-
Name
3-Chloro-2-methylpropene
- EINECS 209-251-2
- CAS No. 563-47-3
- Article Data60
- CAS DataBase
- Density 0.897 g/cm3
- Solubility 0.5 g/L (20 °C) in water
- Melting Point -80 °C
- Formula C4H7Cl
- Boiling Point 72.5 °C at 760 mmHg
- Molecular Weight 90.5526
- Flash Point 9 °F
- Transport Information UN 2554 3/PG 2
- Appearance clear liquid
- Safety 9-16-29-36/37/39-45-61-26
- Risk Codes 11-20/22-34-43-51/53
-
Molecular Structure
-
Hazard Symbols
 F,
F,  C,
C,  N
N
- Synonyms Propene,3-chloro-2-methyl- (6CI,8CI);1-Chloro-2-methyl-2-propene;2-(Chloromethyl)-1-propene;2-Methallyl chloride;2-Methyl-2-propenyl chloride;2-Methyl-3-chloro-1-propene;2-Methyl-3-chloropropene;2-Methylallyl chloride;3-Chloro-2-methyl-1-propene;1-Propene,3-chloro-2-methyl-;Isobutenyl chloride;Methallyl chloride;Methylallyl chloride;NSC 7303;b-Methallyl chloride;g-Chloroisobutylene;
- PSA 0.00000
- LogP 1.80130
Synthetic route

| Conditions | Yield |
|---|---|
| With 1,1,1,3,3,3-hexachloro-propan-2-one; triphenylphosphine In sulfolane from 10 deg C to room temp.; | 95.7% |
| With pyridine; bis(trichloromethyl) carbonate In tetrahydrofuran Ambient temperature; | 65% |
| With 1,1,1,3,3,3-hexachloro-propan-2-one; triphenylphosphine In sulfolane from 10 deg C to room temp., effect of reagents ratio and solvent volume discussed; |
-
-
32670-93-2
2-methallyl diphenylphosphate

-
-
563-47-3
3-Chloro-2-methylpropene

| Conditions | Yield |
|---|---|
| With lithium chloride In N,N-dimethyl-formamide for 0.166667h; Ambient temperature; | 64% |

| Conditions | Yield |
|---|---|
| In dichloromethane O°C, AsPh3 was added, 24 h.; | A 55% B 20% |
| In dichloromethane O°C, AsPh3 was added, 1.33 h.; | A 22% B 2% |
| In dichloromethane O°C, AsPh3 was added, 0.67 h.; | A 10% B 0% |
| In dichloromethane O°C, AsPh3 was added, 0.33 h.; | A 6% B 0% |

-
-
78538-54-2
boric acid trimethallyl ester

-
-
563-47-3
3-Chloro-2-methylpropene

| Conditions | Yield |
|---|---|
| With boron trichloride at 120℃; |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; copper(l) chloride at 30 - 50℃; under 89731.9 Torr; |
-
-
36942-09-3
N,N-dichlorourea

-
-
64-19-7
acetic acid

-
-
115-11-7
isobutene

-
A
-
57576-87-1
acetic acid-(chloro-tert-butyl ester)

-
B
-
563-47-3
3-Chloro-2-methylpropene


| Conditions | Yield |
|---|---|
| With acetic acid | |
| With trichloroacetic acid | |
| With chloroacetic acid |
-
-
473-29-0
N,N-Dichlorobenzenesulfonamide

-
-
60-29-7
diethyl ether

-
-
115-11-7
isobutene

-
-
107-07-3
2-chloro-ethanol

-
A
-
55982-53-1
1-chloro-2-(2-chloroethoxy)-2-methylpropane

-
B
-
563-47-3
3-Chloro-2-methylpropene


| Conditions | Yield |
|---|---|
| Chlorierung; | |
| With chlorine at 0 - 70℃; | |
| With chlorine at 0℃; |
-
-
115-11-7
isobutene

