-
Name
Baicalein
- EINECS
- CAS No. 491-67-8
- Article Data47
- CAS DataBase
- Density 1.548 g/cm3
- Solubility
- Melting Point 256-271 °C(lit.)
- Formula C15H10O5
- Boiling Point 575.932 °C at 760 mmHg
- Molecular Weight 270.241
- Flash Point 225.257 °C
- Transport Information
- Appearance Yellow crystalline solid
- Safety 26-36
- Risk Codes 36/37/38-20/21/22
-
Molecular Structure
-
Hazard Symbols
 Xi,
Xi, Xn
Xn
- Synonyms Baicalein(6CI);Flavone, 5,6,7-trihydroxy- (7CI,8CI);5,6,7-Trihydroxyflavone;NSC 661431;Noroxylin;
- PSA 90.90000
- LogP 2.57680
Synthetic route
-
-
21967-41-9
baicalin

-
-
491-67-8
5,6,7-trihydroxy-2-phenyl-4H-1-benzopyran-4-one

| Conditions | Yield |
|---|---|
| With sulfuric acid; water at 20℃; for 0.166667h; | 93% |
| With sulfuric acid In water at 90℃; for 0.0833333h; | 71.9% |
| With sulfuric acid at 90℃; for 0.166667h; | 29.3% |
-
-
82475-03-4
Baicalin methyl ester

-
-
491-67-8
5,6,7-trihydroxy-2-phenyl-4H-1-benzopyran-4-one

| Conditions | Yield |
|---|---|
| With sulfuric acid In ethanol; water at 95℃; for 8.5h; Inert atmosphere; | 97% |
| With hydrogenchloride In ethanol at 85℃; for 12h; Temperature; Reagent/catalyst; Inert atmosphere; | 91.8% |
-
-
119892-40-9
5,7-dimethoxy-6-hydroxyflavone

-
-
491-67-8
5,6,7-trihydroxy-2-phenyl-4H-1-benzopyran-4-one

| Conditions | Yield |
|---|---|
| With hydrogen bromide at 120 - 130℃; Reagent/catalyst; Temperature; Reflux; | 91% |
| With hydrogen bromide at 120℃; Reagent/catalyst; Temperature; Inert atmosphere; | 88% |
| Multi-step reaction with 2 steps 1: potassium carbonate; acetone 2: aqueous hydriodic acid; acetic acid anhydride / 140 °C View Scheme |

| Conditions | Yield |
|---|---|
| With hydrogen bromide; acetic acid at 130℃; | 92% |
| With hydrogen bromide; acetic acid for 18h; Heating; | 89% |
| With pyridine hydrochloride at 190℃; for 6.5h; Inert atmosphere; | 85% |
-
-
29550-13-8
5,6-dihydroxy-7-methoxyflavone

-
-
491-67-8
5,6,7-trihydroxy-2-phenyl-4H-1-benzopyran-4-one

| Conditions | Yield |
|---|---|
| With water; hydrogen bromide; acetic acid for 18h; Reflux; | 85% |
| With water; hydrogen bromide; Aliquat 336 at 105℃; for 8h; Catalytic behavior; | 80% |
| With hydrogen iodide; acetic anhydride |
-
-
67047-05-6
4-oxo-2-phenyl-4H-1-benzopyran-5,6,7-triyl triacetate

-
-
491-67-8
5,6,7-trihydroxy-2-phenyl-4H-1-benzopyran-4-one

| Conditions | Yield |
|---|---|
| Stage #1: 4-oxo-2-phenyl-4H-1-benzopyran-5,6,7-triyl triacetate With sodium hydroxide In water; acetone at 0℃; for 1h; Stage #2: With hydrogenchloride In water; acetone pH=6 - 7; | 66.7% |

| Conditions | Yield |
|---|---|
| With water; hydrogen bromide; Aliquat 336 at 105℃; for 7h; Catalytic behavior; | 84% |
| With hydrogen bromide; acetic acid for 12h; Heating; | 81% |
-
-
671791-94-9
2',4',5',6'-tetrahydroxyflavone

-
-
491-67-8
5,6,7-trihydroxy-2-phenyl-4H-1-benzopyran-4-one

| Conditions | Yield |
|---|---|
| With hydrogenchloride; lithium hydroxide; sulfuric acid In tetrahydrofuran; acetic acid |

