-
Name
Bromoacetyl bromide
- EINECS 209-923-5
- CAS No. 598-21-0
- Article Data25
- CAS DataBase
- Density 2.373 g/cm3
- Solubility REACTS
- Melting Point 148.5°C (estimate)
- Formula C2H2Br2O
- Boiling Point 148.5 °C at 760 mmHg
- Molecular Weight 201.845
- Flash Point 72 °C
- Transport Information UN 2513 8/PG 2
- Appearance COA
- Safety 26-36/37/39-45-8-30-25
- Risk Codes 34-14
-
Molecular Structure
-
Hazard Symbols
 C
C
- Synonyms Acetyl bromide, bromo-;2-Bromoacetyl bromide;
- PSA 17.07000
- LogP 1.30280
Synthetic route

| Conditions | Yield |
|---|---|
| With bromine |

| Conditions | Yield |
|---|---|
| With oxygen |

| Conditions | Yield |
|---|---|
| With phosphorus; bromine; acetic anhydride | |
| With phosphorus; bromine | |
| With bromine; phosphorus tribromide at 20 - 75℃; Bromination; Hell-Vollhard-Zelinskii reaction; | |
| With phosphorus; bromine; sodium hydroxide at 110℃; Hell-Vollard-Zelinsky halogenation; regioselective reaction; |

| Conditions | Yield |
|---|---|
| With phosphorus tribromide |

| Conditions | Yield |
|---|---|
| With bromine at 100 - 140℃; | |
| With bromine |


| Conditions | Yield |
|---|---|
| at 100 - 105℃; |

-
-
7726-95-6
bromine

-
-
64-19-7
acetic acid

-
A
-
506-96-7
Acetyl bromide

-
B
-
79-08-3
bromoacetic acid

-
C
-
598-21-0
2-Bromoacetyl bromide

-
-
540-49-8
cis+trans-dibromoethylene

-
-
10028-15-6
ozone

-
A
-
631-64-1
dibromoacetic acid

-
B
-
3039-13-2
dibromoacetaldehyde

-
C
-
598-21-0
2-Bromoacetyl bromide

| Conditions | Yield |
|---|---|
| bei der Oxydation; |

-
-
540-49-8
cis+trans-dibromoethylene

-
A
-
463-51-4
Ketene

-
B
-
78957-22-9
bromoketene

-
C
-
598-21-0
2-Bromoacetyl bromide

| Conditions | Yield |
|---|---|
| at -259.15℃; Kinetics; Photolysis; |


| Conditions | Yield |
|---|---|
| With potassium carbonate In dichloromethane; water at 0 - 20℃; for 6.5h; | 100% |
| With 1,2-dichloro-ethane at -10℃; | |
| at -77℃; |

| Conditions | Yield |
|---|---|
| With potassium carbonate In dichloromethane; water at 0 - 20℃; for 6.5h; | 100% |
| In 1,2-dichloro-ethane at -20 - 20℃; for 0.833333h; | 95% |
| In dichloromethane at 0 - 20℃; for 1h; | 92% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In dichloromethane; water at 0 - 20℃; for 6.5h; | 100% |
| In dichloromethane at 0 - 20℃; for 1.5h; Inert atmosphere; | 96% |
| With potassium carbonate In dichloromethane; water at 0 - 20℃; for 12.5h; | 95% |
-
-
108-18-9
diisopropylamine

-
-
598-21-0
2-Bromoacetyl bromide

-
-
51321-61-0
2-bromo-N,N-diisopropylacetamide

| Conditions | Yield |
|---|---|
| In dichloromethane at 0 - 20℃; for 1.5h; Inert atmosphere; | 100% |
| In dichloromethane at -60 - 20℃; for 1h; | 98% |
| In dichloromethane at 0 - 20℃; for 1h; | 88% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In dichloromethane; water at 5 - 20℃; for 12.5h; | 100% |
| With potassium carbonate In dichloromethane; water at 5 - 25℃; for 12.5h; | 100% |
| With sodium carbonate In water pH=9 - 10; | 99% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In dichloromethane; water at 5 - 20℃; for 4h; | 100% |
| With potassium carbonate In dichloromethane; water at 4 - 20℃; for 4.5h; | 100% |
| With potassium carbonate In dichloromethane; water at 0 - 20℃; for 6.5h; | 100% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In dichloromethane; water at 0 - 20℃; for 6.5h; | 100% |
| In tetrahydrofuran; water | 99% |
| In tetrahydrofuran; water | 99% |
| In tetrahydrofuran; water | 99% |
| With 1,2-dichloro-ethane at -10℃; |

| Conditions | Yield |
|---|---|
| With potassium carbonate In dichloromethane; water at 4 - 20℃; for 4.5h; | 100% |
| With 1,2-dichloro-ethane at -10℃; | |
| With triethylamine In dichloromethane at -15℃; | |
| With potassium carbonate In dichloromethane; water at 4 - 20℃; for 12.5h; | |
| With potassium carbonate In dichloromethane; water at 4 - 20℃; for 12h; |
-
-
2216-51-5
(-)-menthol