-
A
-
513-37-1
2-methylprop-1-enyl chloride

-
B
-
594-37-6
1,2-dichloro-2-methylpropane

-
C
-
507-20-0
tertiary butyl chloride

-
D
-
563-47-3
3-Chloro-2-methylpropene

| Conditions | Yield |
|---|---|
| at 80℃; Chlorierung; | |
| With chlorine In tetrachloromethane at 20℃; | A n/a B 6 % Chromat. C n/a D 77 % Chromat. |


| Conditions | Yield |
|---|---|
| bei der Chlorierung; |

| Conditions | Yield |
|---|---|
| With nitrogen trichloride In dichloromethane | |
| With chlorine at 70℃; under 760.051 Torr; for 24h; Inert atmosphere; | A 8.73 %Chromat. B 79.47 %Chromat. |

| Conditions | Yield |
|---|---|
| at 380.1℃; Kinetics; Thermodynamic data; pyrolysis; Ea; var. temp. and initial pressure; |
-
-
115-11-7
isobutene

-
A
-
513-37-1
2-methylprop-1-enyl chloride

-
B
-
1871-57-4
2-chloromethyl-3-chloroprop-1-ene

-
C
-
594-37-6
1,2-dichloro-2-methylpropane

-
D
-
35329-41-0
(E)-1,3-Dichloro-2-methyl-1-propene

-
E
-
35329-40-9
(Z)-1,3-Dichloro-2-methyl-1-propene

-
F
-
563-47-3
3-Chloro-2-methylpropene

| Conditions | Yield |
|---|---|
| With chlorine at 40 - 70℃; Product distribution; various conc.; further products; |

-
-
507-40-4
tert-butylhypochlorite

-
-
122-52-1
triethyl phosphite

-
A
-
78-40-0
triethyl phosphate

-
B
-
507-20-0
tertiary butyl chloride

-
C
-
637-92-3
Ethyl tert-butyl ether

-
D
-
814-49-3
diethyl chlorophosphate

-
E
-
563-47-3
3-Chloro-2-methylpropene

-
F
-
75-65-0
tert-butyl alcohol

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; Rate constant; |


| Conditions | Yield |
|---|---|
| With 1,1,1,3,3,3-hexachloro-propan-2-one; triphenylphosphine In sulfolane from 10 deg C to room temp.; | 95.7% |
| With pyridine; bis(trichloromethyl) carbonate In tetrahydrofuran Ambient temperature; | 65% |
| With 1,1,1,3,3,3-hexachloro-propan-2-one; triphenylphosphine In sulfolane from 10 deg C to room temp., effect of reagents ratio and solvent volume discussed; |
-
-
32670-93-2
2-methallyl diphenylphosphate

-
-
563-47-3
3-Chloro-2-methylpropene

| Conditions | Yield |
|---|---|
| With lithium chloride In N,N-dimethyl-formamide for 0.166667h; Ambient temperature; | 64% |

| Conditions | Yield |
|---|---|
| In dichloromethane O°C, AsPh3 was added, 24 h.; | A 55% B 20% |
| In dichloromethane O°C, AsPh3 was added, 1.33 h.; | A 22% B 2% |
| In dichloromethane O°C, AsPh3 was added, 0.67 h.; | A 10% B 0% |
| In dichloromethane O°C, AsPh3 was added, 0.33 h.; | A 6% B 0% |

-
-
78538-54-2
boric acid trimethallyl ester

-
-
563-47-3
3-Chloro-2-methylpropene

| Conditions | Yield |
|---|---|
| With boron trichloride at 120℃; |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; copper(l) chloride at 30 - 50℃; under 89731.9 Torr; |
-
-
36942-09-3
N,N-dichlorourea

-
-
64-19-7
acetic acid

-
-
115-11-7
isobutene

-
A
-
57576-87-1
acetic acid-(chloro-tert-butyl ester)

-
B
-
563-47-3
3-Chloro-2-methylpropene


| Conditions | Yield |
|---|---|
| With acetic acid | |
| With trichloroacetic acid | |
| With chloroacetic acid |
-
-
473-29-0
N,N-Dichlorobenzenesulfonamide

-
-
60-29-7
diethyl ether

-
-
115-11-7
isobutene

-
-
107-07-3
2-chloro-ethanol

-
A
-
55982-53-1
1-chloro-2-(2-chloroethoxy)-2-methylpropane

-
B
-
563-47-3
3-Chloro-2-methylpropene


| Conditions | Yield |
|---|---|
| Chlorierung; | |
| With chlorine at 0 - 70℃; | |
| With chlorine at 0℃; |
-
-
115-11-7
isobutene