-
-
491-67-8
5,6,7-trihydroxy-2-phenyl-4H-1-benzopyran-4-one

| Conditions | Yield |
|---|---|
| With sulfuric acid; acetic acid for 1h; Reflux; |
-
-
28279-72-3
baicalein 6-O-β-D-glucopyranoside

-
-
491-67-8
5,6,7-trihydroxy-2-phenyl-4H-1-benzopyran-4-one

| Conditions | Yield |
|---|---|
| With hydrogenchloride In methanol at 90℃; for 0.5h; |

-
-
491-67-8
5,6,7-trihydroxy-2-phenyl-4H-1-benzopyran-4-one

| Conditions | Yield |
|---|---|
| With hydrogenchloride In methanol at 90℃; for 0.5h; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 90 percent / BF3-Et2O / 0.25 h / Heating 2: 87 percent / I2; DMSO / 2 h / Heating 3: 89 percent / HBr; glacial AcOH / 18 h / Heating View Scheme | |
| Multi-step reaction with 4 steps 1: 90 percent / BF3-Et2O / 0.25 h / Heating 2: 87 percent / I2; DMSO / 2 h / Heating 3: 88 percent / HBr; glacial AcOH / 2 h / Heating 4: 81 percent / HBr; glacial AcOH / 12 h / Heating View Scheme | |
| Multi-step reaction with 4 steps 1: 90 percent / BF3-Et2O / 0.25 h / Heating 2: 91 percent / HBr; glacial AcOH / 2 h / Heating 3: 46 percent / I2; DMSO / Heating 4: 81 percent / HBr; glacial AcOH / 12 h / Heating View Scheme | |
| Multi-step reaction with 3 steps 1: boron trifluoride diethyl etherate / toluene 2: iodine / dimethyl sulfoxide 3: hydrogen bromide; acetic acid / 130 °C View Scheme | |
| Multi-step reaction with 3 steps 1: boron trifluoride diethyl etherate / dichloromethane / 0.17 h / Reflux 2: iodine / dimethyl sulfoxide / 3 h / Reflux 3: hydrogen bromide; acetic acid / 48 h View Scheme |
-
-
150036-33-2
6,7-dihydroxy-5-methoxyflavone

-
-
491-67-8
5,6,7-trihydroxy-2-phenyl-4H-1-benzopyran-4-one

| Conditions | Yield |
|---|---|
| With hydrogenchloride |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: BF3-Et2O / 0.25 h 2: KOH / ethanol 3: 87 percent / I2; DMSO / 2 h / Heating 4: 89 percent / HBr; glacial AcOH / 18 h / Heating View Scheme | |
| Multi-step reaction with 5 steps 1: BF3-Et2O / 0.25 h 2: KOH / ethanol 3: 87 percent / I2; DMSO / 2 h / Heating 4: 88 percent / HBr; glacial AcOH / 2 h / Heating 5: 81 percent / HBr; glacial AcOH / 12 h / Heating View Scheme | |
| Multi-step reaction with 5 steps 1: BF3-Et2O / 0.25 h 2: KOH / ethanol 3: 91 percent / HBr; glacial AcOH / 2 h / Heating 4: 46 percent / I2; DMSO / Heating 5: 81 percent / HBr; glacial AcOH / 12 h / Heating View Scheme |
-
-
412027-80-6
7-hydroxy-5-methoxy-4-oxo-2-phenyl-4H-chromene-6-carbaldehyde

-
-
491-67-8
5,6,7-trihydroxy-2-phenyl-4H-1-benzopyran-4-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: aq. NaOH solution; aqueous hydrogen peroxide 2: aqueous hydrochloric acid View Scheme |
-
-
22248-14-2
6-hydroxy-2,3,4-trimethoxyacetophenone

