-
Name
Amylcinnamaldehyde
- EINECS 204-541-5
- CAS No. 122-40-7
- Article Data24
- CAS DataBase
- Density 0.963 g/cm3
- Solubility 181.69mg/L at 25℃
- Melting Point 80°C
- Formula C14H18O
- Boiling Point 288.499 °C at 760 mmHg
- Molecular Weight 202.296
- Flash Point 131.139 °C
- Transport Information
- Appearance clear to pale yellow liquid
- Safety 26-37/39
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Cinnamaldehyde,a-amyl- (4CI);Cinnamaldehyde, a-pentyl- (6CI,7CI,8CI);2-(Phenylmethylene)heptanal;2-Benzylideneheptanal;Amylcinnamic acid aldehyde;Amylcinnamic aldehyde;Flomine;Jasminal;Jasminaldehyde;Jasmine aldehyde;Pentylcinnamaldehyde;a-Amyl-b-phenylacrolein;a-Amylcinnamal;a-Amylcinnamaldehyde;a-Pentylcinnamaldehyde;Alpha-amyl cinnamaldehyde;
- PSA 17.07000
- LogP 3.84920
Synthetic route

| Conditions | Yield |
|---|---|
| With benzoic acid; L-proline In neat (no solvent) at 125℃; for 1h; Reagent/catalyst; Time; Aldol Condensation; Inert atmosphere; | 97% |
| With amino-functionalized [Zr6O4(OH)4(O2C-C6H4-CO2)6], UiO-66(NH2) at 159.84℃; for 1h; cross-aldol condensation; chemoselective reaction; | 90% |
| With potassium carbonate; N-benzyl-N,N,N-triethylammonium chloride In dichloromethane for 3h; | 80% |

| Conditions | Yield |
|---|---|
| With chitosan/titanium dioxide microspheres In toluene at 80℃; for 4h; Inert atmosphere; | |
| With chitosan at 160℃; for 8h; chemoselective reaction; | |
| With aluminum oxide In toluene at 120℃; Inert atmosphere; |


| Conditions | Yield |
|---|---|
| With cetyltrimethylammonim bromide; sodium hydroxide In water at 30℃; for 4h; Concentration; |

-
-
111-71-7
heptanal

-
-
100-52-7
benzaldehyde

-
A
-
111-14-8
oenanthic acid

-
B
-
122-40-7
jasminaldehyde

-
C
-
65-85-0
benzoic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water at 30℃; for 72h; |

-
-
111-71-7
heptanal

-
-
100-52-7
benzaldehyde

-
A
-
3021-89-4, 49562-91-6, 49562-92-7
(E)-2-n-pentyl-2-n-nonenal

-
B
-
122-40-7
jasminaldehyde

| Conditions | Yield |
|---|---|
| With potassium hydroxide; Aliquat 336 at 118℃; for 0.0166667h; Irradiation; | A 18% B 82% |
| With calcined-hydrotalcite supported on hexagonal mesoporous silica at 150℃; Mechanism; Kinetics; Aldol condensation; Inert atmosphere; |


| Conditions | Yield |
|---|---|
| at 200℃; |


| Conditions | Yield |
|---|---|
| With 4-dimethylaminopyridine functionalized zirconium-benzene-1,4-dicarboxylate-metal organic framework for 1h; Catalytic behavior; Reagent/catalyst; Aldol Condensation; |

-
-
111-71-7
heptanal

-
-
100-52-7
benzaldehyde

-
A
-
3021-89-4
2-pentyl-2-nonenal

-
B
-
111-14-8
oenanthic acid

-
C
-
122-40-7
jasminaldehyde

| Conditions | Yield |
|---|---|
| With diethylamine for 1h; Catalytic behavior; Reagent/catalyst; Aldol Condensation; |


| Conditions | Yield |
|---|---|
| With formic acid; iron(II) tetrafluoroborate hexahydrate; tris(2-diphenylphosphinoethyl)phosphine In tetrahydrofuran at 60℃; for 2h; Schlenk technique; Inert atmosphere; | 99% |
| With iron(II) fluoro{tris[2-(diphenylphosphino)phenyl]phospino}tetrafluoroborate; hydrogen; trifluoroacetic acid In isopropyl alcohol at 120℃; under 15001.5 Torr; Inert atmosphere; Autoclave; chemoselective reaction; | 98% |
| With Cp*Ir(6,6'-dionato-2,2'-bipyridine)(H2O); isopropyl alcohol at 82℃; for 6h; Inert atmosphere; Schlenk technique; chemoselective reaction; | 97% |
-
-
122-40-7
jasminaldehyde