-
-
598-21-0
2-Bromoacetyl bromide

-
-
16832-20-5, 32815-12-6, 55284-67-8
(1R,2S,5R)-menthol bromoacetate

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane at -78℃; Inert atmosphere; | 100% |
| With potassium carbonate In dichloromethane at 0 - 20℃; Inert atmosphere; | 95% |
| With N,N-dimethyl-aniline In diethyl ether 1.) 0 deg C, 3 h, 2.) reflux, 3 h; | 77% |

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0 - 20℃; | 100% |
| With triethylamine In dichloromethane at 0℃; for 1.25h; Inert atmosphere; | 99% |
| With sodium carbonate In water pH=9 - 10; | 99% |

| Conditions | Yield |
|---|---|
| With pyridine In chloroform at 20℃; for 24h; | 100% |
| With sodium carbonate In water pH=9 - 10; | 99% |
| With potassium carbonate In dichloromethane; water at 0℃; for 1h; | 98% |

| Conditions | Yield |
|---|---|
| With triethylamine In ethyl acetate at 0 - 25℃; for 0.5h; | 100% |
| In dichloromethane at 0 - 20℃; for 1h; | 98% |
| With triethylamine In dichloromethane at 20℃; for 16h; | 88% |

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran at 20℃; for 2h; | 100% |
| With N-ethyl-N,N-diisopropylamine In dichloromethane at 0 - 20℃; for 16h; | 99% |
| In dichloromethane at 0 - 20℃; for 0.5h; Inert atmosphere; | 98% |
-
-
186581-53-3, 908094-01-9
diazomethane

-
-
598-21-0
2-Bromoacetyl bromide

-
-
39755-31-2
1-bromo-3-diazopropan-2-one

| Conditions | Yield |
|---|---|
| With calcium oxide In diethyl ether at 0℃; for 3.08333h; Arndt-Eistert synthesis; | 100% |
| In diethyl ether at 0℃; | 88% |
-
-
98-16-8
3-trifluoromethylaniline

-
-
598-21-0
2-Bromoacetyl bromide

-
-
25625-57-4
2-bromo-N-(3′-(trifluoromethyl)phenyl)acetamide

| Conditions | Yield |
|---|---|
| In dichloromethane at 10 - 20℃; for 1.58333h; | 100% |
| Stage #1: 3-trifluoromethylaniline; 2-Bromoacetyl bromide In dichloromethane at 10 - 20℃; for 1.58333h; Stage #2: With sodium hydrogencarbonate In dichloromethane for 0.666667h; | 100% |
| Stage #1: 3-trifluoromethylaniline; 2-Bromoacetyl bromide In dichloromethane at 10 - 20℃; for 1.58333h; Stage #2: With sodium hydrogencarbonate In dichloromethane; water for 0.833333h; | 100% |
-
-
598-21-0
2-Bromoacetyl bromide

-
-
108-44-1
1-amino-3-methylbenzene

-
-
5439-17-8
2-bromo-N-(3-methylphenyl)acetamide

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0 - 20℃; | 100% |
| With sodium carbonate In water pH=9 - 10; | 99% |
| With triethylamine In dichloromethane at 0℃; for 1h; | 93% |
-
-
34820-01-4
methyl 2,3,6-tri-O-benzoyl-β-D-galactopyranoside

-
-
598-21-0
2-Bromoacetyl bromide

-
-
114682-52-9
methyl 2,3,6-tri-benzoyl-4-O-(bromoacetyl)-β-D-galactopyranoside

| Conditions | Yield |
|---|---|
| With tetramethylurea In 1,2-dimethoxyethane for 24h; Ambient temperature; | 100% |

| Conditions | Yield |
|---|---|
| In dichloromethane at 0 - 20℃; for 1.5h; Inert atmosphere; | 100% |
| With triethylamine In dichloromethane at 0℃; for 2h; Inert atmosphere; | 91% |
| With potassium phosphate In dichloromethane for 3h; Inert atmosphere; | 89% |

