-
Name
3-Dimethylaminoacrolein
- EINECS 213-157-7
- CAS No. 927-63-9
- Article Data25
- CAS DataBase
- Density 0.907 /cm3
- Solubility
- Melting Point
- Formula C5H9NO
- Boiling Point 271.5 °C at 760 mmHg
- Molecular Weight 99.1326
- Flash Point 128.9 °C
- Transport Information UN 3267 8/PG 2
- Appearance
- Safety 26-36/37/39-45
- Risk Codes 34
-
Molecular Structure
-
Hazard Symbols
 C
C
- Synonyms 3-(Dimethylamino)-2-propenal;Acrolein,3-(dimethylamino)- (6CI,7CI,8CI);3-(Dimethylamino)acrylaldehyde;3-(Dimethylamino)propenal;4-Dimethylamino-2-propen-1-al;β-(Dimethylamino)acrolein;(2E)-3-(dimethylamino)prop-2-enal;2-Propenal, 3-(dimethylamino)-;3-(Dimethylamino)-2-propenal;
- PSA 20.31000
- LogP 0.26060
Synthetic route
-
-
104-04-1
N-(4-Nitrophenyl)acetamide

-
-
68-12-2, 33513-42-7
N,N-dimethyl-formamide

-
A
-
1205-59-0
N1,N1-dimethyl-N2-(4-nitro-phenyl)-formamidine

-
B
-
78708-10-8
(E)-3-Dimethylamino-2-formyl-N-(4-nitro-phenyl)-acrylamide

-
C
-
100-01-6
4-nitro-aniline

-
D
-
927-63-9
3-dimethylaminoacrolein

| Conditions | Yield |
|---|---|
| With trichlorophosphate at 75℃; for 1.5h; | A 45% B 53% C 5% D 0.05 g |

-
-
502-49-8
cycloactanone

-
-
5043-86-7
acetal-aminal of β-dimethylaminoacrolein

-
A
-
75143-08-7
2-[(E)-3-Dimethylamino-prop-2-en-(Z)-ylidene]-cyclooctanone

-
B
-
927-63-9
3-dimethylaminoacrolein

| Conditions | Yield |
|---|---|
| With silica gel 1,) 80 deg C to 90 deg C, 1.5 h, 2.) CHCl3, 1 h; | A 49% B n/a |


| Conditions | Yield |
|---|---|
| With methanol |
-
-
105-57-7
diethyl acetal

-
-
75-44-5
phosgene

-
-
107-06-2
1,2-dichloro-ethane

-
-
68-12-2, 33513-42-7
N,N-dimethyl-formamide

-
A
-
13984-13-9, 70669-77-1
3-dimethylaminopropyl-2-ene-1-imino-N,N-dimethylammonium chloride

-
B
-
927-63-9
3-dimethylaminoacrolein

| Conditions | Yield |
|---|---|
| beim Behandeln des Reaktionsgemisches mit Eis sowie anschliessend mit wss. Kaliumcarbonat-Loesung; |


| Conditions | Yield |
|---|---|
| With 1,2-dichloro-ethane und Behandeln des Reaktionsgemisches mit Acetaldehyd-diaethylacetal; |

| Conditions | Yield |
|---|---|
| at 28℃; Product distribution; other temperature; |

-
-
51513-20-3
3-dimethyl-amino-1,1,3-trimethoxypropane

-
-
3603-99-4
cyclotetradecanone

-
A
-
75143-09-8
2-[(E)-3-Dimethylamino-prop-2-en-(Z)-ylidene]-cyclotetradecanone

-
B
-
927-63-9
3-dimethylaminoacrolein

| Conditions | Yield |
|---|---|
| at 95 - 100℃; for 4h; | A 0.12 g B n/a |


-
-
927-63-9
3-dimethylaminoacrolein

| Conditions | Yield |
|---|---|
| With acetic anhydride at 120 - 125℃; for 0.5h; | 100% |

| Conditions | Yield |
|---|---|
| With potassium hydrogencarbonate at 60℃; for 0.5h; Reagent/catalyst; Temperature; Microwave irradiation; Green chemistry; | 97.2% |
| With tetra(n-butyl)ammonium hydroxide In water at 90℃; for 2h; Reagent/catalyst; Temperature; | 97.1% |
| With triethylamine at 120℃; Sonication; | 96.7% |
| With N-butyl-N-ethylpiperidinium bromide at 55℃; for 1h; Microwave irradiation; | 95.6% |
| With trimethyldodecylammonium chloride In dichloromethane; water at 95℃; |

