-
Name
6-Bromo-1H-indole
- EINECS -0
- CAS No. 52415-29-9
- Article Data36
- CAS DataBase
- Density 1.66 g/cm3
- Solubility
- Melting Point 92-96 °C(lit.)
- Formula C8H6BrN
- Boiling Point 316.9 °C at 760 mmHg
- Molecular Weight 196.046
- Flash Point 145.5 °C
- Transport Information
- Appearance white to brown powder
- Safety 26-36-37/39
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms 1H-Indole, 6-bromo-;6-bromo-1H-indole;Bromoindole;
- PSA 15.79000
- LogP 2.93040
Synthetic route
-
-
16732-65-3
6-bromo-indole-2-carboxylic acid(Ref.7.)

-
-
52415-29-9
6-bromo-1H-indole

| Conditions | Yield |
|---|---|
| With quinoline; copper Heating; | 98% |
| With copper In quinoline Heating; | 95% |
| With quinoline; copper(I) bromide |
-
-
99474-21-2
(E)-2-(4-bromo-2-nitrophenyl)-N,N-dimethylethyleneamine

-
-
52415-29-9
6-bromo-1H-indole

| Conditions | Yield |
|---|---|
| With iron(III) chloride hexahydrate; pyrographite; hydrazine hydrate In ethanol at 75℃; for 7h; | 93% |
| With zinc In acetic acid at 85℃; for 2h; | 62% |
| With acetic acid; zinc In methanol; dichloromethane Heating; | 52% |
| With acetic acid; zinc at 75 - 85℃; for 3.5h; | 2.675 g |
-
-
60956-26-5
4-bromo-2-nitrotoluene

-
-
4637-24-5
N,N-dimethyl-formamide dimethyl acetal

-
-
52415-29-9
6-bromo-1H-indole

| Conditions | Yield |
|---|---|
| Stage #1: 4-bromo-2-nitrotoluene; N,N-dimethyl-formamide dimethyl acetal With pyrrolidine In N,N-dimethyl-formamide at 110℃; for 2h; Stage #2: With hydrogenchloride; titanium(III) chloride; ammonium acetate In N,N-dimethyl-formamide at 0℃; for 0.25h; | 90% |
| Stage #1: 4-bromo-2-nitrotoluene; N,N-dimethyl-formamide dimethyl acetal With pyrrolidine In 1,4-dioxane at 102℃; for 3.5h; Leimgruber-Batcho Indole Synthesis; Reflux; Inert atmosphere; Stage #2: With hydrazine hydrate In 1,4-dioxane at 45℃; for 1h; Leimgruber-Batcho Indole Synthesis; | 82% |
| Stage #1: 4-bromo-2-nitrotoluene; N,N-dimethyl-formamide dimethyl acetal With pyrrolidine In N,N-dimethyl-formamide at 105 - 110℃; for 21h; Leimgruber-Batcho Indole Synthesis; Stage #2: With acetic acid; zinc at 70 - 90℃; for 3h; Temperature; Reagent/catalyst; Leimgruber-Batcho Indole Synthesis; | 56% |
-
-
99365-40-9
6-bromo-2,3-dihydro-1H-indole-2-one

-
-
52415-29-9
6-bromo-1H-indole

| Conditions | Yield |
|---|---|
| With bis(trimethylsilyl)amide yttrium(III); 4,4,5,5-tetramethyl-[1,3,2]-dioxaboralane In toluene at 120℃; for 36h; Inert atmosphere; | 90% |
-
-
189265-99-4
6-bromo-1-(4-methylphenylsulfonyl)-1H-indole

-
-
52415-29-9
6-bromo-1H-indole

| Conditions | Yield |
|---|---|
| With sodium hydride In N,N-dimethyl acetamide at 60℃; for 5h; Inert atmosphere; | 85% |
-
-
496-15-1
1-indoline

-
-
52415-29-9
6-bromo-1H-indole

| Conditions | Yield |
|---|---|
| Stage #1: 1-indoline With silver(II) sulfate; sulfuric acid for 0.5h; Stage #2: With bromine for 0.5h; Stage #3: With chloranil In 5,5-dimethyl-1,3-cyclohexadiene for 5h; Reflux; | 73% |
-
-
52448-17-6
(2S)-2-amino-3-(6-bromo-1H-indol-3-yl)propanoic acid

