ammonia / methanol / 48 h / 60 °C / 3750.38 Torr / Autoclave View Scheme |
-
-
114583-17-4
α-(nitromethyl)isobutyronitrile

-
-
324763-51-1
aminopivalinamide

| Conditions | Yield |
|---|---|
| With methanol; Lindlar's catalyst Hydrogenation; | |
| With nickel Hydrogenation; | |
| With hydrogenchloride; iron |
-
-
7647-01-0
hydrogenchloride

-
-
114583-17-4
α-(nitromethyl)isobutyronitrile

-
A
-
859065-87-5
2-(5,5-dimethyl-hexahydro-pyrimidin-2-yl)-2-methyl-propionic acid amide

-
B
-
7328-91-8
2,2-Dimethyl-1,3-diaminopropane

-
C
-
67744-70-1
3-amino-2,2-dimethyl-propionitrile

-
D
-
324763-51-1
aminopivalinamide

| Conditions | Yield |
|---|---|
| at 60 - 70℃; | |
| at 60 - 70℃; |

-
-
67-56-1
methanol

-
-
114583-17-4
α-(nitromethyl)isobutyronitrile

-
A
-
859065-87-5
2-(5,5-dimethyl-hexahydro-pyrimidin-2-yl)-2-methyl-propionic acid amide

-
B
-
7328-91-8
2,2-Dimethyl-1,3-diaminopropane

-
C
-
67744-70-1
3-amino-2,2-dimethyl-propionitrile

-
D
-
324763-51-1
aminopivalinamide

| Conditions | Yield |
|---|---|
| Hydrogenation; | |
| Hydrogenation; |

-
-
67-56-1
methanol

-
-
114583-17-4
α-(nitromethyl)isobutyronitrile

-
A
-
859065-87-5
2-(5,5-dimethyl-hexahydro-pyrimidin-2-yl)-2-methyl-propionic acid amide

-
B
-
7328-91-8
2,2-Dimethyl-1,3-diaminopropane

-
C
-
67744-70-1
3-amino-2,2-dimethyl-propionitrile

-
D
-
324763-51-1
aminopivalinamide

| Conditions | Yield |
|---|---|
| Hydrogenation; | |
| Hydrogenation; |

-
-
114583-17-4
α-(nitromethyl)isobutyronitrile

-
A
-
859065-87-5
2-(5,5-dimethyl-hexahydro-pyrimidin-2-yl)-2-methyl-propionic acid amide

-
B
-
7328-91-8
2,2-Dimethyl-1,3-diaminopropane

-
C
-
67744-70-1
3-amino-2,2-dimethyl-propionitrile

-
D
-
324763-51-1
aminopivalinamide

| Conditions | Yield |
|---|---|
| at 100℃; Hydrogenation; | |
| at 100℃; Hydrogenation; |

-
-
72291-30-6
methyl 2,2-dimethyl-cyanoacetate

-
-
324763-51-1
aminopivalinamide

| Conditions | Yield |
|---|---|
| Stage #1: methyl 2,2-dimethyl-cyanoacetate With ammonia; hydrogen; nickel In 1-methyl-pyrrolidin-2-one; water at 80℃; under 3750.38 Torr; for 1.5h; Stage #2: With ammonia; hydrogen; nickel In 1-methyl-pyrrolidin-2-one; water at 80℃; under 150015 Torr; Product distribution / selectivity; | |
| Stage #1: methyl 2,2-dimethyl-cyanoacetate With ammonia; hydrogen; cobalt In water; butan-1-ol at 80℃; under 3750.38 Torr; for 1.5h; Stage #2: With ammonia; hydrogen; cobalt In water; butan-1-ol at 80℃; under 48754.9 Torr; Product distribution / selectivity; | |
| Stage #1: methyl 2,2-dimethyl-cyanoacetate With ammonia; hydrogen; cobalt In methanol; water at 80℃; under 3750.38 Torr; for 1.5h; Stage #2: With ammonia; hydrogen; cobalt In methanol; water at 80℃; under 48754.9 Torr; Product distribution / selectivity; | |
| Stage #1: methyl 2,2-dimethyl-cyanoacetate With ammonia; hydrogen; nickel In water; butan-1-ol at 80℃; under 3750.38 Torr; for 1.5h; Stage #2: With ammonia; hydrogen; nickel In water; butan-1-ol at 80℃; under 48754.9 Torr; Product distribution / selectivity; |
-
-
72291-30-6
methyl 2,2-dimethyl-cyanoacetate