-
A
-
513-37-1
2-methylprop-1-enyl chloride

-
B
-
594-37-6
1,2-dichloro-2-methylpropane

-
C
-
507-20-0
tertiary butyl chloride

-
D
-
563-47-3
3-Chloro-2-methylpropene

| Conditions | Yield |
|---|---|
| at 80℃; Chlorierung; | |
| With chlorine In tetrachloromethane at 20℃; | A n/a B 6 % Chromat. C n/a D 77 % Chromat. |


| Conditions | Yield |
|---|---|
| bei der Chlorierung; |

| Conditions | Yield |
|---|---|
| With nitrogen trichloride In dichloromethane | |
| With chlorine at 70℃; under 760.051 Torr; for 24h; Inert atmosphere; | A 8.73 %Chromat. B 79.47 %Chromat. |

| Conditions | Yield |
|---|---|
| at 380.1℃; Kinetics; Thermodynamic data; pyrolysis; Ea; var. temp. and initial pressure; |
-
-
115-11-7
isobutene

-
A
-
513-37-1
2-methylprop-1-enyl chloride

-
B
-
1871-57-4
2-chloromethyl-3-chloroprop-1-ene

-
C
-
594-37-6
1,2-dichloro-2-methylpropane

-
D
-
35329-41-0
(E)-1,3-Dichloro-2-methyl-1-propene

-
E
-
35329-40-9
(Z)-1,3-Dichloro-2-methyl-1-propene

-
F
-
563-47-3
3-Chloro-2-methylpropene

| Conditions | Yield |
|---|---|
| With chlorine at 40 - 70℃; Product distribution; various conc.; further products; |

-
-
507-40-4
tert-butylhypochlorite

-
-
122-52-1
triethyl phosphite

-
A
-
78-40-0
triethyl phosphate

-
B
-
507-20-0
tertiary butyl chloride

-
C
-
637-92-3
Ethyl tert-butyl ether

-
D
-
814-49-3
diethyl chlorophosphate

-
E
-
563-47-3
3-Chloro-2-methylpropene

-
F
-
75-65-0
tert-butyl alcohol

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; Rate constant; |

-
-
64-19-7
acetic acid

-
-
3100-04-7
Methylcyclopropen

-
A
-
820-71-3
methallyl acetate

-
B
-
563-47-3
3-Chloro-2-methylpropene

| Conditions | Yield |
|---|---|
| With hydrogenchloride at 0℃; |


| Conditions | Yield |
|---|---|
| With hydrogenchloride In acetic acid at 0℃; Product distribution; var. concentration of HCl and reaction times, other substituted cyclopropene; | |
| With hydrogenchloride In acetic acid at 0℃; |
-
-
115-11-7
isobutene

-
A
-
513-37-1
2-methylprop-1-enyl chloride

-
B
-
594-37-6
1,2-dichloro-2-methylpropane

-
C
-
507-20-0
tertiary butyl chloride

-
D
-
1871-58-5
1,2,3-trichloro-2-methylpropane

-
E
-
563-47-3
3-Chloro-2-methylpropene

| Conditions | Yield |
|---|---|
| With Bu3(PhCH2)NCl*2Cl2 In tetrachloromethane at 20℃; Product distribution; other reagents, other concentration; | A n/a B 69 % Chromat. C n/a D 19 % Chromat. E 9 % Chromat. |

-
-
115-11-7
isobutene

-
A
-
594-37-6
1,2-dichloro-2-methylpropane

-
B
-
1871-58-5
1,2,3-trichloro-2-methylpropane

-
C
-
563-47-3
3-Chloro-2-methylpropene

| Conditions | Yield |
|---|---|
| With Bu3(PhCH2)NCl*2Cl2 In tetrachloromethane at 20℃; | A 74 % Chromat. B 18 % Chromat. C 8 % Chromat. |
| With Bu3(PhCH2)NCl*2Cl2 In tetrachloromethane at 20℃; | A 34 % Chromat. B 62 % Chromat. C 3 % Chromat. |