-
-
927-63-9
3-dimethylaminoacrolein

| Conditions | Yield |
|---|---|
| Stage #1: 1,2-bis(2,2'-bipyrrol-5-yl)benzene; 3-dimethylaminoacrolein With trichlorophosphate In dichloromethane at 0℃; for 1h; Vilsmeier-Haack Formylation; Stage #2: With potassium hydroxide In tetrahydrofuran; water at 20℃; for 1h; | 97% |
-
-
102069-19-2
cyano-acetic acid 2,2-dimethyl-[1,3]dioxolan-4-ylmethyl ester

-
-
111-86-4
n-Octylamine

-
-
927-63-9
3-dimethylaminoacrolein

| Conditions | Yield |
|---|---|
| Stage #1: cyano-acetic acid 2,2-dimethyl-[1,3]dioxolan-4-ylmethyl ester; n-Octylamine With acetic acid In toluene Reflux; Inert atmosphere; Stage #2: 3-dimethylaminoacrolein In toluene for 36h; Reflux; | 97% |

-
-
91107-10-7
4,6-dimethoxy-2,3-diphenyl-1H-indole

-
-
927-63-9
3-dimethylaminoacrolein

| Conditions | Yield |
|---|---|
| With trichlorophosphate In tetrahydrofuran; dichloromethane at -10 - 20℃; for 1h; | 97% |
-
-
98548-92-6
Butylcyanomethylsulfonat

-
-
927-63-9
3-dimethylaminoacrolein

| Conditions | Yield |
|---|---|
| With tetrabutyl-ammonium chloride In water at 90℃; for 1h; | 95.5% |
| With N,N-butyl-methyl-piperidinium bromide at 46℃; for 4h; Microwave irradiation; | 95% |
| With sodium ethanolate at 60℃; Sonication; | 95.7% |
| With pyridine at 40℃; for 2h; Microwave irradiation; Green chemistry; | 94.5% |
-
-
1392321-89-9
triphenylphosphine[5,10,15-tris-(4-methylphenyl)corrolato] cobalt(III)

-
-
927-63-9
3-dimethylaminoacrolein

| Conditions | Yield |
|---|---|
| Stage #1: 3-dimethylaminoacrolein With trichlorophosphate In dichloromethane at 0℃; Vilsmeier Reaction; Inert atmosphere; Stage #2: triphenylphosphine[5,10,15-tris-(4-methylphenyl)corrolato] cobalt(III) In dichloromethane for 0.166667h; Inert atmosphere; | A 4% B 95% |

-
-
14447-18-8
benzyl 2-cyanoacetate

-
-
927-63-9
3-dimethylaminoacrolein

-
-
1107646-58-1
benzyl 2-bromonicotinate

| Conditions | Yield |
|---|---|
| Stage #1: benzyl 2-cyanoacetate; 3-dimethylaminoacrolein With acridine at 120℃; Sonication; Stage #2: With hydrogen bromide | 94.3% |
| Stage #1: benzyl 2-cyanoacetate; 3-dimethylaminoacrolein With 1-methyl-1-hexylpyridinium bromide at 120℃; for 3h; Stage #2: With hydrogen bromide | 92.3% |
-
-
105-56-6
ethyl 2-cyanoacetate

-
-
927-63-9
3-dimethylaminoacrolein

-
-
51513-16-7
5-(N,N-dimethyl)amino-2-cyano-2,4-pentadienoic acid ethyl ester

| Conditions | Yield |
|---|---|
| With N-butyl-N-ethylpiperidinium bromide In water at 55℃; for 1h; Sonication; | 94.2% |
| With triethylamine at 120℃; Sonication; | 93.1% |
| With triethylamine at 50℃; Microwave irradiation; | 90.9% |
-
-
927-63-9
3-dimethylaminoacrolein