-
A
-
324763-51-1
aminopivalinamide

-
B
-
25307-82-8
methyl 3-amino-2,2-dimethylpropionate

| Conditions | Yield |
|---|---|
| With ammonia; hydrogen; mixture of 28percent NiO, 28percent CoO, 13percent CuO, 31percent ZrO2; hydrogenated In methanol at 100℃; under 150015 Torr; Product distribution / selectivity; | |
| With ammonia; hydrogen; mixture of 28percent NiO, 28percent CoO, 13percent CuO, 31percent ZrO2; hydrogenated In tetrahydrofuran at 100℃; under 150015 Torr; Product distribution / selectivity; |
-
-
2941-18-6
4,4-dimethylpyrazolidin-3-one

-
-
324763-51-1
aminopivalinamide

| Conditions | Yield |
|---|---|
| With hydrogen; Raney-Nickel In 2-methyl-propan-1-ol at 55 - 65℃; under 750.075 Torr; Inert atmosphere; Sealed tube; |
-
-
1572-98-1
2-cyano-2-methyl-propionic acid ethyl ester

-
-
324763-51-1
aminopivalinamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: ammonia / methanol / 12 h / 25 - 30 °C 2: hydrogen; ammonia / raney nickel / methanol / 14 h / 40 - 45 °C / 2942.29 Torr View Scheme | |
| Multi-step reaction with 2 steps 1.1: ammonia / methanol / 25 - 35 °C 2.1: ammonia / Raney nickel / methanol / 25 - 35 °C / Autoclave 2.2: 10 h / 60 - 65 °C / 5149.01 - 5884.58 Torr View Scheme | |
| Multi-step reaction with 2 steps 1: ammonia / methanol / 96 h / 35 °C / Sealed tube; Cooling with ice 2: hydrogen; ammonium hydroxide; 5% rhodium on activated aluminium oxide / ethanol; water / 72 h / 3102.97 Torr View Scheme |
-
-
4835-90-9
3-Hydroxy-2,2-dimethylpropanoic acid

-
-
324763-51-1
aminopivalinamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: hydrogen bromide / water / 19 h / 100 °C 2: ammonia / 1,4-dioxane / 16 h / 60 °C / 2250.23 Torr / Autoclave 3: hydrogenchloride / 20 h / 20 °C 4: ammonia / methanol / 48 h / 60 °C / 3750.38 Torr / Autoclave View Scheme |
-
-
19036-43-2
aminopivalic acid

-
-
324763-51-1
aminopivalinamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: hydrogenchloride / 20 h / 20 °C 2: ammonia / methanol / 48 h / 60 °C / 3750.38 Torr / Autoclave View Scheme |
-
-
4300-97-4
3-Chloropivaloyl chloride

-
-
324763-51-1
aminopivalinamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: ammonia / dichloromethane / 2.75 h / 20 °C / Cooling with ice 2: sodium methylate; ammonia / methanol / 50 h / 60 °C / Autoclave View Scheme | |
| Multi-step reaction with 4 steps 1.1: ammonia / dichloromethane / 2.75 h / 20 °C / Cooling with ice 2.1: sodium hydroxide / methanol / 70 h / 50 - 53 °C / Sealed tube 2.2: 0.17 h / 0 - 20 °C 3.1: hydrogenchloride / 20 h / 20 °C 4.1: ammonia / methanol / 48 h / 60 °C / 3750.38 Torr / Autoclave View Scheme |
-
-
2843-17-6
3-bromo-2,2-dimethylpropionic acid