-
-
491-67-8
5,6,7-trihydroxy-2-phenyl-4H-1-benzopyran-4-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: KOH / ethanol 2: 87 percent / I2; DMSO / 2 h / Heating 3: 89 percent / HBr; glacial AcOH / 18 h / Heating View Scheme | |
| Multi-step reaction with 4 steps 1: KOH / ethanol 2: 87 percent / I2; DMSO / 2 h / Heating 3: 88 percent / HBr; glacial AcOH / 2 h / Heating 4: 81 percent / HBr; glacial AcOH / 12 h / Heating View Scheme | |
| Multi-step reaction with 4 steps 1: KOH / ethanol 2: 91 percent / HBr; glacial AcOH / 2 h / Heating 3: 46 percent / I2; DMSO / Heating 4: 81 percent / HBr; glacial AcOH / 12 h / Heating View Scheme |
-
-
6962-57-8
3,6-dihydroxy-2,4-dimethoxyacetophenone

-
-
491-67-8
5,6,7-trihydroxy-2-phenyl-4H-1-benzopyran-4-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: sodium benzoate / 180 °C / Unter vermindertem Druck und anschliessend Erwaermen mit wss.-aethanol. Kalilauge 2: potassium carbonate; acetone 3: aqueous hydriodic acid; acetic acid anhydride / 140 °C View Scheme | |
| Multi-step reaction with 3 steps 1: hydrogenchloride; sodium hydroxide / water / 48 h / 20 °C / Inert atmosphere 2: iodine; sodium hydrogensulfite / dimethylsulfoxide-d6 / 48 h / 120 °C / Cooling with ice 3: hydrogen bromide / 120 °C / Inert atmosphere View Scheme |
-
-
70185-52-3, 74064-14-5
6′-hydroxy-2′,3′,4′-trimethoxychalcone

-
-
491-67-8
5,6,7-trihydroxy-2-phenyl-4H-1-benzopyran-4-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: iodine / dimethyl sulfoxide 2: hydrogen bromide; acetic acid / 130 °C View Scheme | |
| Multi-step reaction with 2 steps 1: iodine / dimethyl sulfoxide / 3 h / Reflux 2: hydrogen bromide; acetic acid / 48 h View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: thionyl chloride / benzene / Reflux 2: boron trifluoride diethyl etherate / toluene 3: iodine / dimethyl sulfoxide 4: hydrogen bromide; acetic acid / 130 °C View Scheme | |
| Multi-step reaction with 4 steps 1: oxalyl dichloride; N,N-dimethyl-formamide / dichloromethane / 2 h / 20 °C / Cooling with ice 2: boron trifluoride diethyl etherate / dichloromethane / 0.17 h / Reflux 3: iodine / dimethyl sulfoxide / 3 h / Reflux 4: hydrogen bromide; acetic acid / 48 h View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: N,N-dimethyl-formamide; oxalyl dichloride / dichloromethane / 2 h / 20 °C / Cooling with ice 2: boron trifluoride diethyl etherate / 0.17 h / Reflux 3: iodine / dimethyl sulfoxide / 3 h / Reflux 4: hydrogen bromide; acetic acid / 48 h / Reflux View Scheme | |
| Multi-step reaction with 3 steps 1: boron trifluoride diethyl etherate; acetic anhydride / 1,2-dichloro-ethane; methanol / 8 h / 90 °C / Reflux 2: iodine / dimethyl sulfoxide / 6 h / 180 °C 3: hydrogen bromide / 120 - 130 °C / Reflux View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: acetic acid; sulfuric acid; sodium bromide; dihydrogen peroxide / 20 - 45 °C 2.1: copper(l) chloride / methanol; N,N-dimethyl-formamide / 100 - 110 °C 3.1: boron trifluoride diethyl etherate / chloroform / 36 h / 20 - 60 °C 3.2: 120 °C / Reflux 4.1: iodine / dimethyl sulfoxide / 6 h / 180 °C 5.1: hydrogen bromide / 120 - 130 °C / Reflux View Scheme | |
| Multi-step reaction with 5 steps 1: acetic acid; sulfuric acid; sodium bromide; dihydrogen peroxide / 20 - 45 °C 2: copper(l) chloride / methanol; N,N-dimethyl-formamide / 100 - 110 °C 3: boron trifluoride diethyl etherate; acetic anhydride / 1,2-dichloro-ethane; methanol / 8 h / 90 °C / Reflux 4: iodine / dimethyl sulfoxide / 6 h / 180 °C 5: hydrogen bromide / 120 - 130 °C / Reflux View Scheme | |
| Multi-step reaction with 6 steps 1: acetic acid; sulfuric acid; sodium bromide; dihydrogen peroxide / 20 - 45 °C 2: copper(l) chloride / methanol; N,N-dimethyl-formamide / 100 - 110 °C 3: boron trifluoride diethyl etherate / chloroform / 10 h / 20 - 60 °C 4: boron trifluoride diethyl etherate; acetic anhydride / chloroform / 36 h / 60 °C / Reflux 5: iodine / dimethyl sulfoxide / 6 h / 180 °C 6: hydrogen bromide / 120 - 130 °C / Reflux View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: copper(l) chloride / methanol; N,N-dimethyl-formamide / 100 - 110 °C 2.1: boron trifluoride diethyl etherate / chloroform / 36 h / 20 - 60 °C 2.2: 120 °C / Reflux 3.1: iodine / dimethyl sulfoxide / 6 h / 180 °C 4.1: hydrogen bromide / 120 - 130 °C / Reflux View Scheme | |
| Multi-step reaction with 4 steps 1: copper(l) chloride / methanol; N,N-dimethyl-formamide / 100 - 110 °C 2: boron trifluoride diethyl etherate; acetic anhydride / 1,2-dichloro-ethane; methanol / 8 h / 90 °C / Reflux 3: iodine / dimethyl sulfoxide / 6 h / 180 °C 4: hydrogen bromide / 120 - 130 °C / Reflux View Scheme | |
| Multi-step reaction with 5 steps 1: copper(l) chloride / methanol; N,N-dimethyl-formamide / 100 - 110 °C 2: boron trifluoride diethyl etherate / chloroform / 10 h / 20 - 60 °C 3: boron trifluoride diethyl etherate; acetic anhydride / chloroform / 36 h / 60 °C / Reflux 4: iodine / dimethyl sulfoxide / 6 h / 180 °C 5: hydrogen bromide / 120 - 130 °C / Reflux View Scheme |
-
-
20491-92-3
2,4,6-trimethoxyphenol