-
-
2274-42-2
methanesulfonyl-acetonitrile

-
-
70682-09-6
2-chloro-3-(methylsulfonyl)pyridine

| Conditions | Yield |
|---|---|
| Stage #1: 3-dimethylaminoacrolein; methanesulfonyl-acetonitrile With N,N-dibutylpiperidinium chloride at 80℃; for 4h; Stage #2: With hydrogenchloride | 94.1% |

| Conditions | Yield |
|---|---|
| 94% |
-
-
105-56-6
ethyl 2-cyanoacetate

-
-
927-63-9
3-dimethylaminoacrolein

-
-
53087-78-8
Ethyl 2-bromo-3-pyridinecarboxylate

| Conditions | Yield |
|---|---|
| Stage #1: ethyl 2-cyanoacetate; 3-dimethylaminoacrolein With 1-dodecyl-3-methylimidazol-1-ium chloride at 90℃; for 4h; Stage #2: With hydrogen bromide | 94% |
| Stage #1: ethyl 2-cyanoacetate; 3-dimethylaminoacrolein With pyridine at 40℃; Sonication; Stage #2: With hydrogen bromide at 40℃; Sonication; | 93.5% |
-
-
105-34-0
methyl 2-cyanoacetate

-
-
927-63-9
3-dimethylaminoacrolein

-
-
40134-18-7
methyl 2-chloropyridine-3-carboxylate

| Conditions | Yield |
|---|---|
| Stage #1: methyl 2-cyanoacetate; 3-dimethylaminoacrolein With N-ethyl-pyridinium tetrafluoroborate at 140℃; for 1h; Microwave irradiation; Stage #2: With hydrogenchloride | 93.9% |
-
-
13654-62-1
2-(ethanesulfonyl)acetonitrile

-
-
927-63-9
3-dimethylaminoacrolein

| Conditions | Yield |
|---|---|
| Stage #1: 2-(ethanesulfonyl)acetonitrile; 3-dimethylaminoacrolein With 1-dodecyl-3-methylimidazol-1-ium chloride at 90℃; for 3h; Stage #2: With hydrogen bromide | 93.9% |

-
-
927-63-9
3-dimethylaminoacrolein

| Conditions | Yield |
|---|---|
| Stage #1: (p-hydroxy) benzylcyanomethyl sulfone; 3-dimethylaminoacrolein With 1-butyl-methylpyrrolidinium bis(trifluoromethylsulfonyl)amide at 110℃; for 1h; Stage #2: With hydrogen bromide | 93.7% |
-
-
927-63-9
3-dimethylaminoacrolein

-
-
126025-73-8
malonaldehyde-potassium salt

| Conditions | Yield |
|---|---|
| With methanol; potassium hydroxide In tetrahydrofuran for 5h; Inert atmosphere; Reflux; | 93% |

-
-
927-63-9
3-dimethylaminoacrolein

| Conditions | Yield |
|---|---|
| Stage #1: (m-fluorobenzyl) cyanomethyl sulfone; 3-dimethylaminoacrolein With 1-butyl-methylpyrrolidinium bis(trifluoromethylsulfonyl)amide at 100℃; for 1.5h; Microwave irradiation; Stage #2: With hydrogen fluoride | 93% |
-
-
105-56-6
ethyl 2-cyanoacetate

-
-
927-63-9
3-dimethylaminoacrolein

-
-
154366-01-5
2-iodonicotinic acid ethyl ester

| Conditions | Yield |
|---|---|
| Stage #1: ethyl 2-cyanoacetate; 3-dimethylaminoacrolein With 1-hydroxyethyl-3-methyl-1H-imidazolium chloride at 110℃; for 3h; Stage #2: With hydrogen iodide | 92.9% |
-
-
1550-50-1
1,1,2,2-tetrafluoro-N,N-dimethylethan-1-amine