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane | 100% |
| With N-ethyl-N,N-diisopropylamine In tetrahydrofuran at 0℃; |
-
-
85695-96-1
(1R,2S,3R,4S)-2-(2,2-dimethylpropoxy)-1,7,7-trimethylbicyclo<2.2.1>heptan-3-ol

-
-
598-21-0
2-Bromoacetyl bromide

-
-
88948-24-7
(1S,2R,3S)-3-(2,2-dimethylpropoxy)-4,7,7-trimethylbicyclo<2.2.1>hept-2-yl bromoacetate

| Conditions | Yield |
|---|---|
| With silver cyanide In benzene for 0.333333h; Heating; | 100% |
| With silver cyanide In benzene at 80℃; for 0.333333h; |
-
-
130533-09-4
2-[(4-methoxybenzyl)amino]-2-phenylacetonitrile

-
-
598-21-0
2-Bromoacetyl bromide

-
-
130533-00-5
N-(4-methoxybenzyl)-N-(1-cyanobenzyl)-2-bromoacetamide

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran at 0℃; for 1h; | 100% |
-
-
130533-04-9
(3R)-2-(4-methoxybenzylamino)-3-benzyloxymethylbutyronitrile

-
-
598-21-0
2-Bromoacetyl bromide

-
-
130533-05-0
N-(4-methoxybenzyl)-N-<(2R)-2-benzyloxymethyl-1-cyanopropyl>-2-bromoacetamide

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran at 0℃; for 1h; | 100% |
-
-
53518-15-3
Coumarin 151

-
-
598-21-0
2-Bromoacetyl bromide

-
-
78277-40-4
7-(bromoacetamido)-4-(trifluoromethyl)coumarin

| Conditions | Yield |
|---|---|
| In tetrahydrofuran for 0.5h; Ambient temperature; | 100% |
| With triethylamine In dichloromethane at 0℃; for 3h; |
-
-
82082-32-4
<5-chloro-2-<(phenylmethyl)amino>phenyl>(2,6-dichlorophenyl)methanone

-
-
598-21-0
2-Bromoacetyl bromide

-
-
82082-41-5
2-bromo-N-<4-chloro-2-(2,6-dichlorobenzoyl)phenyl>-N-(phenylmethyl)acetamide

| Conditions | Yield |
|---|---|
| With pyridine In diethyl ether for 0.333333h; Ambient temperature; | 100% |
-
-
598-21-0
2-Bromoacetyl bromide

-
-
2835-77-0
(2-aminophenyl)(phenyl)methanone

-
-
14439-71-5
2'-benzoyl-2-bromo-acetanilide

| Conditions | Yield |
|---|---|
| 100% | |
| With potassium carbonate In acetonitrile at 20℃; for 1h; | 98% |
| With potassium carbonate In acetonitrile at 20℃; for 1h; | 98% |
-
-
598-21-0
2-Bromoacetyl bromide

-
-
589-08-2
N-Methyl-N-phenethylamine

-
-
73391-97-6
N-methyl-N-2-phenylethyl-2-bromoacetamide

| Conditions | Yield |
|---|---|
| In dichloromethane at -25 - 20℃; | 100% |
| With potassium carbonate In dichloromethane at 0 - 20℃; for 3h; |
-
-
17061-62-0
N,N-bis(p-methoxybenzyl)amine

-
-
598-21-0
2-Bromoacetyl bromide

-
-
180747-36-8
2-Bromo-N,N-bis-(4-methoxy-benzyl)-acetamide

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran at 0℃; for 0.25h; | 100% |
| With triethylamine In dichloromethane at 0 - 20℃; for 4h; Schlenk technique; Inert atmosphere; | |
| With potassium carbonate In dichloromethane at 0 - 20℃; for 0.5h; Inert atmosphere; |
-
-
90719-32-7
(S)-4-Benzyl-2-oxazolidinone