-
-
491-67-8
5,6,7-trihydroxy-2-phenyl-4H-1-benzopyran-4-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: boron trifluoride diethyl etherate / chloroform / 36 h / 20 - 60 °C 1.2: 120 °C / Reflux 2.1: iodine / dimethyl sulfoxide / 6 h / 180 °C 3.1: hydrogen bromide / 120 - 130 °C / Reflux View Scheme | |
| Multi-step reaction with 3 steps 1: boron trifluoride diethyl etherate; acetic anhydride / 1,2-dichloro-ethane; methanol / 8 h / 90 °C / Reflux 2: iodine / dimethyl sulfoxide / 6 h / 180 °C 3: hydrogen bromide / 120 - 130 °C / Reflux View Scheme | |
| Multi-step reaction with 4 steps 1: boron trifluoride diethyl etherate / chloroform / 10 h / 20 - 60 °C 2: boron trifluoride diethyl etherate; acetic anhydride / chloroform / 36 h / 60 °C / Reflux 3: iodine / dimethyl sulfoxide / 6 h / 180 °C 4: hydrogen bromide / 120 - 130 °C / Reflux View Scheme |

| Conditions | Yield |
|---|---|
| With hydrogen iodide; acetic anhydride at 150℃; |

| Conditions | Yield |
|---|---|
| With hydrogen iodide; acetic anhydride at 150℃; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: boron trifluoride diethyl etherate / 0.17 h / Reflux 2: iodine / dimethyl sulfoxide / 3 h / Reflux 3: hydrogen bromide; acetic acid / 48 h / Reflux View Scheme |
-
-
103777-45-3
5-hydroxy-2,4,6-trimethoxyacetophenone