-
-
60-34-4
methylhydrazine

-
-
927-63-9
3-dimethylaminoacrolein

-
-
1094484-55-5
3-(difluoromethyl)-1-methyl-1H-pyrazole-4-carbaldehyde

| Conditions | Yield |
|---|---|
| Stage #1: 1,1,2,2-tetrafluoro-N,N-dimethylethan-1-amine; 3-dimethylaminoacrolein With boron trifluoride In acetonitrile for 2.5h; Stage #2: methylhydrazine In acetonitrile at 5 - 20℃; for 2.5h; | 92.8% |


| Conditions | Yield |
|---|---|
| Stage #1: ethyl 2-cyanoacetate; 3-dimethylaminoacrolein With N-butyl-N-ethylpiperidinium chloride at 80℃; for 6h; Stage #2: With hydrogenchloride | 92.7% |
| Stage #1: ethyl 2-cyanoacetate; 3-dimethylaminoacrolein With acridine at 100℃; Sonication; Stage #2: With hydrogenchloride at 100℃; Sonication; | 92.1% |
-
-
120069-21-8
(1-methylethylsulfonyl)acetonitrile

-
-
927-63-9
3-dimethylaminoacrolein

-
-
139272-28-9
2-chloro-3-isopropylsulfonylpyridine

| Conditions | Yield |
|---|---|
| Stage #1: (1-methylethylsulfonyl)acetonitrile; 3-dimethylaminoacrolein With tetrabutylphosphine bromide at 140℃; for 0.333333h; Stage #2: With hydrogenchloride | 92.5% |

-
-
927-63-9
3-dimethylaminoacrolein

| Conditions | Yield |
|---|---|
| Stage #1: (3,4-dichloro) benzylcyanomethyl sulfone; 3-dimethylaminoacrolein With 1-butyl-methylpyrrolidinium bis(trifluoromethylsulfonyl)amide at 100℃; for 2h; Stage #2: With hydrogenchloride | 92.5% |

-
-
927-63-9
3-dimethylaminoacrolein

| Conditions | Yield |
|---|---|
| Stage #1: (m-methoxy) benzylcyanomethyl sulfone; 3-dimethylaminoacrolein With 1-butyl-methylpyrrolidinium bis(trifluoromethylsulfonyl)amide at 100℃; for 1.5h; Stage #2: With hydrogen fluoride | 92% |

-
-
927-63-9
3-dimethylaminoacrolein

| Conditions | Yield |
|---|---|
| Stage #1: p-bromobenzyl cyanomethyl sulfone; 3-dimethylaminoacrolein With 1-butyl-methylpyrrolidinium bis(trifluoromethylsulfonyl)amide at 100℃; for 1.5h; Microwave irradiation; Stage #2: With hydrogen bromide | 91.8% |
-
-
71888-58-9
cyanoacetic acid octadecyl ester

-
-
927-63-9
3-dimethylaminoacrolein

| Conditions | Yield |
|---|---|
| Stage #1: cyanoacetic acid octadecyl ester; 3-dimethylaminoacrolein With 1-hexyl-3-methylimidazol-1-ium chloride at 200℃; for 0.5h; Microwave irradiation; Stage #2: With hydrogenchloride | 91.1% |

-
-
927-63-9
3-dimethylaminoacrolein

| Conditions | Yield |
|---|---|
| With sodium hydroxide In methanol; dichloromethane; water addn. of dimethylaminoacrolein to soln. of Ni complex, stirring (room temp., 1.5 h), addn. of CH2Cl2, washing (water), concn., addn. of MeOH, addn. of aq. soln. of NaOH (20 %), stirring (room temp., 15 min); quenching (water), extn. into CHCl3, concn. of org. phase, flash chromy.(CH2Cl2/ether 9/1); | 91% |
-
-
13654-62-1
2-(ethanesulfonyl)acetonitrile

-
-
927-63-9
3-dimethylaminoacrolein

| Conditions | Yield |
|---|---|
| Stage #1: 2-(ethanesulfonyl)acetonitrile; 3-dimethylaminoacrolein With 1-butyl-3-methylimidazolium trifluoroacetate at 120℃; for 0.5h; Stage #2: With hydrogen fluoride | 90.7% |
-
-
14447-15-5
cyanoacetic acid n-propyl ester