-
-
52415-29-9
6-bromo-1H-indole

| Conditions | Yield |
|---|---|
| With dipotassium hydrogenphosphate; tryptophanase TnaA at 25℃; pH=7.4; Enzymatic reaction; | 70.4% |

-
-
52415-29-9
6-bromo-1H-indole

| Conditions | Yield |
|---|---|
| With ammonium hydroxide; iron(II) sulfate Heating; | 31% |
-
-
78508-22-2
β-dimethylamino-2-nitrostyrene

-
-
52415-29-9
6-bromo-1H-indole

| Conditions | Yield |
|---|---|
| With acetic acid; zinc In water at 75℃; for 1h; Stirred; | 27% |
| With hydrogen; W-2 Raney nickel In ethanol | 340 mg |
| With acetic acid; zinc | |
| With hydrogen In benzene under 2585.81 Torr; for 26h; | 0.29 g |
-
-
123-75-1
pyrrolidine

-
-
60956-26-5
4-bromo-2-nitrotoluene

-
-
4637-24-5
N,N-dimethyl-formamide dimethyl acetal

-
-
52415-29-9
6-bromo-1H-indole

| Conditions | Yield |
|---|---|
| With ammonium acetate; titanium(III) chloride 1) DMF, 100-110 deg C, 2 h, 2) water, DMF; Yield given. Multistep reaction; | |
| With zinc In hexane; acetic acid; N,N-dimethyl-formamide | |
| With zinc In hexane; acetic acid; N,N-dimethyl-formamide | |
| With zinc In hexane; acetic acid; N,N-dimethyl-formamide |

-
-
63839-24-7
6-bromo-indoline

-
-
52415-29-9
6-bromo-1H-indole

| Conditions | Yield |
|---|---|
| With dimethylsulfide; tert-butylhypochlorite; sodium ethanolate; triethylamine 1.) -65 deg C, CH2Cl2, 1 h, 2.) -65 deg C --> RT, 2 h; Yield given. Multistep reaction; |
-
-
112671-54-2
(5-Bromo-2-trimethylsilanylethynyl-phenyl)-carbamic acid ethyl ester

-
-
52415-29-9
6-bromo-1H-indole

| Conditions | Yield |
|---|---|
| With sodium ethanolate In ethanol Heating; Yield given; |
-
-
108061-73-0
(E)-4-bromo-2-nitro-β-piperidinostyrene

-
-
52415-29-9
6-bromo-1H-indole

| Conditions | Yield |
|---|---|
| With titanium(III) chloride; ammonium acetate buffer In water; acetone for 0.166667h; Yield given; |
-
-
5318-27-4
6-amino-1H-indole

-
-
52415-29-9
6-bromo-1H-indole

| Conditions | Yield |
|---|---|
| With hydrogenchloride; copper(I) bromide; sodium nitrite 1.) 0 deg C, 5 min, 2.) H2O, -10 deg C, 3 h; Yield given. Multistep reaction; |
-
-
60956-26-5
4-bromo-2-nitrotoluene

-
-
52415-29-9
6-bromo-1H-indole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: CuI; DMF / 0.33 h / 180 °C / 6000.6 - 7500.75 Torr / microwave irradiation 2: 52 percent / Zn; AcOH / methanol; CH2Cl2 / Heating View Scheme | |
| Multi-step reaction with 2 steps 1: pyrrolidine / dimethylformamide / 1 h / 110 °C 2: 2.675 g / Zn; aq. HOAc / 3.5 h / 75 - 85 °C View Scheme | |
| Multi-step reaction with 2 steps 1: 4 h / 110 °C 2: 20percent TiCl3, 4M ammonium acetate buffer / H2O; acetone / 0.17 h View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 99 percent / 2 h / Heating 2: 64 percent / aluminum amalgam / H2O / 5 h / Irradiation 3: 1.) 1M aq. HCl, sodium nitrite, 2.) CuBr / 1.) 0 deg C, 5 min, 2.) H2O, -10 deg C, 3 h View Scheme |
-
-
61293-29-6
(E)-2-(2,4-dinitrophenyl)-N,N-dimethyl-1-ethenamine

