-
Name
Diallyl phthalate
- EINECS 205-016-3
- CAS No. 131-17-9
- Article Data29
- CAS DataBase
- Density 1.112 g/cm3
- Solubility Insoluble in water, slightly soluble in petroleum ether and glycerol
- Melting Point -70 °C
- Formula C14H14O4
- Boiling Point 329.066 °C at 760 mmHg
- Molecular Weight 246.263
- Flash Point 164.836 °C
- Transport Information UN 3082 9/PG 3
- Appearance clear colourless to light yellow liquid
- Safety 24/25-60-61
- Risk Codes 22-50/53
-
Molecular Structure
-
Hazard Symbols
 Xn,
Xn, N
N
- Synonyms 1,2-Benzenedicarboxylic acid, di-2-propenyl ester;1,2-Benzenedicarboxylic acid,esters,di-2-propenyl ester;o-Phthalic acid, diallyl ester;DAP monomer (Diallyl Phthalate monomer) manufacturer;Phthalic acid, diallyl ester;
- PSA 52.60000
- LogP 2.37220
Synthetic route

| Conditions | Yield |
|---|---|
| With 3,3′-(2,2-bis(hydroxymethyl)propane-1,3-diyl)bis(1-methyl-1H-imidazol-3-ium) hydrogen sulfate for 2h; Dean-Stark; Reflux; | 100% |
| With diacidic ionic liquid supported on magnetic-silica nanoparticles In neat (no solvent) at 97℃; for 5h; Dean-Stark; | 95% |
| With phosphoric acid |
-
-
182342-88-7
Phthalic acid 1-allyl ester 2-prop-2-ynyl ester

-
-
131-17-9
diallyl phthalate

| Conditions | Yield |
|---|---|
| With indium In ethanol for 40h; Heating; | 95% |

| Conditions | Yield |
|---|---|
| Stage #1: benzene-1,2-dicarboxylic acid With copper; sodium iodide In water under 760.051 Torr; for 1h; Green chemistry; Stage #2: 3-chloroprop-1-ene In water under 760.051 Torr; for 6h; Concentration; Time; Temperature; Reflux; Green chemistry; | 91.7% |

| Conditions | Yield |
|---|---|
| With tetra-(n-butyl)ammonium iodide; potassium carbonate In N,N-dimethyl-formamide at 20℃; for 6h; Inert atmosphere; | 91% |
| Stage #1: benzene-1,2-dicarboxylic acid With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 0.166667h; Inert atmosphere; Stage #2: allyl bromide With tetra-(n-butyl)ammonium iodide In N,N-dimethyl-formamide at 20℃; for 6h; Inert atmosphere; | 91% |
| With potassium hydroxide; Aliquat 336 1.) 60 deg C, 0.1 Torr, 6 h, 2.) 60 deg C, 16 h; Yield given. Multistep reaction; |

| Conditions | Yield |
|---|---|
| With potassium carbonate; Katamin AB at 55 - 60℃; for 3h; Product distribution; or DMF, KOH powder; other catalysts; | 58.4% |
| With potassium carbonate; Katamin AB at 55 - 60℃; for 3h; | 58.4% |

-
-
131-17-9
diallyl phthalate

| Conditions | Yield |
|---|---|
| With netting agent; water at 100℃; |

| Conditions | Yield |
|---|---|
| tin(IV) chloride |

| Conditions | Yield |
|---|---|
| With 1,8-diazabicyclo[5.4.0]undec-7-ene at 20℃; chemoselective reaction; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 0.17 h / 20 °C 2: 1,8-diazabicyclo[5.4.0]undec-7-ene / 20 °C View Scheme |
-
-
827-27-0, 10197-71-4, 15968-01-1
sodium 1,2-benzenedicarboxylate

-
-
107-05-1
3-chloroprop-1-ene

-
-
131-17-9
diallyl phthalate

| Conditions | Yield |
|---|---|
| at 40 - 60℃; | |
| at 65℃; for 3h; |

| Conditions | Yield |
|---|---|
| With phenyl bis(2,4,6-trimethylbenzoyl)phosphine oxide at 20℃; Reagent/catalyst; Temperature; UV-irradiation; | 99% |
-
-
131-17-9
diallyl phthalate