| Conditions | Yield |
|---|---|
| at 0℃; Mechanism; |
-
-
7732-18-5
water

-
-
13898-47-0
hypochloric acid

-
-
115-11-7
isobutene

-
A
-
513-37-1
2-methylprop-1-enyl chloride

-
B
-
558-42-9
3-chloro-2-methylpropan-2-ol

-
C
-
563-47-3
3-Chloro-2-methylpropene

| Conditions | Yield |
|---|---|
| Mechanism; |

-
-
115-11-7
isobutene

-
A
-
513-37-1
2-methylprop-1-enyl chloride

-
B
-
594-37-6
1,2-dichloro-2-methylpropane

-
C
-
507-20-0
tertiary butyl chloride

-
D
-
563-47-3
3-Chloro-2-methylpropene

| Conditions | Yield |
|---|---|
| at 200 - 450℃; Chlorierung; |

-
-
473-29-0
N,N-Dichlorobenzenesulfonamide

-
-
67-66-3
chloroform

-
-
115-11-7
isobutene

-
-
108-95-2
phenol

-
B
-
563-47-3
3-Chloro-2-methylpropene

-
-
7732-18-5
water

-
-
7782-50-5
chlorine

-
-
115-11-7
isobutene

-
A
-
558-42-9
3-chloro-2-methylpropan-2-ol

-
B
-
78-84-2
isobutyraldehyde

-
C
-
563-47-3
3-Chloro-2-methylpropene

| Conditions | Yield |
|---|---|
| at 100℃; |

-
-
36942-09-3
N,N-dichlorourea

-
-
64-19-7
acetic acid

-
-
115-11-7
isobutene

-
A
-
563-47-3
3-Chloro-2-methylpropene

-
-
64-18-6
formic acid

-
-
36942-09-3
N,N-dichlorourea

-
-
115-11-7
isobutene

-
A
-
563-47-3
3-Chloro-2-methylpropene


-
A
-
563-47-3
3-Chloro-2-methylpropene

| Conditions | Yield |
|---|---|
| With oxalic acid Beim Destillieren; | |
| With phosphorus pentoxide Beim Destillieren; |

| Conditions | Yield |
|---|---|
| With titanium tetrachloride |
-
-
513-37-1
2-methylprop-1-enyl chloride

-
-
7664-93-9
sulfuric acid

-
A
-
558-42-9
3-chloro-2-methylpropan-2-ol

-
B
-
563-47-3
3-Chloro-2-methylpropene

-
-
141-97-9
ethyl acetoacetate

-
-
563-47-3
3-Chloro-2-methylpropene

-
-
20962-70-3
ethyl 2-acetyl-4-methylpent-4-enoate

| Conditions | Yield |
|---|---|
| With tetrabutylammomium bromide; potassium carbonate; potassium iodide In N,N-dimethyl-formamide at 40 - 75℃; for 6h; | 100% |
| With tetrabutylammomium bromide; potassium carbonate; potassium iodide In N,N-dimethyl-formamide at 40 - 75℃; for 6h; | 100% |
| With tetrabutylammomium bromide; potassium carbonate; potassium iodide In N,N-dimethyl-formamide at 40 - 75℃; for 6h; | 100% |

| Conditions | Yield |
|---|---|
| With copper(l) iodide; TEA at 60℃; | 100% |
| With copper(l) iodide; N-benzyl-N,N,N-triethylammonium chloride; potassium carbonate; potassium iodide; sodium sulfite In water; benzene at 75 - 80℃; for 6h; | 92% |
| With potassium carbonate; copper(l) iodide; tetrabutyl-ammonium chloride In N,N-dimethyl-formamide for 16h; Product distribution; Ambient temperature; variation of catalysts and reaction time; | 88% |
-
-
106-41-2
4-bromo-phenol

-
-
563-47-3
3-Chloro-2-methylpropene

-
-
5820-27-9
1-(2’-methylallyloxy)-4-bromobenzene

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 70℃; for 18h; Inert atmosphere; | 100% |
| With potassium carbonate In acetone Inert atmosphere; Reflux; | 87% |
| With potassium carbonate; acetone | |
| With potassium carbonate In acetone | |
| With potassium carbonate In N,N-dimethyl-formamide at 70℃; |
-
-
563-47-3
3-Chloro-2-methylpropene

-
-
18147-56-3
trichloro(2-methyl-2-propenyl)silane

| Conditions | Yield |
|---|---|
| With tetrachlorosilane; 1,1,2-trichloro-1,2,2-trimethyldisilane; 1,1,2,2-tetrachloro-1,2-dimethyldisilane; N,N,N,N,N,N-hexamethylphosphoric triamide; nickelocene for 15h; Heating; | 100% |
| With N,N,N,N,N,N-hexamethylphosphoric triamide; tetrachlorosilane; nickelocene for 15h; Heating; | 100% |
| With tetrachlorosilane; triethylamine; copper(l) chloride | 78% |
-
-
75-89-8
2,2,2-trifluoroethanol