-
-
324763-51-1
aminopivalinamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: ammonia / 1,4-dioxane / 16 h / 60 °C / 2250.23 Torr / Autoclave 2: hydrogenchloride / 20 h / 20 °C 3: ammonia / methanol / 48 h / 60 °C / 3750.38 Torr / Autoclave View Scheme |
-
-
324763-51-1
aminopivalinamide

-
-
324763-46-4
(3S,5S)-5-{(1S,3S)-1-azido-3-{[4-methoxy-3-(3-methoxypropoxy)phenyl]methyl}-4-methylpentyl}-dihydro-3-(1-methylethyl)furan-2(3H)-one

| Conditions | Yield |
|---|---|
| With 2-hydroxypyridin; triethylamine at 90℃; for 16h; After distillation of a part of Et3N over a period of 0.5 h further 8.5 h heating at 90 °ree;C; | 100% |
-
-
324763-51-1
aminopivalinamide

-
-
1309922-72-2
(3S,5R)-5-((1S,3S)-1-azido-3-(4-methoxy-3-(methoxypropoxy)benzyl)-4-methylpentyl)-3-isopropyl-dihydrofuran-2(3H)-one

-
-
1607844-94-9
(2S,4R,5S,7S)-N-(3-amino-2,2-dimethyl-3-oxopropyl)-5-azido-4-hydroxy-2-isopropyl-7-(4-methoxy-3-(methoxypropoxy)benzyl)-8-methylnonanamide

| Conditions | Yield |
|---|---|
| With propionic acid at 110℃; for 2h; | 95% |
-
-
866030-35-5
tert-butyl {(1S,3S)-1-((2S,4S)-4-isopropyl-5-oxotetrahydrofuran-2-yl)-3-[4-methoxy-3-(3-methoxypropoxy)benzyl]-4-methylpentyl}carbamate

-
-
324763-51-1
aminopivalinamide

-
-
173338-07-3
tert-butyl (3S,5S,6S,8S)-8-(3-amino-2,2-dimethyl-3-oxopropylcarbamoyl)-6-hydroxy-3-(4-methoxy-3-(3-methoxypropoxy)benzyl)-2,9-dimethyldecan-5-ylcarbamate

| Conditions | Yield |
|---|---|
| With 2-Ethylhexanoic acid In n-heptane at 60℃; Reagent/catalyst; Temperature; Solvent; Time; | 94% |
| With 2-Ethylhexanoic acid In n-heptane at 60℃; for 40h; Solvent; Reagent/catalyst; | 93% |
| With 2-Ethylhexanoic acid In n-heptane at 70℃; for 8h; Inert atmosphere; | 92% |
-
-
324763-51-1
aminopivalinamide


| Conditions | Yield |
|---|---|
| 2-Ethylhexanoic acid In tetrahydrofuran at 100℃; for 6h; Product distribution / selectivity; | 93% |
-
-
324763-51-1
aminopivalinamide

-
-
324763-46-4
(3S,5S)-5-{(1S,3S)-1-azido-3-{[4-methoxy-3-(3-methoxypropoxy)phenyl]methyl}-4-methylpentyl}-dihydro-3-(1-methylethyl)furan-2(3H)-one

| Conditions | Yield |
|---|---|
| With propionic acid at 120℃; for 1.5h; | 87% |
-
-
324763-51-1
aminopivalinamide

-
-
1236549-00-0
{(1S,3S)-1-((2S,4S)-4-isopropyl-5-oxo-furanidin-2-yl)-3-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-4-methyl-pentyl}-carbamic acid benzyl ester

