-
Name
Ammonium carbamate
- EINECS 214-185-2
- CAS No. 1111-78-0
- Article Data47
- CAS DataBase
- Density 1.6 g/cm3
- Solubility Soluble in water, ethanol
- Melting Point 59-61°C (subl.)
- Formula CH3NO2·H3N
- Boiling Point 251 °C at 760 mmHg
- Molecular Weight 78.0708
- Flash Point 105.6 °C
- Transport Information
- Appearance white crystalline solid
- Safety 22-24/25
- Risk Codes 22
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms Ammoniumcarbamate (6CI,7CI);Carbamic acid, monoammonium salt (8CI,9CI);Carbamic acid, ammoniumsalt (1:1);
- PSA 66.56000
- LogP 0.64730
Synthetic route

| Conditions | Yield |
|---|---|
| With ammonia at 0℃; for 8h; | 90% |
| With ammonia | |
| With ammonia; water; urea |
-
-
14066-73-0
benzophenone semicarbazone

-
A
-
110-21-4
hydrazodicarboxamide

-
B
-
16240-68-9
1,5-dibenzhydrylidene-carbonohydrazide

-
C
-
1111-78-0
ammonium carbamate

-
D
-
983-79-9
benzophenone azine

| Conditions | Yield |
|---|---|
| bei Erhitzen ueber den Schmelzpunkt; |


-
-
1111-78-0
ammonium carbamate

| Conditions | Yield |
|---|---|
| With alkaline potassium permanganate |
-
-
15719-64-9, 15719-76-3, 97762-63-5
methylammonium carbonate

-
A
-
4366-93-2
potassium carbamate

-
B
-
1111-78-0
ammonium carbamate

| Conditions | Yield |
|---|---|
| With potassium ammonium Nebenproduktd wird entfernt durch Erwaermen im Vakuum auf 50grad; |
-
-
15719-64-9, 15719-76-3, 97762-63-5
methylammonium carbonate

-
-
1111-78-0
ammonium carbamate

| Conditions | Yield |
|---|---|
| With ammonia | |
| With ammonia Waermetoenung; | |
| With ethanol; ammonia at 100 - 110℃; |

| Conditions | Yield |
|---|---|
| With alkaline potassium permanganate |

| Conditions | Yield |
|---|---|
| With alkaline potassium permanganate |

| Conditions | Yield |
|---|---|
| With phosphate-EDTA-buffer; urea amidohydrolase EC 3.5.1.5 at 25℃; rates of urea amidohydrolase catalyzed hydrolysis; pH 7.0; | |
| With water |
-
-
124-38-9
carbon dioxide

-
A
-
50-00-0
formaldehyd

-
B
-
64-18-6
formic acid

-
C
-
463-77-4
carbamic Acid

-
D
-
1111-78-0
ammonium carbamate

| Conditions | Yield |
|---|---|
| With ammonia; water at -258.15℃; bombardment with 1 MeV protons; Further byproducts given; |


-
-
1111-78-0
ammonium carbamate

| Conditions | Yield |
|---|---|
| With ammonia; water auch bei Sauerstoff-Ausschluss; |

| Conditions | Yield |
|---|---|
| With ammonia at 182℃; under 116262 Torr; for 0.333333h; Conversion of starting material; Industry scale; | |
| With ammonia In water at 182℃; under 116262 Torr; for 0.333333h; Industry scale; Compressed gas(es); | |
| With ammonia at 182℃; under 116262 Torr; for 0.333333h; Conversion of starting material; Industry scale; | |
| With ammonia | |
| With ammonia Product distribution / selectivity; |

| Conditions | Yield |
|---|---|
| With ammonia Product distribution / selectivity; Industry scale; |


| Conditions | Yield |
|---|---|
| With ammonium hydroxide In methanol at 37℃; for 3h; | 94% |