-
-
563-47-3
3-Chloro-2-methylpropene

-
-
74407-77-5
2-methyl-3-(2',2',2'-trifluoroethoxy)-prop-1-ene

| Conditions | Yield |
|---|---|
| With sodium hydroxide; tetra(n-butyl)ammonium hydrogensulfate; tetra-(n-butyl)ammonium iodide | 100% |
| Stage #1: 2,2,2-trifluoroethanol With potassium hydroxide at 40℃; Stage #2: 3-Chloro-2-methylpropene In water at 40℃; | 78% |
| With potassium carbonate In N,N-dimethyl-formamide |
-
-
286376-23-6
4-methyl-N-(prop-1-yn-1-yl)benzenesulfonamide

-
-
563-47-3
3-Chloro-2-methylpropene

-
-
292607-11-5
N-(but-2-ynyl)-4-methyl-N-(2-methylallyl)benzenesulfonamide

| Conditions | Yield |
|---|---|
| Stage #1: 4-methyl-N-(prop-1-yn-1-yl)benzenesulfonamide With sodium hydride In tetrahydrofuran; N,N-dimethyl-formamide at 0℃; for 1h; Stage #2: 3-Chloro-2-methylpropene With sodium iodide In tetrahydrofuran; N,N-dimethyl-formamide at 20℃; for 9h; Further stages.; | 100% |

| Conditions | Yield |
|---|---|
| With zinc In tetrahydrofuran at 20℃; for 4h; | 100% |
-
-
73396-92-6
2-iodo-N,N-diallylaniline

-
-
563-47-3
3-Chloro-2-methylpropene

-
-
882788-93-4
N,N-diallyl-2-(2-methylprop-2-en-1-yl)aniline

| Conditions | Yield |
|---|---|
| Stage #1: 2-iodo-N,N-diallylaniline With TurboGrignard In tetrahydrofuran at -10℃; for 0.5h; Stage #2: 3-Chloro-2-methylpropene In tetrahydrofuran at -10 - 20℃; for 12h; | 100% |
| Stage #1: 2-iodo-N,N-diallylaniline With TurboGrignard In tetrahydrofuran at -10℃; for 1h; Stage #2: 3-Chloro-2-methylpropene With copper(I) cyanide lithium chloride In tetrahydrofuran at -10 - 20℃; for 25h; | |
| Stage #1: 2-iodo-N,N-diallylaniline With TurboGrignard In tetrahydrofuran at -10℃; for 0.5h; Inert atmosphere; Stage #2: 3-Chloro-2-methylpropene With copper(I) cyanide di(lithium chloride) In tetrahydrofuran at -10 - 20℃; | 568 mg |
| Stage #1: 2-iodo-N,N-diallylaniline With isopropylmagnesium chloride In tetrahydrofuran at -10℃; Stage #2: 3-Chloro-2-methylpropene With copper(l) cyanide; lithium chloride at -10 - 20℃; |
-
-
563-47-3
3-Chloro-2-methylpropene

-
-
104131-54-6
2-methyl-2-propenyl azide

| Conditions | Yield |
|---|---|
| With sodium azide In dimethyl sulfoxide at 20℃; for 2.5h; | 100% |
| With sodium azide In dimethyl sulfoxide at 70℃; for 15h; | |
| With sodium azide In dimethyl sulfoxide at 70℃; for 15h; |

| Conditions | Yield |
|---|---|
| With copper(l) iodide; tetrabutylammomium bromide; potassium carbonate In N,N-dimethyl-formamide at 20℃; for 24h; | 100% |
-
-
107033-46-5
(R)-(tert-butyldiphenylsilyloxy)methyloxirane

-
-
563-47-3
3-Chloro-2-methylpropene

-
-
945468-53-1
C23H32O2Si

| Conditions | Yield |
|---|---|
| Stage #1: 3-Chloro-2-methylpropene With magnesium In tetrahydrofuran at 24℃; for 1h; Stage #2: (R)-(tert-butyldiphenylsilyloxy)methyloxirane In tetrahydrofuran at -40℃; for 5h; Further stages.; | 100% |