-
-
491-67-8
5,6,7-trihydroxy-2-phenyl-4H-1-benzopyran-4-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: boron trifluoride diethyl etherate; acetic anhydride / chloroform / 36 h / 60 °C / Reflux 2: iodine / dimethyl sulfoxide / 6 h / 180 °C 3: hydrogen bromide / 120 - 130 °C / Reflux View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: boron trifluoride diethyl etherate / chloroform / 36 h / 20 - 60 °C 1.2: 120 °C / Reflux 2.1: iodine / dimethyl sulfoxide / 6 h / 180 °C 3.1: hydrogen bromide / 120 - 130 °C / Reflux View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: sodium dithionite / water / 3 h / 20 °C 2: boron trifluoride diethyl etherate / chloroform / 10 h / 20 - 90 °C 3: hydrogenchloride; sodium hydroxide / water / 48 h / 20 °C / Inert atmosphere 4: iodine; sodium hydrogensulfite / dimethylsulfoxide-d6 / 48 h / 120 °C / Cooling with ice 5: hydrogen bromide / 120 °C / Inert atmosphere View Scheme |
-
-
15233-65-5
2,6-dimethoxy-1,4-hydroquinone

-
-
491-67-8
5,6,7-trihydroxy-2-phenyl-4H-1-benzopyran-4-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: boron trifluoride diethyl etherate / chloroform / 10 h / 20 - 90 °C 2: hydrogenchloride; sodium hydroxide / water / 48 h / 20 °C / Inert atmosphere 3: iodine; sodium hydrogensulfite / dimethylsulfoxide-d6 / 48 h / 120 °C / Cooling with ice 4: hydrogen bromide / 120 °C / Inert atmosphere View Scheme |

| Conditions | Yield |
|---|---|
| With hydrogenchloride In tetrahydrofuran; water for 2h; | 100% |
-
-
491-67-8
5,6,7-trihydroxy-2-phenyl-4H-1-benzopyran-4-one

-
-
74-88-4
methyl iodide

-
-
973-67-1
5,6,7-trimethoxyflavone

| Conditions | Yield |
|---|---|
| With pyridine; potassium carbonate; potassium iodide In acetone at 60℃; for 8h; Reflux; | 95% |
| With potassium carbonate In acetone for 8h; Heating; | 82% |
| With potassium carbonate; acetone |
-
-
491-67-8
5,6,7-trihydroxy-2-phenyl-4H-1-benzopyran-4-one

-
-
108-24-7
acetic anhydride

-
-
67047-05-6
4-oxo-2-phenyl-4H-1-benzopyran-5,6,7-triyl triacetate

| Conditions | Yield |
|---|---|
| With pyridine at 70℃; for 6h; | 94% |
| With sodium acetate at 80℃; for 2h; | 92% |
| With sodium acetate at 75℃; | 91.1% |
-
-
110-91-8
morpholine

-
-
50-00-0
formaldehyd

-
-
491-67-8
5,6,7-trihydroxy-2-phenyl-4H-1-benzopyran-4-one

-
-
852333-36-9
8-morpholinemethylene baicalein

| Conditions | Yield |
|---|---|
| In methanol at 55℃; | 93.5% |
| In methanol; water at 20℃; for 2h; Mannich reaction; | 69% |

-
-
123-75-1
pyrrolidine

-
-
50-00-0
formaldehyd

-
-
491-67-8
5,6,7-trihydroxy-2-phenyl-4H-1-benzopyran-4-one

-
-
1060171-73-4
5,6,7-trihydroxy-2-phenyl-8-(pyrrolidin-1-ylmethyl)-4H-chromen-4-one

| Conditions | Yield |
|---|---|
| In methanol at 45℃; | 92.5% |
| In methanol; water at 20℃; for 2h; Mannich reaction; | 71% |
| In methanol; water at 70℃; for 8h; Mannich Aminomethylation; |

-
-
50-00-0
formaldehyd

-
-
491-67-8
5,6,7-trihydroxy-2-phenyl-4H-1-benzopyran-4-one

-
-
142-84-7
di-n-propylamine

| Conditions | Yield |
|---|---|
| In methanol at 45℃; | 92.5% |


-
-
491-67-8
5,6,7-trihydroxy-2-phenyl-4H-1-benzopyran-4-one

| Conditions | Yield |
|---|---|
| With dmap; benzotriazol-1-ol; dicyclohexyl-carbodiimide In dichloromethane at 20℃; | 92% |
-
-
2051-90-3
Dichlorodiphenylmethane