-
-
1111-78-0
ammonium carbamate

| Conditions | Yield |
|---|---|
| With ammonia In methanol; water at 20℃; for 7h; | 94% |

| Conditions | Yield |
|---|---|
| With ammonium hydroxide In methanol at 37℃; for 5h; | 93% |

| Conditions | Yield |
|---|---|
| With ammonium hydroxide In methanol at 37℃; for 15h; | 93% |

| Conditions | Yield |
|---|---|
| In methanol at 20℃; for 2h; | 93% |
-
-
1111-78-0
ammonium carbamate

-
-
16712-16-6, 64165-14-6, 65387-22-6, 70179-79-2, 540-69-2
ammonium formate

| Conditions | Yield |
|---|---|
| With 5% Pd/C; hydrogen In ethanol; water at 20℃; under 20627.1 Torr; for 8h; Kinetics; Catalytic behavior; Solvent; Pressure; Reagent/catalyst; Temperature; Time; | 91.7% |
| With 5%-palladium/activated carbon; hydrogen In ethanol; water at 20℃; under 20627.1 Torr; for 8h; Solvent; Temperature; Pressure; Time; Reagent/catalyst; | 91.7% |

| Conditions | Yield |
|---|---|
| In methanol at 37℃; for 16h; | 91% |
-
-
63-29-6, 487-44-5, 575-64-4, 14362-29-9, 18281-92-0, 26623-21-2
D-glucurono-6,3-lactone

-
-
1111-78-0
ammonium carbamate

| Conditions | Yield |
|---|---|
| In methanol at 20℃; for 16h; | 89.5% |
-
-
1111-78-0
ammonium carbamate

-
-
999-97-3
1,1,1,3,3,3-hexamethyl-disilazane

-
-
35342-88-2
N,O-bis<(trimethylsilyl)oxy>acetamide

| Conditions | Yield |
|---|---|
| With sulfuric acid In chloroform at 35 - 40℃; for 15h; | 89.1% |
| With sulfuric acid In chloroform at 35 - 40℃; for 12h; Product distribution; other temperatures; other reaction time; other solvents: CCl4, THF, DMF.; |

| Conditions | Yield |
|---|---|
| With ammonium hydroxide In methanol at 37℃; for 24h; | 89% |
-
-
60839-94-3
2-fluoro-methylthiobenzene

-
-
1111-78-0
ammonium carbamate

-
A
-
1280126-02-4
(fluoromethyl)(imino)(phenyl)-λ6-sulfanone

| Conditions | Yield |
|---|---|
| With [bis(acetoxy)iodo]benzene In methanol at 20℃; for 0.5h; | A 89% B n/a |

-
-
1111-78-0
ammonium carbamate

-
-
81931-98-8
1-<(Difluoromethyl)thio>-4-methoxybenzene

| Conditions | Yield |
|---|---|
| With [bis(acetoxy)iodo]benzene In methanol at 20℃; for 12h; | A 88% B n/a |

-
-
1111-78-0
ammonium carbamate

-
-
499-40-1, 554-91-6, 585-99-9, 4233-70-9, 5077-31-6, 5188-47-6, 6614-35-3, 7286-49-9, 13117-25-4, 17296-19-4, 19940-03-5, 20113-42-2, 21216-58-0, 25538-26-5, 28447-39-4, 34097-00-2, 40246-35-3, 40592-71-0, 52611-34-4, 71184-87-7, 77881-87-9, 96844-88-1, 117249-74-8, 117249-83-9, 117249-87-3, 117249-92-0, 117249-96-4, 117250-05-2, 117250-10-9, 117250-14-3, 117250-19-8, 117306-23-7, 117306-27-1, 117306-32-8, 117306-37-3, 117306-42-0, 117306-47-5, 117469-04-2, 117469-05-3, 117469-10-0
melibiose

| Conditions | Yield |
|---|---|
| With ammonium hydroxide In methanol at 37℃; for 10h; | 87% |

| Conditions | Yield |
|---|---|
| With ammonium hydroxide In methanol at 37℃; for 12h; | 85% |

| Conditions | Yield |
|---|---|
| With ammonium hydroxide In methanol at 37℃; for 20h; | 85% |

| Conditions | Yield |
|---|---|
| In methanol; water at 5 - 37℃; for 64h; | 83% |

| Conditions | Yield |
|---|---|
| With ammonium hydroxide In methanol at 37℃; for 25h; | 82% |