-
-
563-47-3
3-Chloro-2-methylpropene

| Conditions | Yield |
|---|---|
| With hydrogenchloride; sodium hydroxide; potassium iodide In methanol; N,N-dimethyl-formamide | 100% |
| With sodium hydroxide; concentrated hydrochloric acid; potassium iodide In methanol; sodium hydride; N,N-dimethyl-formamide |

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: 5-chloro-2-hydroxybenzoic acid With potassium carbonate; potassium iodide In N,N-dimethyl-formamide at 65℃; for 0.0833333h; Inert atmosphere; Stage #2: 3-Chloro-2-methylpropene In N,N-dimethyl-formamide at 65℃; for 21h; | 100% |
| With potassium carbonate In N-methyl-acetamide |
-
-
30030-83-2
1-<4-(1H-indol-3-yl)piperidino>ethanone

-
-
563-47-3
3-Chloro-2-methylpropene

-
-
648882-71-7
1-{4-[1-(2-methylallyl)-1H-indol-3-yl]-piperidin-1-yl}-ethanone

| Conditions | Yield |
|---|---|
| Stage #1: 1-<4-(1H-indol-3-yl)piperidino>ethanone With sodium hydride In DMF (N,N-dimethyl-formamide) at 20℃; for 0.333333h; Stage #2: 3-Chloro-2-methylpropene In DMF (N,N-dimethyl-formamide) at 20℃; | 100% |
-
-
121-71-1
3-Hydroxyacetophenone