-
-
406911-89-5
C12H10O4

| Conditions | Yield |
|---|---|
| With C46H54Cl2FeN2O3Ru In dichloromethane at 35℃; for 8h; | 97% |
| With C34H50B10Cl2N2O2Ru In dichloromethane at 40℃; for 8h; Reagent/catalyst; Time; Inert atmosphere; | 96% |
| With C96H111Cl6N9O3Ru3 In dichloromethane at 35℃; for 12h; Schlenk technique; Inert atmosphere; | 96% |
-
-
131-17-9
diallyl phthalate

| Conditions | Yield |
|---|---|
| [2-((2,6-iPr2-Ph-imino)methyl)phenol][p-cymene][=CHPh]Ru2Cl3 In various solvent(s) at 70℃; for 4h; Product distribution; Further Variations:; Catalysts; Temperatures; | 96% |
| With diazomethyl-trimethyl-silane; ruthenium Schiff-base In toluene at 85℃; for 17h; ring-closing metathesis; | 94% |
| With diazomethyl-trimethyl-silane; ruthenium In toluene at 85℃; for 17h; Product distribution; Further Variations:; Catalysts; | 94% |
| hybrid N,O-bidentate Schiff base ruthenium complex In toluene at 85℃; for 17h; | 94% |
| Schiff base substituted ruthenium benzylidene; 1,3-bis(2,4,6-trimethylphenyl)imidazolidin-2-ylidene In benzene-d6 at 55℃; for 4h; ring-closing metathesis; | 87 % Spectr. |

| Conditions | Yield |
|---|---|
| at -25 - 7℃; for 4.16h; | 94% |
-
-
131-17-9
diallyl phthalate

-
-
1608129-88-9
C26H24O8

| Conditions | Yield |
|---|---|
| With tricyclohexylphosphine[1,3-bis(2,4,6-trimethylphenyl)-4,5-dihydroimidazol-2-ylidine][benzylidene]ruthenium(II) dichloride; titanium(IV)isopropoxide In toluene at 80℃; for 0.75h; Inert atmosphere; | 91% |

| Conditions | Yield |
|---|---|
| With bis(1,5-cyclooctadiene)diiridium(I) dichloride In dichloromethane at 20℃; Glovebox; | 80% |

| Conditions | Yield |
|---|---|
| With palladium(II) acetylacetonate; triphenylphosphine In tetrahydrofuran at 50℃; for 5h; | 59% |

| Conditions | Yield |
|---|---|
| With palladium(II) acetylacetonate; triphenylphosphine; zinc In tetrahydrofuran at 60℃; for 5h; | 54% |
-
-
131-17-9
diallyl phthalate

-
-
1147550-11-5
ammonium thiocyanate

-
-
369-57-3
benzenediazonium tetrafluoroborate

| Conditions | Yield |
|---|---|
| copper(II) bis(tetrafluoroborate) In water; acetone at -15 - -10℃; for 2.5h; | 48% |
| With copper(II) bis(tetrafluoroborate) In water; acetone at -20 - -15℃; for 2.5h; | 48% |

-
-
131-17-9
diallyl phthalate

-
-
459-64-3
4-methoxybenzenediazonium tetrafluoroborate

-
-
1147550-11-5
ammonium thiocyanate

| Conditions | Yield |
|---|---|
| copper(II) bis(tetrafluoroborate) In water; acetone | 44% |
| With copper(II) bis(tetrafluoroborate) In water; acetone at -20 - -15℃; for 2.5h; | 44% |


| Conditions | Yield |
|---|---|
| With tricyclohexylphosphine[1,3-bis(2,4,6-trimethylphenyl)-4,5-dihydroimidazol-2-ylidine][benzylidene]ruthenium(II) dichloride; titanium(IV) isopropylate In toluene at 80℃; Inert atmosphere; | A 42% B 38% |

-
-
131-17-9
diallyl phthalate

-
-
2093-46-1
2-methylphenyl diazonium tetrafluoroborate

-
-
1147550-11-5
ammonium thiocyanate

| Conditions | Yield |
|---|---|
| copper(II) bis(tetrafluoroborate) In water; acetone | 41% |
| With copper(II) bis(tetrafluoroborate) In water; acetone at -20 - -15℃; for 2.5h; | 41% |

-
-
131-17-9
diallyl phthalate

-
-
1147550-11-5
ammonium thiocyanate

-
-
459-44-9
4-methylbenzenediazonium tetrafluoroborate

| Conditions | Yield |
|---|---|
| copper(II) bis(tetrafluoroborate) In water; acetone | 40% |
| With copper(II) bis(tetrafluoroborate) In water; acetone at -20 - -15℃; for 2.5h; | 40% |