-
-
927-63-9
3-dimethylaminoacrolein

-
-
1011481-58-5
propyl 2-chloronicotinate

| Conditions | Yield |
|---|---|
| Stage #1: cyanoacetic acid n-propyl ester; 3-dimethylaminoacrolein With sodium t-butanolate at 80℃; Sonication; Stage #2: With hydrogenchloride at 80℃; Sonication; | 90.5% |
-
-
105-56-6
ethyl 2-cyanoacetate

-
-
927-63-9
3-dimethylaminoacrolein

-
-
113898-56-9
3-(ethoxycarbonyl)-2-fluoropyridine

| Conditions | Yield |
|---|---|
| Stage #1: ethyl 2-cyanoacetate; 3-dimethylaminoacrolein With 1-butyl-3-methylimidazolium trifluoroacetate at 120℃; for 2h; Stage #2: With hydrogen fluoride | 90.4% |

| Conditions | Yield |
|---|---|
| With acetic acid In ethanol; water at 22 - 50℃; for 18.5h; Mannich reaction; | 90% |

3-Dimethylaminoacrolein Specification
The 3-Dimethylaminoacrolein, with the CAS registry number 927-63-9 and EINECS registry number 213-157-7, has the IUPAC name of (E)-3-(dimethylamino)prop-2-enal. It belongs to the following product categories: C1 to C6; Aldehydes; Carbonyl Compounds. And the molecular formula of the chemical is C5H9NO. What's more, it should be stored at 2-8°C.
The physical properties of 3-Dimethylaminoacrolein are as followings: (1)ACD/LogP: 0.41; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): -0.07; (4)ACD/LogD (pH 7.4): 0.4; (5)ACD/BCF (pH 5.5): 1; (6)ACD/BCF (pH 7.4): 1.18; (7)ACD/KOC (pH 5.5): 13.21; (8)ACD/KOC (pH 7.4): 38.94; (9)#H bond acceptors: 2; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 2; (12)Polar Surface Area: 20.31 Å2; (13)Index of Refraction: 1.446; (14)Molar Refractivity: 29.17 cm3; (15)Molar Volume: 109.2 cm3; (16)Polarizability: 11.56×10-24cm3; (17)Surface Tension: 28.6 dyne/cm; (18)Density: 0.907 g/cm3; (19)Flash Point: 128.9 °C; (20)nthalpy of Vaporization: 50.97 kJ/mol; (21)Boiling Point: 271.5 °C at 760 mmHg; (22)Vapour Pressure: 0.00643 mmHg at 25°C.
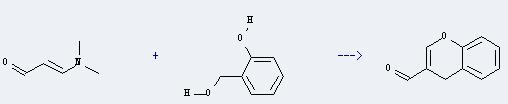
Uses of 3-Dimethylaminoacrolein: It can react with 2-hydroxymethyl-phenol to produce 4H-chromene-3-carbaldehyde. This reaction will need reagent acetic anhydride, and the menstruum acetic acid. The reaction time is 2 hours with heating, and the yield is about 40%.
You should be cautious while dealing with this chemical. It may cause burns. Therefore, you had better take the following instructions: Wear suitable protective clothing, gloves and eye/face protection; In case of contact with eyes, rinse immediately with plenty of water and seek medical advice; In case of accident or if you feel unwell, seek medical advice immediately (show label where possible).
You can still convert the following datas into molecular structure:
(1)SMILES: O=C\C=C\N(C)C
(2)InChI: InChI=1/C5H9NO/c1-6(2)4-3-5-7/h3-5H,1-2H3/b4-3+
(3)InChIKey: RRLMPLDPCKRASL-ONEGZZNKBT
Related Products
- 3-Dimethylaminoacrolein
- 92764-22-2
- 927-67-3
- 927-68-4
- 927684-97-7
- 927689-99-4
- 927691-21-2
- 92771-38-5
- 92-77-3
- 927-73-1
- 927-74-2
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View