-
-
52415-29-9
6-bromo-1H-indole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 64 percent / aluminum amalgam / H2O / 5 h / Irradiation 2: 1.) 1M aq. HCl, sodium nitrite, 2.) CuBr / 1.) 0 deg C, 5 min, 2.) H2O, -10 deg C, 3 h View Scheme |
-
-
119-32-4
4-Methyl-3-nitroanilin

-
-
52415-29-9
6-bromo-1H-indole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 1.) sodium nitrite, 16percent HBr, 2.) copper(I) bromide / 1.) water, -1 deg C, 2.) water, 70 deg C, 1 h 2: 4 h / 110 °C 3: 20percent TiCl3, 4M ammonium acetate buffer / H2O; acetone / 0.17 h View Scheme | |
| Multi-step reaction with 2 steps 1.1: hydrogen bromide / water / 0.33 h / Reflux 1.2: 0.25 h / 0 °C 1.3: 0 °C / Heating 2.1: pyrrolidine / N,N-dimethyl-formamide / 21 h / 105 - 110 °C 2.2: 3 h / 70 - 90 °C View Scheme | |
| Multi-step reaction with 3 steps 1.1: hydrogen bromide / water / 0.33 h / Reflux 1.2: 0.25 h / 0 °C 1.3: 0 °C / Heating 2.1: 5.5 h / 110 °C 3.1: hydrogen / benzene / 26 h / 2585.81 Torr View Scheme | |
| Multi-step reaction with 3 steps 1.1: hydrogen bromide / water / 0.33 h / Reflux 1.2: 0.25 h / 0 °C 1.3: 0 °C / Heating 2.1: N,N-dimethyl-formamide / 21 h / 105 - 110 °C 3.1: acetic acid; zinc / 3 h / 70 - 90 °C View Scheme |
-
-
881-50-5
N-(4-bromo-2-nitrophenyl)acetamide

-
-
52415-29-9
6-bromo-1H-indole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: 3N KOH / 24 h / Heating 2: 1.) sodium nitrite, 5N HCl; 2.) aq. KI / 1.) 0-10 deg C; 2.) RT, 30 min 3: 69 percent / SnCl2, conc. HCl / ethanol / 0.25 h / 50 - 60 °C 4: 72 percent / pyridine / 3 h / 0-10 deg C 5: CuI, Et3N / Pd(PPh3)2Cl2 / 60 °C 6: NaOEt / ethanol / Heating View Scheme |
-
-
103-88-8
4-bromoacetanilide

-
-
52415-29-9
6-bromo-1H-indole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: HNO3 / 8 min below 0 deg C, 8 min, RT. 2: 3N KOH / 24 h / Heating 3: 1.) sodium nitrite, 5N HCl; 2.) aq. KI / 1.) 0-10 deg C; 2.) RT, 30 min 4: 69 percent / SnCl2, conc. HCl / ethanol / 0.25 h / 50 - 60 °C 5: 72 percent / pyridine / 3 h / 0-10 deg C 6: CuI, Et3N / Pd(PPh3)2Cl2 / 60 °C 7: NaOEt / ethanol / Heating View Scheme |
-
-
875-51-4
4-Bromo-2-nitroaniline

-
-
52415-29-9
6-bromo-1H-indole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 1.) sodium nitrite, 5N HCl; 2.) aq. KI / 1.) 0-10 deg C; 2.) RT, 30 min 2: 69 percent / SnCl2, conc. HCl / ethanol / 0.25 h / 50 - 60 °C 3: 72 percent / pyridine / 3 h / 0-10 deg C 4: CuI, Et3N / Pd(PPh3)2Cl2 / 60 °C 5: NaOEt / ethanol / Heating View Scheme |
-
-
112671-42-8
1-iodo-4-bromo-2-nitrobenzene

-
-
52415-29-9
6-bromo-1H-indole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 69 percent / SnCl2, conc. HCl / ethanol / 0.25 h / 50 - 60 °C 2: 72 percent / pyridine / 3 h / 0-10 deg C 3: CuI, Et3N / Pd(PPh3)2Cl2 / 60 °C 4: NaOEt / ethanol / Heating View Scheme |
-
-
64085-52-5
5-bromo-2-iodo-aniline