-
-
1111-78-0
ammonium carbamate

| Conditions | Yield |
|---|---|
| In methanol at 20℃; for 48h; | 82% |
-
-
1535-67-7
difluoromethyl phenyl sulfide

-
-
1111-78-0
ammonium carbamate

-
A
-
1333375-53-3
(difluoromethyl)(imino)(phenyl)-λ6-sulfanone

| Conditions | Yield |
|---|---|
| With [bis(acetoxy)iodo]benzene In methanol at 20℃; for 8h; | A 82% B n/a |


-
-
1111-78-0
ammonium carbamate

| Conditions | Yield |
|---|---|
| With ammonia In methanol; water at 20℃; for 48h; | 81% |
-
-
65325-64-6
1-[(fluoromethyl)sulfanyl]-4-methylbenzene

-
-
1111-78-0
ammonium carbamate

| Conditions | Yield |
|---|---|
| With [bis(acetoxy)iodo]benzene In methanol at 20℃; for 0.5h; | A 80% B n/a |

-
-
1111-78-0
ammonium carbamate

-
-
93-91-4
1-phenylbutan-1,3-dione

-
-
23652-90-6
(2Z)-3-amino-1-phenylbut-2-en-1-one

| Conditions | Yield |
|---|---|
| In methanol at 20℃; for 24h; | 75% |
-
-
151-50-8
potassium cyanide

-
-
1111-78-0
ammonium carbamate

-
-
142924-43-4
(S)-N-(3-oxo-1-phenylbutan-2-yl)acetamide

| Conditions | Yield |
|---|---|
| With ammonium carbonate In ethanol; water at 45℃; for 3h; sonification; | 74% |


| Conditions | Yield |
|---|---|
| In chloroform at 20℃; | 73% |

| Conditions | Yield |
|---|---|
| With ammonium carbonate In ethanol; water at 45℃; for 3h; sonification; | 73% |

-
-
1111-78-0
ammonium carbamate

-
-
76043-69-1
2,2-dichloro-3-phenylpropionaldehyde

-
-
18166-56-8
α-chloro-β-phenylpropionamide

| Conditions | Yield |
|---|---|
| With 2-(2,4,6-trichlorophenyl)-2,5,6,7-tetrahydropyrrolo[2,1-c][1,2,4]triazol-4-ylium tetrafluoroborate In tetrahydrofuran at 20℃; for 0.75h; | 73% |

| Conditions | Yield |
|---|---|
| In methanol at 20℃; for 24h; | 71% |
-
-
1111-78-0
ammonium carbamate

-
-
28637-56-1
2,2-dimethyl-5,6,7,8-tetrahydro-4H-benzo[d][1,3]dioxin-4-one

-
-
141772-31-8
2-fluoro-4-chloro-5-methoxycarbonylaniline

| Conditions | Yield |
|---|---|
| Stage #1: 2,2-dimethyl-5,6,7,8-tetrahydro-4H-benzo[d][1,3]dioxin-4-one; 2-fluoro-4-chloro-5-methoxycarbonylaniline In 5,5-dimethyl-1,3-cyclohexadiene at 140℃; for 10h; Stage #2: ammonium carbamate In methanol at 60℃; for 12h; Stage #3: With orthoformic acid triethyl ester for 12h; Reflux; | 69% |


-
-
1111-78-0
ammonium carbamate

| Conditions | Yield |
|---|---|
| With [bis(acetoxy)iodo]benzene In methanol at 20℃; for 3h; | A 68% B n/a |