-
-
1236549-06-6
(1S,2S,4S)-4-(2-carbamoyl-2-methylpropyl-carbamoyl)-2-hydroxy-1-{(S)-2-[4-methoxy-3-(3-methoxypropoxy)-benzyl]-3-methylbutyl}-5-methylhexyl-carbamic acid benzyl ester

| Conditions | Yield |
|---|---|
| With 2-Ethylhexanoic acid In di-isopropyl ether at 60℃; Solvent; Reagent/catalyst; | 86% |
| With 2-Ethylhexanoic acid In di-isopropyl ether at 60℃; Reagent/catalyst; Solvent; | 86% |
| With 2-hydroxypyridin; triethylamine In toluene for 16h; Reflux; Inert atmosphere; | 82% |

| Conditions | Yield |
|---|---|
| With 2-Ethylhexanoic acid at 120℃; for 0.833333h; | 84% |
-
-
142-08-5
2-hydroxypyridin

-
-
324763-51-1
aminopivalinamide

-
-
324763-46-4
(3S,5S)-5-{(1S,3S)-1-azido-3-{[4-methoxy-3-(3-methoxypropoxy)phenyl]methyl}-4-methylpentyl}-dihydro-3-(1-methylethyl)furan-2(3H)-one

-
-
324763-47-5
(2S,4S,5S,7S)-N-(3-amino-2,2-dimethyl-3-oxopropyl)-5-azido-4-hydroxy-2-isopropyl-7-(4-methoxy-3-(methoxypropoxy)benzyl)-8-methylnonanamide

| Conditions | Yield |
|---|---|
| With triethylamine | 79% |

-
-
324763-51-1
aminopivalinamide


| Conditions | Yield |
|---|---|
| With triethylamine In toluene at 80 - 90℃; for 20h; | 78.5% |
-
-
324763-51-1
aminopivalinamide

-
-
1000047-42-6
N-{(S)-2-[2,2-dimethyl-4-(2-methylphenyl)-5-oxopiperazin-1-yl]-1-[(2S,4S)-4-isopropyl-5-oxotetrahydrofuran-2-yl]ethyl}-2-nitrobenzenesulfonamide

-
-
1000047-43-7
(2S,4S,5S)-6-[2,2-dimethyl-4-(2-methylphenyl)-5-oxopiperazin-1-yl]-4-hydroxy-2-isopropyl-5-(2-nitrobenzenesulfonylamino)hexanoic acid (2-carbamoyl-2-methylpropyl)amide

| Conditions | Yield |
|---|---|
| With 2-hydroxypyridin; triethylamine at 80℃; for 15h; | 78% |
-
-
324763-51-1
aminopivalinamide

-
-
324763-46-4
(3S,5S)-5-{(1S,3S)-1-azido-3-{[4-methoxy-3-(3-methoxypropoxy)phenyl]methyl}-4-methylpentyl}-dihydro-3-(1-methylethyl)furan-2(3H)-one

-
-
324763-47-5
(2S,4S,5S,7S)-N-(3-amino-2,2-dimethyl-3-oxopropyl)-5-azido-4-hydroxy-2-isopropyl-7-(4-methoxy-3-(methoxypropoxy)benzyl)-8-methylnonanamide

| Conditions | Yield |
|---|---|
| With 2-hydroxypyridin; triethylamine at 80℃; | 76% |
| With 2-hydroxypyridin; triethylamine | 59% |
-
-
324763-51-1
aminopivalinamide


| Conditions | Yield |
|---|---|
| With 2-hydroxypyridin; triethylamine at 80℃; for 2h; | 73% |
-
-
324763-51-1
aminopivalinamide

-
-
1663-61-2
triethoxymethylbenzene

| Conditions | Yield |
|---|---|
| With acetic acid In ethanol at 110℃; for 24h; Inert atmosphere; | 72% |
-
-
324763-51-1
aminopivalinamide

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| With 2-hydroxypyridin; hydrogenchloride; triethylamine In methanol at 80℃; | 68% |
-
-
324763-51-1
aminopivalinamide