-
-
52415-29-9
6-bromo-1H-indole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 72 percent / pyridine / 3 h / 0-10 deg C 2: CuI, Et3N / Pd(PPh3)2Cl2 / 60 °C 3: NaOEt / ethanol / Heating View Scheme |
-
-
112671-48-4
ethyl (5-bromo-2-iodophenyl)carbamate

-
-
52415-29-9
6-bromo-1H-indole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: CuI, Et3N / Pd(PPh3)2Cl2 / 60 °C 2: NaOEt / ethanol / Heating View Scheme |
-
-
99365-39-6
3-(4-bromo-2-nitrophenyl)-2-oxopropanoic acid

-
-
52415-29-9
6-bromo-1H-indole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: Fe(OH)2; H2O 2: quinoline; copper (I)-bromide View Scheme |

-
-
52415-29-9
6-bromo-1H-indole

| Conditions | Yield |
|---|---|
| With acetic acid; zinc at 75 - 85℃; for 3h; | 1.7 g |
| With acetic acid; zinc at 70 - 90℃; for 3h; Reagent/catalyst; | 7.19 g |
-
-
25408-61-1
Ν,Ν-dimethylacetamide dimethyl acetal

-
-
60956-26-5
4-bromo-2-nitrotoluene

-
-
52415-29-9
6-bromo-1H-indole

| Conditions | Yield |
|---|---|
| Stage #1: Ν,Ν-dimethylacetamide dimethyl acetal; 4-bromo-2-nitrotoluene With pyrrolidine In N,N-dimethyl-formamide at 110℃; for 3h; Stage #2: With acetic acid; zinc In water at 75 - 85℃; for 2h; |
-
-
73-22-3
L-Tryptophan

-
-
52415-29-9
6-bromo-1H-indole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: sodium bromide; disodium hydrogenphosphate; tryptophan halogenase / 72 h / 25 °C / pH 7.4 / Enzymatic reaction 2: tryptophanase TnaA; dipotassium hydrogenphosphate / 25 °C / pH 7.4 / Enzymatic reaction View Scheme |
-
-
120-72-9
indole

-
-
52415-29-9
6-bromo-1H-indole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: acetic acid; sodium cyanoborohydride / 2 h / 15 °C 2.1: sulfuric acid; silver(II) sulfate / 0.5 h 2.2: 0.5 h 2.3: 5 h / Reflux View Scheme |
-
-
52415-29-9
6-bromo-1H-indole

-
-
68-12-2, 33513-42-7
N,N-dimethyl-formamide

-
-
17826-04-9
6-bromo-1H-indole-3-carbaldehyde

| Conditions | Yield |
|---|---|
| With trichlorophosphate at 0 - 40℃; for 2.5h; Inert atmosphere; | 100% |
| With trichlorophosphate at 0 - 20℃; Vilsmeier-Haack Formylation; Inert atmosphere; | 99% |
| With sodium hydroxide; trichlorophosphate Vilsmeier-Haack reaction; | 98% |
-
-
52415-29-9
6-bromo-1H-indole

-
-
18162-48-6
tert-butyldimethylsilyl chloride

-
-
184637-11-4
6-bromo-1-(tert-butyldimethylsilyl)-1H-indole

| Conditions | Yield |
|---|---|
| Stage #1: 6-bromo-1H-indole With sodium hydride In N,N-dimethyl-formamide; mineral oil at 0 - 20℃; for 0.5h; Stage #2: tert-butyldimethylsilyl chloride In N,N-dimethyl-formamide; mineral oil at 0 - 20℃; for 1h; | 100% |
| Stage #1: 6-bromo-1H-indole With sodium hydride In tetrahydrofuran; mineral oil at 0 - 20℃; for 0.75h; Inert atmosphere; Stage #2: tert-butyldimethylsilyl chloride In tetrahydrofuran; mineral oil at 0 - 20℃; for 12h; Inert atmosphere; | 90% |
| Stage #1: 6-bromo-1H-indole With sodium hydride In N,N-dimethyl-formamide at 0 - 10℃; for 0.5h; Inert atmosphere; Stage #2: tert-butyldimethylsilyl chloride In N,N-dimethyl-formamide at 20℃; Inert atmosphere; | 73% |
| With n-butyllithium 1.) THF, hexane, -78 deg C, 15 min, 2.) THF, hexane, RT, 4 h; Yield given. Multistep reaction; |
-
-
52415-29-9
6-bromo-1H-indole