Ammonium carbamate Consensus Reports
Ammonium carbamate Specification
Ammonium carbamate is an organic compound with the formula CH3NO2·H3N, and its systematic name is the same with the product name. With the CAS registry number 1111-78-0, it is also named as Carbamic acid, ammoniumsalt (1:1). It belongs to the product category of Industrial/Fine Chemicals. Its EINECS number is 214-185-2. In addition, the molecular weight is 78.07. This chemical is stable at common pressure and temperature, and it should be sealed and stored in a cool and dry place. Moreover, it should be protected from oxides, acids, alkali and water. This chemical will decompose slowly in the air and release ammonia, and it can slightly volatilize at room temperature and completely sublimate and decompose at the temperature of 50 °C. Moreover, it will turn into ammonium carbonate when placed in damp air or aqueous solution. When heated, it will produce urea. This chemical can be used as aluminum phosphide intermediate, and it is also used in medicine.
Physical properties of Ammonium carbamate are: (1)ACD/LogP: -1.194; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): -1.94; (4)ACD/LogD (pH 7.4): -3.73; (5)ACD/BCF (pH 5.5): 1.00; (6)ACD/BCF (pH 7.4): 1.00; (7)ACD/KOC (pH 5.5): 1.00; (8)ACD/KOC (pH 7.4): 1.00; (9)#H bond acceptors: 3; (10)#H bond donors: 3; (11)#Freely Rotating Bonds: 0; (12)Polar Surface Area: 40.54 Å2; (13)Flash Point: 105.6 °C; (14)Enthalpy of Vaporization: 53.77 kJ/mol; (15)Boiling Point: 251 °C at 760 mmHg; (16)Vapour Pressure: 0.00662 mmHg at 25°C.
Preparation: this chemical can be prepared by anhydrous liquid ammonia and drikold. Moreover, 400 mL liquid ammonia can produce 200-300 g Ammonium carbamate.
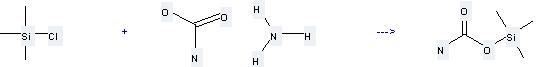
Uses of Ammonium carbamate: it can be used to produce trimethylsilyl carbamate at the temperature of 20 °C. It will need solvent CHCl3. The yield is about 73%.
When you are using this chemical, please be cautious about it as the following:
It is harmful if swallowed. You should not breathe dust. When using it, you must avoid contact with eyes.
You can still convert the following datas into molecular structure:
(1)SMILES: [O-]C(=O)N.[NH4+]
(2)Std. InChI: InChI=1S/CH3NO2.H3N/c2-1(3)4;/h2H2,(H,3,4);1H3
(3)Std. InChIKey: BVCZEBOGSOYJJT-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intravenous | 77mg/kg (77mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD | American Journal of Veterinary Research. Vol. 29, Pg. 897, 1968. |
| rat | LD50 | intravenous | 39mg/kg (39mg/kg) | American Journal of Veterinary Research. Vol. 29, Pg. 897, 1968. | |
| rat | LD50 | oral | > 681mg/kg (681mg/kg) | LUNGS, THORAX, OR RESPIRATION: DYSPNEA BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) SKIN AND APPENDAGES (SKIN): HAIR: OTHER | National Technical Information Service. Vol. OTS0535595, |
Related Products
- Ammonium (-)-3-bromo-8-camphorsulfonate
- Ammonium (S)-5-((4-amino-4-carboxy-1-oxobutyl)amino)-2-nitrobenzoate
- Ammonium 1-pyrrolidinedithiocarbamate
- Ammonium 2,4-dichlorophenoxyacetate
- Ammonium 2-hydroxyethanesulphonate
- Ammonium 4-nitrobenzoate dihydrate
- Ammonium acetate
- Ammonium adipate
- Ammonium alginate
- Ammonium azide
- 111-18-2
- 11118-25-5
- 11118-57-3
- 1111-88-2
- 1111-89-3
- 1111-92-8
- 111-19-3
- 111196-50-0
- 11119-67-8
- 111196-80-6
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View