-
-
491-67-8
5,6,7-trihydroxy-2-phenyl-4H-1-benzopyran-4-one

-
-
18107-18-1
diazomethyl-trimethyl-silane

| Conditions | Yield |
|---|---|
| Stage #1: Dichlorodiphenylmethane; 5,6,7-trihydroxy-2-phenyl-4H-1-benzopyran-4-one at 170℃; for 1h; Stage #2: diazomethyl-trimethyl-silane In tetrahydrofuran; methanol; hexane at 20℃; for 24h; | 90% |

-
-
491-67-8
5,6,7-trihydroxy-2-phenyl-4H-1-benzopyran-4-one

-
-
35132-20-8
(R,R)-1,2-diphenylethylenediamine

| Conditions | Yield |
|---|---|
| In ethanol at 20℃; for 168h; | 90% |
-
-
109-01-3
1-methyl-piperazine

-
-
50-00-0
formaldehyd

-
-
491-67-8
5,6,7-trihydroxy-2-phenyl-4H-1-benzopyran-4-one

-
-
1060171-92-7
5,6,7-trihydroxy-8-((4-methylpiperazin-1-yl)methyl)-2-phenyl-4H-chromen-4-one

| Conditions | Yield |
|---|---|
| In methanol at 50℃; | 89.5% |
| In methanol; water at 20℃; for 2h; Mannich reaction; | 72.2% |
| In methanol at 55℃; for 4h; |


| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone for 24h; Heating; | 87% |
| With potassium carbonate In acetone for 24h; Heating / reflux; | 87% |

| Conditions | Yield |
|---|---|
| In ethanol; water at 90℃; for 8h; Condensation; | 85% |
-
-
491-67-8
5,6,7-trihydroxy-2-phenyl-4H-1-benzopyran-4-one

-
-
7584-85-2, 7792-96-3, 30048-41-0, 66701-54-0, 71328-30-8, 103531-01-7, 149342-82-5
β-D-galactopyranosyl-(1->4)-α-D-glucopyranosyl fluoride

| Conditions | Yield |
|---|---|
| With Tris buffer at 37℃; pH=7.0; | 84% |
| With Tris-HCl buffer at 25℃; pH=7.8; Enzyme kinetics; |
-
-
491-67-8
5,6,7-trihydroxy-2-phenyl-4H-1-benzopyran-4-one

-
-
108-24-7
acetic anhydride

-
-
731817-58-6
5-hydroxy-4-oxo-2-phenyl-4H-1-benzopyran-6,7-diyl diacetate

| Conditions | Yield |
|---|---|
| With pyridine; sodium acetate at 120℃; for 8h; | 84% |
| With pyridine; dmap for 24h; |
-
-
392-95-0
1-chloro-2,4-dinitro-6-trifluoromethylbenzene

-
-
491-67-8
5,6,7-trihydroxy-2-phenyl-4H-1-benzopyran-4-one

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In N,N-dimethyl-formamide at 20℃; | 83% |
-
-
491-67-8
5,6,7-trihydroxy-2-phenyl-4H-1-benzopyran-4-one

| Conditions | Yield |
|---|---|
| Stage #1: 5,6,7-trihydroxy-2-phenyl-4H-1-benzopyran-4-one With [D]-sodium hydroxide; platinum on activated charcoal; water-d2 at 130℃; for 12h; Inert atmosphere; Stage #2: With formic acid at 130℃; for 12h; Temperature; Inert atmosphere; | 83% |

| Conditions | Yield |
|---|---|
| In ethanol; ethyl acetate at 20 - 50℃; | 82.6% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone for 30h; Heating; | 82% |
| With potassium carbonate In acetone for 30h; Heating / reflux; | 82% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone for 24h; Heating; | 82% |
| With potassium carbonate In acetone for 24h; Heating / reflux; | 82% |

| Conditions | Yield |
|---|---|
| at 170℃; for 1h; | 81% |
-
-
331-39-5
caffeic acid

-
-
491-67-8
5,6,7-trihydroxy-2-phenyl-4H-1-benzopyran-4-one

-
-
1266114-33-3
C23H18O7

| Conditions | Yield |
|---|---|
| With cerium(III) chloride heptahydrate; sodium iodide In ethanol; acetonitrile for 0.2h; Reflux; | 80% |
-
-
50-00-0
formaldehyd