-
-
372077-73-1
3-iodo-6-bromoindole

| Conditions | Yield |
|---|---|
| With potassium hydroxide; iodine In N,N-dimethyl-formamide at 20℃; | 100% |
| With potassium hydroxide; iodine In N,N-dimethyl-formamide Ambient temperature; | |
| Stage #1: 6-bromo-1H-indole With sodium hydroxide In methanol at 20℃; for 0.166667h; Stage #2: With iodine; potassium iodide In methanol at 20℃; for 3h; | 134 mg |

| Conditions | Yield |
|---|---|
| With potassium hydroxide In acetone at 0 - 25℃; | 100% |
| Stage #1: 6-bromo-1H-indole With sodium hydride In 1,2-dimethoxyethane; dimethyl sulfoxide at 20℃; for 0.5h; Stage #2: methyl iodide In 1,2-dimethoxyethane; dimethyl sulfoxide at 20℃; | 95% |
| Stage #1: 6-bromo-1H-indole With sodium hydride In N,N-dimethyl-formamide at 0℃; for 0.5h; Inert atmosphere; Stage #2: methyl iodide In N,N-dimethyl-formamide at 20℃; for 2h; Inert atmosphere; | 93.1% |
-
-
52415-29-9
6-bromo-1H-indole

-
-
73430-27-0
(E)-N,N-dimethyl-2-nitroethenamine

-
-
222173-67-3
3-<(E)-2-nitroethenyl>-6-bromoindole

| Conditions | Yield |
|---|---|
| Stage #1: (E)-N,N-dimethyl-2-nitroethenamine In trifluoroacetic acid at 20℃; for 0.0833333h; Michael Addition; Inert atmosphere; Stage #2: 6-bromo-1H-indole In dichloromethane at 20℃; for 0.75h; Inert atmosphere; | 100% |
| With trifluoroacetic acid at 20℃; for 0.5h; | 96% |
-
-
52415-29-9
6-bromo-1H-indole

-
-
375853-82-0, 286961-14-6
tert-Butyl 4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-3,6-dihydro-1(2H)-pyridinecarboxylate

| Conditions | Yield |
|---|---|
| With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium carbonate In 1,4-dioxane; water for 2h; Inert atmosphere; | 100% |
-
-
52415-29-9
6-bromo-1H-indole

-
-
98-59-9
p-toluenesulfonyl chloride

-
-
189265-99-4
6-bromo-1-(4-methylphenylsulfonyl)-1H-indole

| Conditions | Yield |
|---|---|
| Stage #1: 6-bromo-1H-indole With sodium hydride In acetonitrile at 0℃; for 0.166667h; Stage #2: p-toluenesulfonyl chloride In acetonitrile at 0 - 20℃; for 4h; | 99% |
| With sodium hydride In acetonitrile at 0 - 20℃; for 4h; | 99% |
| With potassium hydroxide; tetra(n-butyl)ammonium hydrogensulfate In toluene for 4h; | 98% |
-
-
52415-29-9
6-bromo-1H-indole

-
-
13154-24-0
triisopropylsilyl chloride

-
-
299432-99-8
6-bromo-1-(triisopropylsilyl)-1H-indole

| Conditions | Yield |
|---|---|
| Stage #1: 6-bromo-1H-indole With sodium hydride In tetrahydrofuran at 20℃; for 0.166667h; Stage #2: triisopropylsilyl chloride In tetrahydrofuran at 20℃; for 0.5h; | 99% |
| Stage #1: 6-bromo-1H-indole With sodium hydride In tetrahydrofuran at 20℃; for 0.166667h; Stage #2: triisopropylsilyl chloride In tetrahydrofuran at 20℃; for 0.5h; | 99% |
| Stage #1: 6-bromo-1H-indole With sodium hydride In tetrahydrofuran; mineral oil at 0 - 20℃; for 0.5h; Stage #2: triisopropylsilyl chloride In tetrahydrofuran; mineral oil at 0 - 20℃; | 96% |
-
-
52415-29-9
6-bromo-1H-indole