-
-
131-17-9
diallyl phthalate

-
-
1147550-11-5
ammonium thiocyanate

-
-
1422-76-0
3-methylbenzenediazonium tetrafluoroborate

| Conditions | Yield |
|---|---|
| copper(II) bis(tetrafluoroborate) In water; acetone | 38% |
| With copper(II) bis(tetrafluoroborate) In water; acetone at -20 - -15℃; for 2.5h; | 38% |

-
-
131-17-9
diallyl phthalate

-
-
6407-35-8
N-cyclohexylidenemethylamine

-
A
-
94-66-6
2-allylcyclohexan-1-one

-
B
-
115782-91-7
(Z)-2,6-diallyl-1-cyclohexanone

-
-
36040-02-5, 115782-91-7, 115782-92-8
(E)-2,6-diallyl-1-cyclohexanone

| Conditions | Yield |
|---|---|
| With ethylmagnesium bromide; triphenylphosphine; palladium(II) acetylacetonate 1.) ether, 2.) 36-40 deg C, 5 h; Yield given. Multistep reaction; |

-
-
131-17-9
diallyl phthalate

-
-
6407-36-9
N-isopropyliden-cyclohexyl amine

-
A
-
109-49-9
1-hexen-5-one

-
B
-
74912-33-7
nona-1,8-dien-5-one

| Conditions | Yield |
|---|---|
| With ethylmagnesium bromide Multistep reaction; |

-
-
131-17-9
diallyl phthalate

-
-
29246-20-6
3,4,5,6-tetrahydrobenzo[c][1,6]dioxecine-1,8-dione

| Conditions | Yield |
|---|---|
| Grubbs catalyst first generation In 1,2-dichloro-ethane at 150℃; for 0.5h; MW irradiation; | 79 % Chromat. |

| Conditions | Yield |
|---|---|
| With Grubbs catalyst first generation In dichloromethane |

