-
Name
Ethyl levulinate
- EINECS 208-728-2
- CAS No. 539-88-8
- Article Data233
- CAS DataBase
- Density 0.996 g/cm3
- Solubility Soluble in water.
- Melting Point <25 °C
- Formula C7H12O3
- Boiling Point 205.499 °C at 760 mmHg
- Molecular Weight 144.17
- Flash Point 919 °C
- Transport Information
- Appearance clear yellowish liquid
- Safety 24/25
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms FEMA No. 2442;Ethyl ketovalerate;Ethyl 4-oxopentanoate;Levulinic acid, ethyl ester;Ethyl 4-oxovalerate;Ethyl levulinate (natural);Ethyl 4-ketovalerate;Ethyl 3-acetylpropionate;Ethyl acetylpropanoate;Ethyl-4-oxovalerate;
- PSA 43.37000
- LogP 0.91870
Synthetic route

| Conditions | Yield |
|---|---|
| With Zirconium Exchanged Phosphotungstic Acid at 120℃; for 2h; Temperature; | 100% |
| With sulfuric acid for 16h; Reflux; | 100% |
| With naphthalene; N,N,N-triethyl-N-butanesulfonic acid ammonium hydrogen sulfate at 140℃; for 0.5h; | 99% |

| Conditions | Yield |
|---|---|
| With Amberlyst 15 at 140℃; for 5h; | 99% |
| With naphthalene; N,N,N-triethyl-N-butanesulfonic acid ammonium hydrogen sulfate at 140℃; for 24h; | 85% |
| With Amberlyst-15 at 120℃; | 74% |

| Conditions | Yield |
|---|---|
| With MCM-41; amberlyst-15 In ethyl acetate at 15 - 80℃; for 5h; Temperature; Solvent; Reagent/catalyst; | 96.92% |
| With tetrachloromethane; iron(III)-acetylacetonate at 70℃; | 95% |
| With Amberlyst-15 at 120℃; | 95% |

| Conditions | Yield |
|---|---|
| at 20 - 80℃; for 5h; Temperature; Reagent/catalyst; | 96.65% |


| Conditions | Yield |
|---|---|
| With tungsten(VI) oxide In ethanol under 30003 Torr; for 2h; Reagent/catalyst; Autoclave; Inert atmosphere; Sealed tube; | 93.3% |
| With sulphated alumina In ethanol at 200℃; for 3h; Catalytic behavior; Temperature; Reagent/catalyst; Time; | 80.6% |
| With divinylbenzene polymer with acid ionic liquid In ethanol at 150℃; under 760.051 Torr; for 12h; Solvent; Inert atmosphere; | 75% |
-
-
78932-46-4
2-Methoxy-2-methyl-cyclopropanecarboxylic acid ethyl ester

-
-
539-88-8
4-oxopentanoic acid ethyl ester

| Conditions | Yield |
|---|---|
| With sulfuric acid In water; acetone for 2h; Ambient temperature; | 93% |
-
-
98-00-0
(2-furyl)methyl alcohol

-
-
64-17-5
ethanol

-
A
-
6270-56-0
2-(ethoxymethyl)furan

-
B
-
1446756-00-8
4,5,5-triethoxypentan-2-one

-
C
-
539-88-8
4-oxopentanoic acid ethyl ester

| Conditions | Yield |
|---|---|
| at 110℃; for 2h; Temperature; Reagent/catalyst; Autoclave; Ionic liquid; | A n/a B n/a C 92% |
| With hydrothermally treated graphene oxide (GO-HT) at 120℃; for 6h; Autoclave; | A 38.8% B 11.7% C 39.7% |

-
-
67-47-0
5-hydroxymethyl-2-furfuraldehyde

-
-
64-17-5
ethanol

-
A
-
1917-65-3
5-(ethoxymethyl)furfural

-
B
-
539-88-8
4-oxopentanoic acid ethyl ester

| Conditions | Yield |
|---|---|
| With dual acidic Glu-TsOH-Ti catalyst at 90℃; for 6h; Reagent/catalyst; | A 91% B n/a |
| With mesoporous silica Z-SBA-15 catalyst at 140℃; for 5h; | A 76% B 23% |
| With partially reduced graphene oxide (S-RGO) at 140℃; for 24h; | A 71% B 22% |