-
-
598-21-0
2-Bromoacetyl bromide

-
-
129549-13-9
4S-3-(2-bromoacetyl)-4-(phenylmethyl)-2-oxazolidinone

| Conditions | Yield |
|---|---|
| With n-butyllithium at -78 - 20℃; for 0.5h; | 100% |
| Stage #1: (S)-4-Benzyl-2-oxazolidinone With n-butyllithium In tetrahydrofuran; hexane at -78℃; for 1h; Inert atmosphere; Stage #2: 2-Bromoacetyl bromide In tetrahydrofuran; hexane at -78 - 20℃; for 16.5h; Inert atmosphere; | 92% |
| Stage #1: (S)-4-Benzyl-2-oxazolidinone With n-butyllithium In tetrahydrofuran; hexane at -78 - -60℃; for 0.5h; Stage #2: 2-Bromoacetyl bromide In tetrahydrofuran; hexane at -78℃; for 1h; | 87% |
-
-
598-21-0
2-Bromoacetyl bromide

-
-
611-21-2
N,2-dimethylaniline

-
-
13508-79-7
N-methyl-N-(2-methylphenyl)-2-bromoacetamide

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane for 1h; Ambient temperature; | 100% |
-
-
115196-83-3
methyl 4-azido-3-O-benzyl-4,6-dideoxy-α-D-mannopyranoside

-
-
598-21-0
2-Bromoacetyl bromide

| Conditions | Yield |
|---|---|
| With tetramethylurea In dichloromethane Ambient temperature; | 100% |
Bromoacetyl bromide Specification
The CAS registry number of Bromoacetyl bromide is 598-21-0. The IUPAC name is 2-bromoacetyl bromide. Its EINECS registry number is 209-923-5. In addition, the molecular formula is C2H2Br2O and the molecular weight is 201.84. It is a kind of dark brown-yellow liquid and belongs to the classes of Pharmaceutical Intermediates; Organics; Acid Halides; Carbonyl Compounds; Organic Building Blocks.
Physical properties about this chemical are: (1)ACD/LogP: 1.32; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 1.32; (4)ACD/LogD (pH 7.4): 1.32; (5)ACD/BCF (pH 5.5): 5.93; (6)ACD/BCF (pH 7.4): 5.93; (7)ACD/KOC (pH 5.5): 124.42; (8)ACD/KOC (pH 7.4): 124.42; (9)#H bond acceptors: 1; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 1; (12)Polar Surface Area: 17.07 Å2; (13)Index of Refraction: 1.543; (14)Molar Refractivity: 26.8 cm3; (15)Molar Volume: 85 cm3; (16)Polarizability: 10.62 ×10-24cm3; (17)Surface Tension: 45.6 dyne/cm ; (18)Density: 2.373 g/cm3; (19)Flash Point: 72 °C; (20)Enthalpy of Vaporization: 38.54 kJ/mol; (21)Boiling Point: 148.5 °C at 760 mmHg; (22)Vapour Pressure: 4.21 mmHg at 25°C.
Preparation of Bromoacetyl bromide: It can be prepared from acetic acid and bromine in the presence of phosphorus. Firstly, dry the red phosphorus for 2 hours. Then mix it with glacial acetic acid. Next, add the bromine with stirring. when add half of the bromine, you should heat it immediately. After adding the bromine, heat it to 140 °C for reaction 2 hours. By means of cooling and distilling you can get the product.
![]()
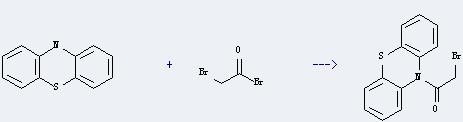
Uses of Bromoacetyl bromide: it is mainly used in medicine intermediates. And it can react with 10H-phenothiazine to get 10-bromoacetyl-10H-phenothiazine. This reaction will need reagent pyridine and solvent toluene. The reaction time is 12 hours at reaction ambient temperature. In addition, the yield is about 45%.
When you are using this chemical, please be cautious about it as the following:
This chemical can react violently with water and cause burns. So you should never add water to this product.During using it, wear suitable protective clothing, gloves and eye/face protection and avoid contact with eyes. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. And in case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.). What's more, you should keep container dry.
You can still convert the following datas into molecular structure:
(1)SMILES: BrC(=O)CBr
(2)InChI: InChI=1/C2H2Br2O/c3-1-2(4)5/h1H2
(3)InChIKey: LSTRKXWIZZZYAS-UHFFFAOYAE
Related Products
- Bromoacetyl bromide
- Bromoacetyl chloride
- 598-22-1
- 598-23-2
- 59-82-5
- 598-25-4
- 59827-64-4
- 598-30-1
- 5983-09-5
- 59831-02-6
- 598-31-2
- 59831-64-0
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View