-
-
1000046-88-7
tert-butyl {(1S)-2-[4-(2-chlorophenyl)-2,2-dimethyl-5-oxopiperazin-1-yl]-1-[(2S,4S)-4-isopropyl-5-oxotetrahydrofuran-2-yl]ethyl}carbamate

-
-
1000046-90-1
tert-butyl {(2S,3S,5S)-5-[(3-amino-2,2-dimethyl-3-oxopropyl)carbamoyl]-1-[4-(2-chlorophenyl)-2,2-dimethyl-5-oxopiperazin-1-yl]-3-hydroxy-6-methylheptan-2-yl}carbamate

| Conditions | Yield |
|---|---|
| With 2-hydroxypyridin; triethylamine at 80℃; for 18h; | 66% |
| With 2-hydroxypyridin; triethylamine at 80℃; for 18h; | 66% |
-
-
324763-51-1
aminopivalinamide

-
-
1236549-00-0
{(1S,3S)-1-((2S,4S)-4-isopropyl-5-oxo-furanidin-2-yl)-3-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-4-methyl-pentyl}-carbamic acid benzyl ester

-
A
-
1236549-06-6
(1S,2S,4S)-4-(2-carbamoyl-2-methylpropyl-carbamoyl)-2-hydroxy-1-{(S)-2-[4-methoxy-3-(3-methoxypropoxy)-benzyl]-3-methylbutyl}-5-methylhexyl-carbamic acid benzyl ester

| Conditions | Yield |
|---|---|
| With 2-hydroxypyridin In tert-butyl methyl ether at 65℃; | A 66% B n/a |

-
-
953820-47-8
tert-butyl (1S,3S)-1-((2S,4S)-4-isopropyl-5-oxotetrahydrofuran-2-yl)-3-((1-(3-methoxypropyl)-1H-indazol-6-yl)methyl)-4-methylpentylcarbamate

-
-
324763-51-1
aminopivalinamide

-
-
953820-48-9
tert-butyl (3S,5S,6S,8S)-8-(3-amino-2,2-dimethyl-3-oxopropylcarbamoyl)-6-hydroxy-3-((1-(3-methoxypropyl)-1H-indazol-6-yl)methyl)-2,9-dimethyldecan-5-ylcarbamate

| Conditions | Yield |
|---|---|
| With 2-hydroxypyridin; triethylamine at 80℃; | 65% |
-
-
324763-51-1
aminopivalinamide

-
-
325740-70-3
(3S,5S)-5-((1S,3S)-1-azido-3-(4-methoxy-3-(3-methoxypropoxy)benzyl)-4-methylpentyl)-3-isopropyldihydrofuran-2(3H)-one

-
-
325154-31-2
(2S,4S,5R,7S)-5-Azido-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| With 2-hydroxypyridin; triethylamine | 59% |
-
-
324763-51-1
aminopivalinamide


-
-
361460-41-5
(2S,4S,5R,7S)-5-Benzylamino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| With 2-hydroxypyridin; triethylamine at 80℃; for 72h; | 53% |
-
-
324763-51-1
aminopivalinamide

-
-
1000047-28-8
{(S)-1-[(2S,4S)-4-isopropyl-5-oxotetrahydrofuran-2-yl]-2-[4-(2-methoxymethoxyphenyl)-2,2-dimethyl-5-oxopiperazin-1-yl]ethyl}carbamic acid benzyl ester

-
-
1000047-29-9
{(1S,2S,4S)-4-(2-carbamoyl-2-methylpropylcarbamoyl)-2-hydroxy-1-[4-(2-methoxymethoxyphenyl)-2,2-dimethyl-5-oxopiperazin-1-ylmethyl]-5-methylhexyl}carbamic acid benzyl ester

| Conditions | Yield |
|---|---|
| With 2-hydroxypyridin; triethylamine at 80℃; for 11h; | 52% |
-
-
324763-51-1
aminopivalinamide