-
-
14583-98-3
1-tributylstannanyl-propan-2-one

-
-
105-36-2
ethyl bromoacetate

-
-
539-88-8
4-oxopentanoic acid ethyl ester

| Conditions | Yield |
|---|---|
| With N,N,N,N,N,N-hexamethylphosphoric triamide In benzene at 25℃; for 7h; | 90% |
| In benzene for 1h; Ambient temperature; Irradiation; | 50% |
| With bis-triphenylphosphine-palladium(II) chloride In tetrahydrofuran at 100℃; for 9h; | 41% |
-
-
98-00-0
(2-furyl)methyl alcohol

-
-
64-17-5
ethanol

-
A
-
60-29-7
diethyl ether

-
B
-
539-88-8
4-oxopentanoic acid ethyl ester

| Conditions | Yield |
|---|---|
| Amberlyst 15 at 125℃; for 1.16667h; Conversion of starting material; | A 4% B 89% |
| Amberlyst 46 at 125℃; for 0.55 - 1.1h; Conversion of starting material; | A 0.4% B 68% |
| Amberlyst 36 at 125℃; for 0.533333 - 1.11667h; Conversion of starting material; | A 1.2% B 60% |
| Purolite MN500 at 125℃; for 0.883333 - 1.16667h; Conversion of starting material; | A 0.4% B 38% |


| Conditions | Yield |
|---|---|
| With multi-walled carbon nanotubes/PTFE-supported Candida antarctica lipase B In toluene for 2h; Green chemistry; Enzymatic reaction; | 85% |
| lithium carbonate at 100℃; under 41404.1 Torr; for 3h; Product distribution / selectivity; | |
| potassium carbonate at 100℃; under 41404.1 Torr; for 3h; Product distribution / selectivity; |

| Conditions | Yield |
|---|---|
| at 160℃; for 0.5h; Sealed vessel; | 85% |
| at 160℃; for 0.5h; sealed tube; | 84.7% |

| Conditions | Yield |
|---|---|
| With poly(p-styrenesulfonic acid)-grafted carbon nanotubes at 120℃; for 24h; Reagent/catalyst; Sealed tube; Green chemistry; chemoselective reaction; | 84% |
| With 3-methyl-1-(4-sulfobutyl)imidazol-3-ium bis((trifluoromethyl)sulfonyl)azanide; naphthalene at 140℃; | 77% |
| With sulfated zirconia In glycerol at 210℃; for 4h; | 47.46% |
| With H-beta 19 at 150 - 160℃; under 15001.5 Torr; for 20h; Reagent/catalyst; Pressure; Autoclave; Inert atmosphere; | 48 %Chromat. |

| Conditions | Yield |
|---|---|
| Stage #1: diiodomethane With diethylzinc; trifluoroacetic acid In dichloromethane for 0.5h; Stage #2: ethyl acetoacetate In dichloromethane at 20℃; for 4h; | 81% |
-
-
67-47-0
5-hydroxymethyl-2-furfuraldehyde

-
-
64-17-5
ethanol

-
A
-
1917-65-3
5-(ethoxymethyl)furfural

-
B
-
539-88-8
4-oxopentanoic acid ethyl ester

-
C
-
109-94-4
formic acid ethyl ester

| Conditions | Yield |
|---|---|
| With sulfuric acid at 75℃; for 24h; Reagent/catalyst; Temperature; Sealed tube; | A 81% B 16% C n/a |

-
-
64-17-5
ethanol

-
-
50-99-7, 59-23-4, 921-60-8, 1949-88-8, 1990-29-0, 2152-76-3, 2595-97-3, 2595-98-4, 3458-28-4, 4205-23-6, 5934-56-5, 5978-95-0, 5987-68-8, 6027-89-0, 6038-51-3, 7635-11-2, 10030-80-5, 15572-79-9, 19163-87-2, 23567-25-1, 26566-61-0, 30077-17-9, 31103-86-3, 39665-52-6, 40866-07-7, 58367-01-4, 58407-05-9, 58407-06-0, 83198-69-0, 83198-70-3, 83198-71-4, 93780-23-5, 145920-48-5
2,3,4,5,6-pentahydroxy-hexanal

-
-
539-88-8
4-oxopentanoic acid ethyl ester

| Conditions | Yield |
|---|---|
| With tin(IV) oxide at 180℃; for 3h; Temperature; Reagent/catalyst; | 81% |

| Conditions | Yield |
|---|---|
| With 3C12H30N2O6S2(2+)*2O40PW12(3-) at 120℃; for 12h; Reagent/catalyst; | 80% |
| With propylsulfonic acid functionalized phenyl bridged organosilicacore/ silica shell structured nanosphere-2 at 140℃; for 4h; Reagent/catalyst; Autoclave; | |
| With per-rhenic acid at 160℃; for 16h; Reagent/catalyst; Concentration; Temperature; Time; Schlenk technique; | 80 %Spectr. |
-
-
98-00-0
(2-furyl)methyl alcohol