-
-
92368-90-6
(±)-2-benzyl-1-heptanol

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate; palladium diacetate In methanol at 20℃; for 0.5h; | 98% |
-
-
122-40-7
jasminaldehyde

| Conditions | Yield |
|---|---|
| With 2-pentafluorophenyl-6,7-dihydro-5H-pyrrolo[2,1-c][1,2,4]triazol-2-ium tetrafluoroborate; water-d2; potassium acetate In dichloromethane at 50℃; for 12h; | 96% |
-
-
1352211-40-5
(1-bromovinyl)triisopropylsilane

-
-
122-40-7
jasminaldehyde

-
-
1352210-55-9
(E)-4-benzylidene-2-(triisopropylsilyl)non-1-en-3-ol

| Conditions | Yield |
|---|---|
| Stage #1: (1-bromovinyl)triisopropylsilane With n-butyllithium In tetrahydrofuran; hexane at -78℃; for 1h; Inert atmosphere; Stage #2: jasminaldehyde In tetrahydrofuran; hexane at -78℃; Inert atmosphere; | 94% |
-
-
24762-04-7
2-Diazo-3-oxo-butyric acid methyl ester

-
-
69739-34-0
t-butyldimethylsiyl triflate

-
-
122-40-7
jasminaldehyde

| Conditions | Yield |
|---|---|
| With 2,6-dimethylpyridine; zinc trifluoromethanesulfonate In dichloromethane at -78 - 20℃; Mukaiyama reaction; Inert atmosphere; regioselective reaction; | 92% |


| Conditions | Yield |
|---|---|
| With chlorine[2-(4,5-dihydro-1H-imidazol-2-yl)-6-methoxypyridine](pentamethylcyclopentadienyl)iridium(III) chloride; sodium formate In water at 80℃; for 0.5h; Schlenk technique; chemoselective reaction; | A 92% B n/a |

| Conditions | Yield |
|---|---|
| With iron(III) chloride In acetic acid methyl ester Heating; | 90% |

| Conditions | Yield |
|---|---|
| iron(III) chloride In acetic acid for 3h; Heating / reflux; | 87% |

| Conditions | Yield |
|---|---|
| With copper(II) bis(trifluoromethanesulfonate) In toluene at 70℃; for 6h; Schlenk technique; Inert atmosphere; | 86% |


| Conditions | Yield |
|---|---|
| With bis[dichloro(pentamethylcyclopentadienyl)iridium(III)] In toluene at 120℃; for 12h; Inert atmosphere; | 84% |

| Conditions | Yield |
|---|---|
| With palladium on activated charcoal; hydrogen In methanol at 20℃; for 20h; | 75% |
| With 5%-palladium/activated carbon; hydrogen; potassium carbonate In methanol at 39.84℃; under 3750.38 Torr; for 0.833333h; Autoclave; chemoselective reaction; | 97.6 %Chromat. |
-
-
92138-20-0, 102518-79-6, 103735-86-0, 120786-18-7, 130791-77-4, 132435-40-6
natural Huperzine A

-
-
122-40-7
jasminaldehyde

| Conditions | Yield |
|---|---|
| With triethylamine In methanol at 60℃; | 72.8% |

| Conditions | Yield |
|---|---|
| With acetic acid In ethanol at 20℃; for 0.5h; | 66% |
-
-
122-40-7
jasminaldehyde

-
-
95-54-5
1,2-diamino-benzene

-
A
-
5851-48-9
2-hexylbenzimidazole

-
B
-
716-79-0
2-phenyl-1H-benzoimidazole

| Conditions | Yield |
|---|---|
| With tetra(n-butyl)ammonium hydroxide; water at 150℃; for 0.166667h; Microwave irradiation; Green chemistry; | A 61% B 50% |


| Conditions | Yield |
|---|---|
| With diethyl 2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate; (4s,6s)-2,4,5,6-tetra(9H-carbazol-9-yl)isophthalonitrile; (2S,4S)-2,4-bis(diphenylphosphino)pentane; N-ethyl-N,N-diisopropylamine; cobalt(II) bromide In tetrahydrofuran at 20℃; for 24h; Irradiation; Inert atmosphere; Schlenk technique; stereoselective reaction; | 10% |

| Conditions | Yield |
|---|---|
| With sodium acetate; acetic anhydride |

| Conditions | Yield |
|---|---|
| With piperidine |

| Conditions | Yield |
|---|---|
| (i) Zn, benzene, ether, (ii) KOH, EtOH; Multistep reaction; |
-
-
543-82-8
1,5-dimethylhexylamine

-
-
122-40-7
jasminaldehyde

-
-
30121-84-7
(1,5-Dimethyl-hexyl)-[2-[1-phenyl-meth-(E)-ylidene]-hept-(E)-ylidene]-amine
-
-
5397-03-5
hydrazinecarbodithioic acid methyl ester

-
-
122-40-7
jasminaldehyde

-
-
26174-31-2
N'-[2-[1-Phenyl-meth-(E)-ylidene]-hept-(E)-ylidene]-hydrazinecarbodithioic acid methyl ester
-
-
95-80-7
4-methylbenzene-1,3-diamine