-
-
563-47-3
3-Chloro-2-methylpropene

-
-
1017048-68-8
1-[3-(2-methylallyloxy)phenyl]ethanone

| Conditions | Yield |
|---|---|
| With tetra-(n-butyl)ammonium iodide; potassium carbonate In N,N-dimethyl-formamide at 20 - 80℃; | 100% |
| With potassium carbonate In acetonitrile for 17h; Reflux; |
3-Chloro-2-methylpropene Chemical Properties
The molecular structure of 3-Chloro-2-methylpropene (CAS NO.563-47-3):
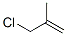
IUPAC Name: 3-Chloro-2-methylprop-1-ene
Molecular Weight: 90.55138 g/mol
Molecular Formula: C4H7Cl
Density: 0.897 g/cm3
Melting Point: -80 °C
Boiling Point: 72.5 °C at 760 mmHg
Flash Point: 9 °F
Index of Refraction: 1.41
Molar Refractivity: 24.99 cm3
Molar Volume: 100.8 cm3
Surface Tension: 20.6 dyne/cm
Enthalpy of Vaporization: 30.08 kJ/mol
Vapour Pressure: 130 mmHg at 25 °C
Storage Temp.: refrigerator (+4 °C) + flammables area
Water Solubility: 0.5 g/L (20 °C)
XLogP3-AA: 2.1
Rotatable Bond Count: 1
Exact Mass: 90.023628
MonoIsotopic Mass: 90.023628
Canonical SMILES: CC(=C)CCl
InChI: InChI=1S/C4H7Cl/c1-4(2)3-5/h1,3H2,2H3
InChIKey: OHXAOPZTJOUYKM-UHFFFAOYSA-N
EINECS: 209-251-2
Product Categories: Industrial/Fine Chemicals
3-Chloro-2-methylpropene Uses
3-Chloro-2-methylpropene (CAS NO.563-47-3) is used to make plastics and pharmaceuticals. It is important organic intermediates. It also can be widely used in medicine, pesticides, perfume, synthetic materials.
3-Chloro-2-methylpropene Toxicity Data With Reference
| 1. | mmo-sat 6 µmol/plate | BCPCA6 Biochemical Pharmacology. 29 (1980),2611. | ||
| 2. | dns-hmn:hla 1 mmol/L | CALEDQ Cancer Letters (Shannon, Ireland). 20 (1983),263. | ||
| 3. | orl-rat TDLo:77,250 mg/kg/2Y-I:CAR | NTPTR* National Toxicology Program Technical Report Series. (Research Triangle Park, NC 27709) No. NTP-TR-300 ,1986. | ||
| 4. | orl-mus TDLo:51,500 mg/kg/2Y-I:CAR | NTPTR* National Toxicology Program Technical Report Series. (Research Triangle Park, NC 27709) No. NTP-TR-300 ,1986. | ||
| 5. | orl-rat TD:77,250 mg/kg/2Y-I:NEO,REP | NTPTR* National Toxicology Program Technical Report Series. (Research Triangle Park, NC 27709) No. NTP-TR-300 ,1986. |
3-Chloro-2-methylpropene Consensus Reports
NTP 10th Report on Carcinogens. NTP Carcinogenesis Studies (gavage); Clear Evidence: mouse, rat NTPTR* National Toxicology Program Technical Report Series. (Research Triangle Park, NC 27709) No. NTP-TR-300 ,1986. . Reported in EPA TSCA Inventory.
3-Chloro-2-methylpropene Safety Profile
Hazard Codes:  F,
F,  C,
C,  N
N
Risk Statements: 11-20/22-34-43-51/53
R11:Highly flammable.
R20/22:Harmful by inhalation and if swallowed.
R34:Causes burns.
R43:May cause sensitization by skin contact.
R51/53:Toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment.
Safety Statements: 9-16-29-36/37/39-45-61-26
S9:Keep container in a well-ventilated place.
S16:Keep away from sources of ignition.
S29:Do not empty into drains.
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection.
S45:In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
S61:Avoid release to the environment. Refer to special instructions / safety data sheets.
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
RIDADR: UN 2554 3/PG 2
WGK Germany: 2
RTECS: UC8050000
HazardClass: 3
PackingGroup: II
HS Code: 29032900
Confirmed carcinogen with experimental carcinogenic, neoplastigenic, and tumorigenic data. Experimental reproductive effects. An irritant. Human mutation data reported. Very dangerous fire hazard when exposed to heat, flame, or oxidizers. Moderately explosive when exposed to heat or flame. Can react vigorously with oxidizing materials. To fight fire, use alcohol foam, CO2, dry chemical. When heated to decomposition it emits toxic fumes of Cl−. See also CHLORINATED HYDROCARBONS, ALIPHATIC; and ALLYL COMPOUNDS.
3-Chloro-2-methylpropene Standards and Recommendations
DFG MAK: Confirmed Animal Carcinogen with Unknown Relevance to Humans
DOT Classification: 3; Label: Flammable Liquid
3-Chloro-2-methylpropene Specification
3-Chloro-2-methylpropene (CAS NO.563-47-3) is also named as 1-Chloro-2-methyl-2-propene ; 2-Methallyl chloride ; 2-Methyl-2-propenyl chloride ; 2-Methyl-allylchlorid ; 2-Methyl-allylchlorid [German] ; 3-Chlor-2-methyl-prop-1-en ; 3-Chlor-2-methyl-prop-1-en [German] ; 3-Chloro-2-methyl-1-propene ; 3-Chloroisobutylene ; 3-Cloro-2-metil-prop-1-ene ; 3-Cloro-2-metil-prop-1-ene [Italian] ; 4-01-00-00803 (Beilstein Handbook Reference) ; AI3-14901 ; BRN 0878160 ; CCRIS 869 ; Chlorue de methallyle ; Chlorue de methallyle [French] ; Chlorure de methallyle ; Chlorure de methallyle [French] ; Cloruro di metallile ; Cloruro di metallile [Italian] ; HSDB 1149 ; Isobutenyl chloride ; MAC ; Methallyl chloride ; NCI-C54820 ; NSC 7303 ; Propene, 3-chloro-2-methyl- ; beta-Methylallyl chloride ; gamma-Chloroisobutylene . 3-Chloro-2-methylpropene (CAS NO.563-47-3) is clear liquid with a sharp penetrating odor. It is insoluble in water. It may be toxic by ingestion. Irritating to skin and eyes. It is highly flammable. 3-Chloro-2-methylpropene may react with water at elevated temperatures. It is sensitive to light. 3-Chloro-2-methylpropene can react vigorously with oxidizers. It is incompatible with strong bases.Inhalation causes irritation of nose and throat. Contact of vapor or liquid with eyes causes irritation. Liquid irritates skin. Ingestion causes irritation of mouth and stomach.
Related Products
- 3-Chloro-2-methylpropene
- 56-34-8
- 563-52-0
- 56353-15-2
- 56353-97-0
- 56353-99-2
- 56354-06-4
- 563-54-2
- 56355-62-5
- 5635-67-6
- 56-35-9
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View