-
-
64-17-5
ethanol

-
A
-
6270-56-0
2-(ethoxymethyl)furan

-
B
-
539-88-8
4-oxopentanoic acid ethyl ester

| Conditions | Yield |
|---|---|
| With sulfonic acid-functionalized MIL-101(Cr) at 140℃; for 2h; Temperature; Reagent/catalyst; Autoclave; | A n/a B 79.2% |
| With hierarchical-HZ-5 at 99.84℃; for 4h; Catalytic behavior; Green chemistry; | A 26% B 73% |
| With hierarchical-HZ-5 at 99.84℃; for 4h; Catalytic behavior; Green chemistry; | A 49% B 41% |

-
-
57-48-7
D-Fructose

-
-
64-17-5
ethanol

-
A
-
1917-65-3
5-(ethoxymethyl)furfural

-
B
-
539-88-8
4-oxopentanoic acid ethyl ester

| Conditions | Yield |
|---|---|
| With poly(p-styrenesulfonic acid)-grafted carbon nanotubes at 100℃; for 12h; Sealed tube; Green chemistry; chemoselective reaction; | A n/a B 79% |
| With dual acidic Glu-TsOH-Ti catalyst at 120℃; for 30h; | A 66% B 18% |
| In hexane at 100℃; for 1.33333h; Ionic liquid; Sealed tube; Green chemistry; | A 54% B 6% |

-
-
64-17-5
ethanol

-
-
123-76-2
levulinic acid

-
A
-
591-11-7
5-methyl-5H-furan-2-one

-
B
-
539-88-8
4-oxopentanoic acid ethyl ester

| Conditions | Yield |
|---|---|
| With tin modified silicotungstic acid/Ta2O5 at 70℃; for 3h; Catalytic behavior; Reagent/catalyst; | A n/a B 78% |


| Conditions | Yield |
|---|---|
| With 5-isopropyl-2-methylbenzenesulfonic acid In Isopropylbenzene at 50℃; for 20h; | 76% |
| With Amberlyst-15 at 77℃; for 23h; Reagent/catalyst; | 75% |
| With H-ZSM-5 | |
| With amberlyst36 at 75℃; for 3h; Inert atmosphere; | 65.8 %Chromat. |
-
-
14583-98-3
1-tributylstannanyl-propan-2-one

-
-
105-39-5
chloroacetic acid ethyl ester

-
-
539-88-8
4-oxopentanoic acid ethyl ester

| Conditions | Yield |
|---|---|
| With N,N,N,N,N,N-hexamethylphosphoric triamide In benzene at 25℃; for 24h; | 75% |
-
-
14583-98-3
1-tributylstannanyl-propan-2-one

-
-
623-48-3
ethyl iodoacetae

-
-
539-88-8
4-oxopentanoic acid ethyl ester

| Conditions | Yield |
|---|---|
| In benzene for 1h; Ambient temperature; Irradiation; | 74% |
-
-
39131-44-7
5-bromomethyl-furan-2-carbaldehyde

-
-
64-17-5
ethanol

-
A
-
1917-65-3
5-(ethoxymethyl)furfural

-
B
-
539-88-8
4-oxopentanoic acid ethyl ester

| Conditions | Yield |
|---|---|
| With calcium carbonate for 1h; Reflux; | A 74% B 6% |
| In water at 70℃; for 0.5h; Overall yield = 98 %; |

-
-
470-23-5
D-fructose

-
-
64-17-5
ethanol

-
A
-
67-47-0
5-hydroxymethyl-2-furfuraldehyde

-
B
-
1917-65-3
5-(ethoxymethyl)furfural

-
C
-
539-88-8
4-oxopentanoic acid ethyl ester

| Conditions | Yield |
|---|---|
| With SO3H-CD carbon In tetrahydrofuran at 120℃; for 6h; Catalytic behavior; Temperature; Time; Solvent; Reagent/catalyst; Sonication; | A n/a B 74% C n/a |
| With alkaline lignin acidic carbonaceous catalyst at 110℃; for 15h; Sealed tube; | A 7.4% B 55.9% C 8.1% |
| With sulfuric acid at 110℃; for 15h; Sealed tube; | A 8.4% B 49.8% C 7.3% |


| Conditions | Yield |
|---|---|
| With divinylbenzene polymer with acid ionic liquid In ethanol at 150℃; under 760.051 Torr; for 12h; Solvent; Inert atmosphere; | 73% |