| Conditions | Yield |
|---|---|
| Stage #1: diallyl phthalate With dimethylaluminum chloride In toluene at 110℃; Inert atmosphere; Stage #2: With hydrogenchloride In water; toluene at 20℃; | |
| Stage #1: diallyl phthalate With dimethylaluminum chloride In toluene at 110℃; Inert atmosphere; Stage #2: With hydrogenchloride In water; toluene at 20℃; |
Diallyl phthalate Consensus Reports
NTP Carcinogenesis Studies (gavage); Equivocal Evidence: rat NTPTR* National Toxicology Program Technical Report Series. (Research Triangle Park, NC 27709) No. NTP-TR-284 ,1985. . Reported in EPA TSCA Inventory.
Diallyl phthalate Specification
Diallyl phthalate is an organic compound with the formula C14H14O4, and its systematic name is the same with the product name. With the CAS registry number 131-17-9, it is also named as 1,2-Benzenedicarboxylic acid, di-2-propenyl ester. It belongs to the product categories of C12 to C63; Carbonyl Compounds; Esters; Alpha Sort; Analytical Standards; AromaticsVolatiles/ Semivolatiles; Chemical Class; Esters Analytical Standards; Plasticizers; Ester series. Its EINECS number is 205-016-3. In addition, the molecular weight is 246.26. Its classification codes are: (1)Mutation data; (2)Skin / Eye Irritant; (3)Tumor data. This chemical should be sealed and stored in a cool, ventilated and dry place. Moreover, it should be protected from oxides, heat and fire. It is used to produced DAP polymerization fluid and DAP resin, and it is also used as crosslinking agent.
Physical properties of Diallyl phthalate are: (1)ACD/LogP: 3.228; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 3.23; (4)ACD/LogD (pH 7.4): 3.23; (5)ACD/BCF (pH 5.5): 167.36; (6)ACD/BCF (pH 7.4): 167.36; (7)ACD/KOC (pH 5.5): 1359.23; (8)ACD/KOC (pH 7.4): 1359.23; (9)#H bond acceptors: 4; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 8; (12)Polar Surface Area: 52.6 Å2; (13)Index of Refraction: 1.524; (14)Molar Refractivity: 67.781 cm3; (15)Molar Volume: 221.433 cm3; (16)Polarizability: 26.871×10-24cm3; (17)Surface Tension: 39.21 dyne/cm; (18)Density: 1.112 g/cm3; (19)Flash Point: 164.836 °C; (20)Enthalpy of Vaporization: 57.152 kJ/mol; (21)Boiling Point: 329.066 °C at 760 mmHg; (22)Vapour Pressure: 0 mmHg at 25°C.
Preparation: this chemical can be prepared by phthalic acid anhydride and 3-bromo-propene at the temperature of 55 - 60 °C. This reaction will need reagent aqueous potash with the reaction time of 3 hours. This reaction will also need catalyst Katamin AB. The yield is about 58.4%.
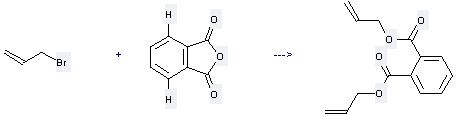
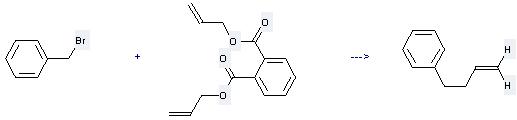
Uses of Diallyl phthalate: it can be used to produce but-3-enyl-benzene at the temperature of 60 °C. It will need reagents Zn, triphenylphosphine, Pd(acac)2 and solvent tetrahydrofuran with the reaction time of 5 hours. The yield is about 54%.
When you are using this chemical, please be cautious about it as the following:
This chemical is harmful if swallowed. It is very toxic to aquatic organisms as it may cause long-term adverse effects in the aquatic environment. When using it, you must avoid contact with eyes. This material and its container must be disposed of as hazardous waste. You should avoid releasing it to the environment, and you need to refer to special instructions/safety data sheet.
You can still convert the following datas into molecular structure:
(1)SMILES: O=C(OC\C=C)c1ccccc1C(=O)OC\C=C
(2)Std. InChI: InChI=1S/C14H14O4/c1-3-9-17-13(15)11-7-5-6-8-12(11)14(16)18-10-4-2/h3-8H,1-2,9-10H2
(3)Std. InChIKey: QUDWYFHPNIMBFC-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| dog | LDLo | oral | 800mg/kg (800mg/kg) | National Technical Information Service. Vol. OTS0521092, | |
| mouse | LDLo | oral | 681mg/kg (681mg/kg) | National Toxicology Program Technical Report Series. Vol. NTP-TR-242, Pg. 1983, | |
| rabbit | LD50 | oral | 1700mg/kg (1700mg/kg) | "Industrial Hygiene and Toxicology," 2nd ed., Patty, F.A., ed., New York, John Wiley & Sons, Inc., 1958-63Vol. 2, Pg. 1904, 1963. | |
| rabbit | LD50 | skin | 3300mg/kg (3300mg/kg) | National Technical Information Service. Vol. OTS0521092, | |
| rabbit | LDLo | subcutaneous | 1gm/kg (1000mg/kg) | LIVER: "HEPATITIS (HEPATOCELLULAR NECROSIS), DIFFUSE" LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES | Federation Proceedings, Federation of American Societies for Experimental Biology. Vol. 5, Pg. 191, 1946. |
| rat | LC50 | inhalation | 5200mg/m3/1H (5200mg/m3) | LUNGS, THORAX, OR RESPIRATION: DYSPNEA GASTROINTESTINAL: CHANGES IN STRUCTURE OR FUNCTION OF SALIVARY GLANDS SENSE ORGANS AND SPECIAL SENSES: OTHER: EYE | National Technical Information Service. Vol. OTS0521092, |
| rat | LD50 | oral | 656mg/kg (656mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) | National Toxicology Program Technical Report Series. Vol. NTP-TR-284, Pg. 1985, |
Related Products
- Diallyl 2,2'-oxydiethyl dicarbonate
- Diallyl bisphenol A
- Diallyl chlorendate
- Diallyl isophthalate
- Diallyl maleate
- DIALLYL PEROXYDICARBONATE
- Diallyl phosphite
- Diallyl phthalate
- Diallyl selenide
- Diallyl sulfide
- 13117-94-7
- 131-18-0
- 131180-45-5
- 131180-52-4
- 131180-57-9
- 131184-73-1
- 131189-22-5
- 1311-93-9
- 13119-76-1
- 13119-86-3
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View