-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
147621-26-9
6-bromoindole-1-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With dmap In tetrahydrofuran at 20℃; for 4h; | 99% |
| With dmap In tetrahydrofuran at 20℃; Inert atmosphere; | 99% |
| With dmap In dichloromethane | 98% |

| Conditions | Yield |
|---|---|
| With Ti0.97Pd0.03O1.97; potassium carbonate In water at 100℃; for 6h; Suzuki-Miyaura Coupling; | 99% |
| With C20H20N2O2Pd; sodium hydrogencarbonate In water at 80℃; for 6h; Suzuki-Miyaura Coupling; | 94% |
| With potassium carbonate In water for 0.5h; Suzuki-Miyaura Coupling; Heating; | 90% |
-
-
52415-29-9
6-bromo-1H-indole

-
-
6482-24-2
2-Bromoethyl methyl ether

| Conditions | Yield |
|---|---|
| Stage #1: 6-bromo-1H-indole With sodium hydride In 1,2-dimethoxyethane; dimethyl sulfoxide at 20℃; for 0.5h; Stage #2: 2-Bromoethyl methyl ether In 1,2-dimethoxyethane; dimethyl sulfoxide at 20℃; | 99% |
-
-
52415-29-9
6-bromo-1H-indole

-
-
106-95-6
allyl bromide

-
-
945399-52-0
6-bromo-1-(prop-2-en-1-yl)-1H-indole

| Conditions | Yield |
|---|---|
| Stage #1: 6-bromo-1H-indole With sodium hydride In N,N-dimethyl-formamide at 0℃; for 0.5h; Stage #2: allyl bromide In N,N-dimethyl-formamide at 20℃; for 0.5h; | 99% |
| Stage #1: 6-bromo-1H-indole With sodium hydride In N,N-dimethyl-formamide at 20℃; Stage #2: allyl bromide In N,N-dimethyl-formamide for 2h; | 98% |
| Stage #1: 6-bromo-1H-indole With sodium hydride In N,N-dimethyl-formamide; mineral oil at 20℃; for 1h; Stage #2: allyl bromide In N,N-dimethyl-formamide; mineral oil at 20℃; for 0.75h; | 3.2 g |

| Conditions | Yield |
|---|---|
| With iodine; potassium iodide In ethanol; water at 20 - 60℃; | 99% |
| With iodine; potassium iodide In ethanol; water at 60℃; for 72h; Schlenk technique; Inert atmosphere; | 77% |
-
-
52415-29-9
6-bromo-1H-indole

-
-
63839-24-7
6-bromo-indoline

| Conditions | Yield |
|---|---|
| With sodium cyanoborohydride; acetic acid at 20℃; | 99% |
| With sodium cyanoborohydride; acetic acid for 1h; | 79% |
| With sodium cyanoborohydride; acetic acid at 10℃; for 1h; | 70% |
-
-
52415-29-9
6-bromo-1H-indole

-
-
115666-47-2
6-iodo-1 H-indole

| Conditions | Yield |
|---|---|
| With copper(l) iodide; sodium iodide; N,N`-dimethylethylenediamine In 1,4-dioxane at 110℃; for 22h; Inert atmosphere; Sealed tube; | 99% |
| With copper(l) iodide; sodium iodide; N,N`-dimethylethylenediamine In 1,4-dioxane at 110℃; for 22h; Inert atmosphere; | 99% |
| With copper(l) iodide; sodium iodide; N,N`-dimethylethylenediamine In 1,4-dioxane at 110℃; for 22h; Microwave irradiation; Sealed tube; Inert atmosphere; | 97% |
-
-
52415-29-9
6-bromo-1H-indole

-
-
1533443-74-1
ethyl 5-methyl-2-oxo-3,4-dihydro-2H-benzo[b][1,4]oxazine-3-carboxylate