-
-
122-40-7
jasminaldehyde

-
-
134668-01-2
4-Methyl-N1,N3-bis-[2-[1-phenyl-meth-(E)-ylidene]-hept-(E)-ylidene]-benzene-1,3-diamine

| Conditions | Yield |
|---|---|
| In ethanol for 5h; Heating; also ZnCl2, 160 deg C, 0.5 h, 180 deg C, 5 min; |
-
-
122-40-7
jasminaldehyde

-
A
-
16252-10-1
2-methyl-1-phenyl-heptane

| Conditions | Yield |
|---|---|
| With ethanol; palladium Hydrogenation; |
-
-
122-40-7
jasminaldehyde

-
-
134667-74-6
C39H46N2O2S2

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: aq. ethanol / 5 h / Heating; also ZnCl2, 160 deg C, 0.5 h, 180 deg C, 5 min 2: 61 percent / benzene / 6 h / Heating View Scheme |
-
-
122-40-7
jasminaldehyde

-
-
134667-88-2
C41H50N2O2S2

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: aq. ethanol / 5 h / Heating; also ZnCl2, 160 deg C, 0.5 h, 180 deg C, 5 min 2: 64 percent / benzene / 6 h / Heating View Scheme |
-
-
122-40-7
jasminaldehyde

-
-
53394-47-1
3-Pentyl-4-phenyl-buta-1,3-dien

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: (i) Zn, benzene, ether, (ii) KOH, EtOH 2: 98 °C / 20 Torr / (thermolysis) View Scheme |
Amylcinnamaldehyde Consensus Reports
Amylcinnamaldehyde Specification
The Amylcinnamaldehyde, with the CAS registry number 122-40-7, is also known as Heptanal, 2-(phenylmethylene)-. It belongs to the product categories of Pharmaceutical Intermediates; Aldehydes; C10 to C21; Carbonyl Compounds; A-B; Alphabetical Listings; Flavors and Fragrances. Its EINECS registry number is 204-541-5. This chemical's molecular formula is C14H18O and molecular weight is 202.29. What's more, its systematic name is called 2-Benzylideneheptanal. It is widely used in making various types of daily chemical flavor, allocation of jasmine, lily of the valley, lilac, etc.
Physical properties about Amylcinnamaldehyde are: (1)ACD/LogP: 4.357; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 4.36; (4)ACD/LogD (pH 7.4): 4.36; (5)ACD/BCF (pH 5.5): 1204.95; (6)ACD/BCF (pH 7.4): 1204.95; (7)ACD/KOC (pH 5.5): 5584.16; (8)ACD/KOC (pH 7.4): 5584.16; (9)#H bond acceptors: 1; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 6; (12)Polar Surface Area: 17.07 Å2; (13)Index of Refraction: 1.534; (14)Molar Refractivity: 65.336 cm3; (15)Molar Volume: 210.063 cm3; (16)Polarizability: 25.901×10-24cm3; (17)Surface Tension: 35.909 dyne/cm; (18)Density: 0.963 g/cm3; (19)Flash Point: 131.139 °C; (20)Enthalpy of Vaporization: 52.774 kJ/mol; (21)Boiling Point: 288.499 °C at 760 mmHg; (22)Vapour Pressure: 0.002 mmHg at 25 °C.
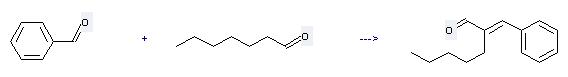
Preparation of Amylcinnamaldehyde: this chemical can be prepared by benzaldehyde with heptanal. This reaction needs reagent K2CO3, catalyst benzyltriethylammonium chloride and solvent CH2Cl2. The reaction time is 3 hours. The yield is 80 %.
When you are dealing with this chemical, you should be very careful. This chemical may cause inflammation to the skin or other mucous membranes. And it is irritating to eyes, respiratory system and skin. Therefore, you should wear gloves and eye/face protection. In case of contacting with eyes, you should rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
(1) SMILES: O=CC(=Cc1ccccc1)CCCCC
(2) InChI: InChI=1S/C14H18O/c1-2-3-5-10-14(12-15)11-13-8-6-4-7-9-13/h4,6-9,11-12H,2-3,5,10H2,1H3
(3) InChIKey: HMKKIXGYKWDQSV-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| rat | LD50 | oral | 3730mg/kg (3730mg/kg) | SENSE ORGANS AND SPECIAL SENSES: OTHER CHANGES: OLFACTION BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) SENSE ORGANS AND SPECIAL SENSES: OTHER: EYE | Food and Cosmetics Toxicology. Vol. 2, Pg. 327, 1964. |
Related Products
- Amylcinnamaldehyde
- 122413-01-8
- 122423-79-4
- 122-42-9
- 122429-13-4
- 122-43-0
- 122431-37-2
- 122433-29-8
- 122433-41-4
- 12244-16-5
- 12244-32-5
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View