-
-
539-88-8
4-oxopentanoic acid ethyl ester

| Conditions | Yield |
|---|---|
| With niobium pentachloride | 72% |
-
-
67-47-0
5-hydroxymethyl-2-furfuraldehyde

-
-
64-17-5
ethanol

-
A
-
38641-99-5
2-(diethoxymethyl)-5-(ethoxymethyl)furan

-
B
-
1917-65-3
5-(ethoxymethyl)furfural

-
C
-
539-88-8
4-oxopentanoic acid ethyl ester

| Conditions | Yield |
|---|---|
| With Amberlyst-15 resin at 110℃; Sealed tube; | A 10% B 71% C 16% |
| With partially reduced graphene oxide (S-RGO) at 140℃; for 24h; Reagent/catalyst; Temperature; | A 12% B 58% C 30% |
| With Amberlyst-15 resin at 75℃; for 24h; Sealed tube; | A 27% B 52% C 8% |


| Conditions | Yield |
|---|---|
| With titanium(IV) oxide at 150℃; under 15001.5 Torr; for 3h; Inert atmosphere; Green chemistry; | 71% |
-
-
14794-31-1
ethyl 3-(chloroformyl)propionate

-
-
75-16-1
methylmagnesium bromide

-
-
539-88-8
4-oxopentanoic acid ethyl ester

| Conditions | Yield |
|---|---|
| Stage #1: methylmagnesium bromide With tributylphosphine In tetrahydrofuran at 20℃; Stage #2: ethyl 3-(chloroformyl)propionate In tetrahydrofuran at -40℃; for 0.333333h; | 70% |
-
-
539-88-8
4-oxopentanoic acid ethyl ester

-
-
99631-16-0
ethyl (S)-4-hydroxypentanoate

| Conditions | Yield |
|---|---|
| With magnesium chloride In isopropyl alcohol at 25℃; pH=7; Catalytic behavior; | 100% |
| With asymmetric dehydrogenase enzyme; NADPH4 at 20℃; for 24h; pH=6.8 - 7.2; Inert atmosphere; Enzymatic reaction; enantioselective reaction; | 74% |
| With recombinant alcohol dehydrogenase from Stenotrophomonas maltophilia SmADH2; isopropyl alcohol; NADH In aq. phosphate buffer at 30℃; pH=7; Green chemistry; Enzymatic reaction; stereoselective reaction; | n/a |
-
-
13175-68-3
1,2-bis (triethylsilyloxy) ethane

-
-
539-88-8
4-oxopentanoic acid ethyl ester

-
-
941-43-5
Ethyl levulinate ethylene ketal

| Conditions | Yield |
|---|---|
| With trimethylsilyl trifluoromethanesulfonate In dichloromethane at -78℃; for 4.5h; | 100% |
-
-
542-92-7
cyclopenta-1,3-diene

-
-
539-88-8
4-oxopentanoic acid ethyl ester

-
-
1190404-31-9
ethyl 4-(2,4-cyclopentadien-1-ylidene)valerate

| Conditions | Yield |
|---|---|
| Stage #1: cyclopenta-1,3-diene; 4-oxopentanoic acid ethyl ester With pyrrolidine In methanol at 20℃; Inert atmosphere; Stage #2: With acetic acid In methanol for 0.15h; | 100% |
-
-
539-88-8
4-oxopentanoic acid ethyl ester

-
-
62-53-3
aniline

-
-
6724-71-6
5-methyl-1-phenylpyrrolidin-2-one

| Conditions | Yield |
|---|---|
| With platinum doped titanium oxide; hydrogen In neat (no solvent) at 150℃; under 3750.38 Torr; for 18h; Time; Temperature; Pressure; chemoselective reaction; | 100% |
| With platinum doped titanium oxide; hydrogen In neat (no solvent) at 450℃; under 7500.75 Torr; Kinetics; Reagent/catalyst; Calcination; chemoselective reaction; | |
| Multi-step reaction with 2 steps 1: / neat (no solvent) / Green chemistry 2: hydrogen; / neat (no solvent) / 12 h / 85 °C / 760.05 Torr / Green chemistry View Scheme |

| Conditions | Yield |
|---|---|
| Stage #1: 4-oxopentanoic acid ethyl ester; adenosine With hydrogenchloride; orthoformic acid triethyl ester In 1,4-dioxane; N,N-dimethyl-formamide at 20℃; Stage #2: With calf intestine adenosine deaminase In water; glycerol at 20℃; for 72h; Enzymatic reaction; | 100% |
-
-
57-55-6
propylene glycol