-
-
1533444-23-3
ethyl (R)-5-methyl-3-(5-methyl-1H-indol-3-yl)-2-oxo-3,4-dihydro-2H-benzo[b][1,4]oxazine-3-carboxylate

| Conditions | Yield |
|---|---|
| With C38H33O4P In toluene at -40℃; for 4h; enantioselective reaction; | 99% |
-
-
52415-29-9
6-bromo-1H-indole

| Conditions | Yield |
|---|---|
| With C53H40N2O2; copper(II) bis(trifluoromethanesulfonate) In ethylbenzene at 20℃; for 24h; Friedel-Crafts Alkylation; Inert atmosphere; enantioselective reaction; | 99% |
-
-
52415-29-9
6-bromo-1H-indole

-
-
530-93-8
1,2,3,4-tetrahydronaphthalen-2-one

| Conditions | Yield |
|---|---|
| With L-Tartaric acid; 1,1-Dimethylurea at 70℃; for 3h; | 99% |

| Conditions | Yield |
|---|---|
| With (2-dicyclohexylphosphino-2’,6’-diisopropoxy-1,1‘-biphenyl)[2-(2’-methylamino-1,1’-biphenyl)]palladium(II) methanesulfonate; caesium carbonate In tetrahydrofuran; water at 80℃; Suzuki Coupling; | 99% |
-
-
52415-29-9
6-bromo-1H-indole

-
-
1189-71-5
isocyanate de chlorosulfonyle

-
-
224434-83-7
6-bromo-1H-indole-3-carbonitrile

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at -50 - 20℃; Inert atmosphere; | 99% |
-
-
52415-29-9
6-bromo-1H-indole

-
-
198012-02-1
4-methyl-N-(4-trifluoromethylbenzylidene)-benzenesulfonamide

| Conditions | Yield |
|---|---|
| With (Rp)-4,12-di(4-(3',5'-bis(trifluoromethyl))-phenyl-3-yl)-[2.2]paracyclophane-hydrogenphosphate In toluene at -20℃; for 12h; Molecular sieve; enantioselective reaction; | 99% |
-
-
52415-29-9
6-bromo-1H-indole

-
-
224434-83-7
6-bromo-1H-indole-3-carbonitrile

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at -50 - 20℃; for 1.5h; Inert atmosphere; | 99% |
-
-
52415-29-9
6-bromo-1H-indole

| Conditions | Yield |
|---|---|
| Stage #1: 3-(1-phenylvinyl)-1H-indole With C76H57F12NO6P2 In 1,2-dichloro-ethane at 0℃; for 0.166667h; Molecular sieve; Stage #2: 6-bromo-1H-indole In 1,2-dichloro-ethane at 0℃; for 24h; Molecular sieve; enantioselective reaction; | 99% |
-
-
52415-29-9
6-bromo-1H-indole

-
-
1033591-96-6
1-(1,3-benzothiazol-2-yl)-2,2,2-trifluoroethane-1,1-diol

| Conditions | Yield |
|---|---|
| With (R)-6,6'-bis(2,4,6-triisopropylphenyl)-1,1'-spirobiindanyl-7,7'-diyl hydrogen phosphate In dichloromethane at 20℃; Friedel-Crafts Alkylation; Molecular sieve; enantioselective reaction; | 99% |

| Conditions | Yield |
|---|---|
| With Ti0.97Pd0.03O1.97; potassium carbonate In water at 100℃; for 6h; Suzuki-Miyaura Coupling; | 98% |
-
-
52415-29-9
6-bromo-1H-indole

-
-
13081-18-0
ethyl-3,3,3-trifluoropyruvate

| Conditions | Yield |
|---|---|
| With (3aR,11aR,14aS,16bS)-2,2,13,13-tetra-isopropyl-3a,11a,14a,16b-tetrahydro-bis(1,3-dioxolano[4',5':3,4]pyrrolo)[1,2-a:1',2'-a]naphtho[1,2-d:8,7-d']diimidazole; copper(II) bis(trifluoromethanesulfonate) at 25℃; for 1h; Friedel-Crafts Alkylation; Inert atmosphere; Schlenk technique; stereoselective reaction; | 97.3% |
-
-
52415-29-9
6-bromo-1H-indole