-
-
78-39-7
Triethyl orthoacetate

| Conditions | Yield |
|---|---|
| With acetic acid In ethanol at 110℃; for 24h; Inert atmosphere; | 50% |
-
-
324763-51-1
aminopivalinamide

-
-
1200341-46-3
(2R,2'S,3S,4'S,5S)-3-isopropyl-5-(4-isopropyl-5-oxo-tetrahydro-furan-2-yl)-2-(4-methoxy-3-(3-methoxypropoxy)-phenyl)-pyrrolidine-1-sulfonic acid 2,2,2-trichloro-ethyl ester

-
-
1219468-79-7
(2R,3S,5S)-2,2,2-trichloroethyl 5-((1S,3S)-3-(3-amino-2,2-dimethyl-3-oxopropylcarbamoyl)-1-hydroxy-4-methylpentyl)-3-isopropyl-2-(4-methoxy-3-(3-methoxypropoxy)phenyl)pyrrolidine-1-sulfonate

| Conditions | Yield |
|---|---|
| With 2-hydroxypyridin; triethylamine at 85℃; Inert atmosphere; | 47% |
| With 2-hydroxypyridin; triethylamine at 85℃; for 72h; Inert atmosphere; |
-
-
324763-51-1
aminopivalinamide

-
-
115-80-0
Triethyl orthopropionate

| Conditions | Yield |
|---|---|
| With acetic acid In ethanol at 110℃; for 24h; Inert atmosphere; | 44% |
-
-
24964-76-9
triethyl orthobutyrate

-
-
324763-51-1
aminopivalinamide

| Conditions | Yield |
|---|---|
| With acetic acid In ethanol at 110℃; for 24h; Inert atmosphere; | 36% |
-
-
324763-51-1
aminopivalinamide


| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In acetonitrile at 180℃; for 5h; Microwave irradiation; | 29% |
-
-
324763-51-1
aminopivalinamide

-
-
19036-43-2
aminopivalic acid

| Conditions | Yield |
|---|---|
| With sulfuric acid |
3-Amino-2,2-dimethylpropionamide Chemical Properties
Product Name: 3-Amino-2,2-dimethylpropionamide (CAS NO.324763-51-1)
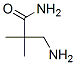
Molecular Formula: C5H12N2O
Molecular Weight: 116.16g/mol
Mol File: 324763-51-1.mol
Melting Point: 74-75 °C
Boiling point: 270.7 °C at 760 mmHg
Flash Point: 117.5 °C
Density: 1.004 g/cm3
Surface Tension: 39.2 dyne/cm
Enthalpy of Vaporization: 50.88 kJ/mol
Vapour Pressure: 0.00675 mmHg at 25°C
3-Amino-2,2-dimethylpropionamide Specification
3-Amino-2,2-dimethylpropionamide , its CAS NO. is 324763-51-1, the synonyms are 2-(Aminomethyl)-2-methylpropanamide ; 3-Amino-2,2-dimethylpropionic acid amide .
Related Products
- 3-Amino-2(1H)-pyridinethione
- 3-Amino-2-(2-(diisopropylamino)ethoxy) butyrophenonedihydrochloride
- 3-Amino-2-(Phenylethynyl)pyridine
- 3-Amino-2,2,4,4-tetramethylthietane
- 3-Amino-2,2-difluoropropionic acid hydrochloride
- 3-Amino-2,2-dimethyl-1-propanol
- 3-Amino-2,2-dimethylpropionamide
- 3-Amino-2,2-dimethyl-propionic acid ethyl ester
- 3-Amino-2,3,4,5-Tetrahydro-1H-1-benzazepin-2-one
- 3-Amino-2,3,4,5-tetrahydro-2-oxo-1H-1-benzazepine-1-acetic acid ter-butyl ester
- 324767-92-2
- 324769-02-0
- 324769-06-4
- 324769-07-5
- 3247-70-9
- 32477-35-3
- 324773-66-2
- 32479-45-1
- 324795-53-1
- 3248-05-3
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View