-
-
491-67-8
5,6,7-trihydroxy-2-phenyl-4H-1-benzopyran-4-one

-
-
124-40-3
dimethyl amine

-
-
516484-10-9
8-((dimethylamino)methyl)-5,6,7-trihydroxy-2-phenyl-4H-chromen-4-one

| Conditions | Yield |
|---|---|
| In methanol; water at 20℃; for 2h; Mannich reaction; | 79% |
| In methanol at 20℃; for 1.5h; Mannich Aminomethylation; | 10.5% |

-
-
393-75-9
4-chloro-3,5-dinitrobenzotrifluoride

-
-
491-67-8
5,6,7-trihydroxy-2-phenyl-4H-1-benzopyran-4-one

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In N,N-dimethyl-formamide at 20℃; | 78% |
Baicalein Specification
The Baicalein, with the CAS registry number 491-67-8, is also known as 5,6,7-Trihydroxy-2-phenyl-4H-chromen-4-one. It belongs to the product categories of Tri-substituted Flavones; The group of Scutellaria; Inhibitors; Aromatics; Heterocycles. This chemical's molecular formula is C15H10O5 and molecular weight is 270.24. What's more, its IUPAC name is called 5,6,7-Trihydroxy-2-phenylchromen-4-one. It should be stored in a cool, dry and well-ventilated place. This chemical is a flavone, a type of flavonoid, originally isolated from the roots of Scutellaria baicalensis. It is used as an inhibitor of 12-lipoxygenase, leukotriene biosynthesis and release of lysosomal enzymes. Baicalein also inhibits cellular Ca2+ uptake and mobilization and adjuvant-induced arthritis. It is the flavonoid component of Nepalese and Sino-Japanese crude d.
Physical properties about Baicalein are: (1)ACD/LogP: 3.641; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 3.55; (4)ACD/LogD (pH 7.4): 2.21; (5)ACD/BCF (pH 5.5): 281.14; (6)ACD/BCF (pH 7.4): 12.89; (7)ACD/KOC (pH 5.5): 1859.72; (8)ACD/KOC (pH 7.4): 85.24; (9)#H bond acceptors: 5; (10)#H bond donors: 3; (11)#Freely Rotating Bonds: 4; (12)Polar Surface Area: 86.99 Å2; (13)Index of Refraction: 1.732; (14)Molar Refractivity: 69.852 cm3; (15)Molar Volume: 174.563 cm3; (16)Polarizability: 27.691×10-24cm3; (17)Surface Tension: 79.60 dyne/cm; (18)Density: 1.548 g/cm3; (19)Flash Point: 225.257 °C; (20)Enthalpy of Vaporization: 89.452 kJ/mol; (21)Boiling Point: 575.932 °C at 760 mmHg; (22)Vapour Pressure: 0 mmHg at 25 °C.
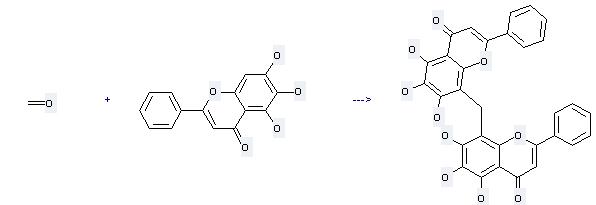
Uses of Baicalein: it is used to produce other chemicals. For example, it can react with formaldehyde to get bis(5,6,7-trihydroxyflavon-8-yl)methane. This reaction needs solvents H2O and ethanol at temperature of 90 °C. The reaction time is 8 hours. The yield is 85 %.
When you are dealing with this chemical, you should be very careful. This chemical is inflammation to the skin, eyes and respiratory system. It may cause damage to health by inhalation, in contacting with skin and if swallowed. Therefore, you should wear suitable protective clothing. In case of contacting with eyes, you should rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
(1) SMILES: O=C\1c3c(O)c(O)c(O)cc3O/C(=C/1)c2ccccc2
(2) InChI: InChI=1S/C15H10O5/c16-9-6-11(8-4-2-1-3-5-8)20-12-7-10(17)14(18)15(19)13(9)12/h1-7,17-19H
(3) InChIKey: FXNFHKRTJBSTCS-UHFFFAOYSA-N
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View