-
-
98-09-9
benzenesulfonyl chloride

-
-
679794-03-7
1‐(benzenesulfonyl)‐6‐bromo‐1H‐indole

| Conditions | Yield |
|---|---|
| With sodium hydroxide; tetra(n-butyl)ammonium hydrogensulfate In dichloromethane at 0 - 20℃; for 3h; | 97% |
| With tetra(n-butyl)ammonium hydrogensulfate; sodium hydroxide In dichloromethane at 20℃; for 3.16667h; Cooling with ice; | 95% |
| With sodium hydride In N,N-dimethyl-formamide at 0 - 20℃; | 92% |
-
-
52415-29-9
6-bromo-1H-indole

-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
182344-70-3
5-bromo-indole-1-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With dmap In dichloromethane at 20℃; | 97% |
| With dmap In dichloromethane at 20℃; for 1h; Addition; | 90% |
-
-
52415-29-9
6-bromo-1H-indole

-
-
56-45-1
L-serin

-
-
52448-17-6
(2S)-2-amino-3-(6-bromo-1H-indol-3-yl)propanoic acid

| Conditions | Yield |
|---|---|
| With Pf0A9 enzyme In aq. phosphate buffer; water; dimethyl sulfoxide at 75℃; for 12h; pH=8; Enzymatic reaction; | 97% |
| With ammonium sulfate; pyridoxal 5'-phosphate; sodium sulfite In phosphate buffer; dimethyl sulfoxide at 37℃; for 6h; pH=7.8; | |
| Stage #1: 6-bromo-1H-indole; L-serin With acetic anhydride; acetic acid at 73℃; for 3h; Inert atmosphere; Stage #2: With cobalt(II) chloride; sodium hydroxide In aq. buffer at 37℃; for 48h; pH=8; Enzymatic reaction; |
6-Bromoindole Chemical Properties
Molecule structure of 6-Bromoindole (CAS NO.52415-29-9):
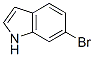
IUPAC Name: 6-Bromo-1H-indole
Molecular Weight: 196.04394 g/mol
Molecular Formula: C8H6BrN
Density: 1.66 g/cm3
Melting Point: 92-96 °C(lit.)
Boiling Point: 316.9 °C at 760 mmHg
Flash Point: 145.5 °C
Index of Refraction: 1.711
Molar Refractivity: 46.21 cm3
Molar Volume: 118 cm3
Polarizability: 18.32×10-24 cm3
Surface Tension: 54.8 dyne/cm
Enthalpy of Vaporization: 53.61 kJ/mol
Vapour Pressure: 0.000738 mmHg at 25 °C
XLogP3: 3.3
H-Bond Donor :1
Exact Mass: 194.968362
MonoIsotopic Mass: 194.968362
Topological Polar Surface Area: 15.8
Heavy Atom Count: 10
Canonical SMILES: C1=CC(=CC2=C1C=CN2)Br
InChI: InChI=1S/C8H6BrN/c9-7-2-1-6-3-4-10-8(6)5-7/h1-5,10H
InChIKey: MAWGHOPSCKCTPA-UHFFFAOYSA-N
Product Categories: blocks; Bromides; IndolesOxindoles; Indole/indoline/oxindole; Indoles and derivatives; pharmacetical; Indole; Indoles; Heterocyclic Compounds; Halogenated; Organohalides; Simple Indoles; Halogenated Heterocycles; Heterocyclic Building Blocks; IndolesBuilding Blocks
6-Bromoindole Safety Profile
Hazard Codes:  Xi
Xi
Risk Statements: 36/37/38
R36/37/38:Irritating to eyes, respiratory system and skin.
Safety Statements: 26-36-37/39
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36:Wear suitable protective clothing.
S37/39:Wear suitable gloves and eye/face protection.
WGK Germany: 3
Hazard Note: Irritant
HazardClass: IRRITANT
6-Bromoindole Specification
6-Bromoindole (CAS NO.52415-29-9) is also named as 1H-indole, 6-bromo- ; 6-Brom-1H-indol ; 6-Bromo-1H-indole . 6-Bromoindole (CAS NO.52415-29-9) is white to brown powder.
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View