-
-
539-88-8
4-oxopentanoic acid ethyl ester

-
-
5413-49-0
2,4-dimethyl-1,3-dioxolane-2-propionic acid ethyl ester

| Conditions | Yield |
|---|---|
| sulfuric acid In water at 95 - 106℃; under 30 - 80 Torr; Product distribution / selectivity; | 99.8% |
| With sulfuric acid at 110 - 170℃; under 10 - 15 Torr; |
-
-
141-43-5
ethanolamine

-
-
539-88-8
4-oxopentanoic acid ethyl ester

-
-
136749-84-3
(7aRS)-5-oxo-7a-methyl-2,3,5,6,7,7a-hexahydropyrrolo<2,1-b>oxazole

| Conditions | Yield |
|---|---|
| With 3 A molecular sieve In methanol for 48h; Ambient temperature; | 99% |
| In toluene Heating; | 53% |
| With potassium carbonate In toluene for 36h; Heating; | 40% |
| In toluene at 110℃; for 24h; |

| Conditions | Yield |
|---|---|
| With iron(II) triflate; formic acid; tris(2-diphenylphosphinoethyl)phosphine In 1,4-dioxane at 140℃; for 24h; Reagent/catalyst; Temperature; | 99% |
| With hydrogen In ethanol at 200℃; under 45004.5 Torr; for 6h; Reagent/catalyst; Temperature; Pressure; Solvent; Time; Autoclave; | 99% |
| With formic acid; triphenylphosphine In tetrahydrofuran at 160℃; for 8h; | 99% |

-
-
539-88-8
4-oxopentanoic acid ethyl ester

| Conditions | Yield |
|---|---|
| With L-proline In dimethyl sulfoxide at 20℃; for 168h; Mannich Aminomethylation; enantioselective reaction; | 99% |
-
-
107-21-1
ethylene glycol

-
-
539-88-8
4-oxopentanoic acid ethyl ester

-
-
941-43-5
Ethyl levulinate ethylene ketal

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In benzene for 18h; Heating; | 98% |
| With toluene-4-sulfonic acid In benzene Inert atmosphere; | 98% |
| Stage #1: ethylene glycol With toluene-4-sulfonic acid In benzene for 1h; Reflux; Dean-Stark; Inert atmosphere; Stage #2: 4-oxopentanoic acid ethyl ester In benzene for 18h; Reflux; Dean-Stark; Inert atmosphere; | 98% |

| Conditions | Yield |
|---|---|
| With methanol; sodium tetrahydroborate In tetrahydrofuran at 0 - 5℃; for 2h; | 98% |
| With sodium tetrahydroborate In tetrahydrofuran; water | 95% |
| With nickel at 100℃; under 88260.9 Torr; Hydrogenation; |

| Conditions | Yield |
|---|---|
| With platinum doped titanium oxide; hydrogen In neat (no solvent) at 120℃; under 7500.75 Torr; for 2h; chemoselective reaction; | 98% |
| With platinum on carbon; hydrogen In methanol at 25℃; for 10h; | 93% |
| Multi-step reaction with 2 steps 1: / neat (no solvent) / Green chemistry 2: hydrogen; / neat (no solvent) / 12 h / 85 °C / 760.05 Torr / Green chemistry View Scheme |
-
-
539-88-8
4-oxopentanoic acid ethyl ester

-
-
126-30-7
2,2-Dimethyl-1,3-propanediol

-
-
16837-24-4
3-(2,5,5-trimethyl-[1,3]dioxan-2-yl)-propionic acid ethyl ester

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid; orthoformic acid triethyl ester In dichloromethane at 20℃; for 19h; Inert atmosphere; | 98% |
| With toluene-4-sulfonic acid; orthoformic acid triethyl ester In dichloromethane Inert atmosphere; | 98% |

| Conditions | Yield |
|---|---|
| With hydrogen In hexane at 180℃; for 4h; Temperature; Reagent/catalyst; Solvent; Autoclave; | 98% |
| Multi-step reaction with 2 steps 1: hydrogen / 1,4-dioxane / 2 h / 190 °C / 30003 Torr / Autoclave 2: hydrogen / 1,4-dioxane / 5 h / 190 °C / 30003 Torr / Autoclave View Scheme | |
| Multi-step reaction with 2 steps 1: hydrogen / 1,4-dioxane / 2 h / 190 °C / 30003 Torr / Autoclave 2: hydrogen / 1,4-dioxane / 5 h / 190 °C / 30003 Torr / Autoclave View Scheme |

-
-
539-88-8
4-oxopentanoic acid ethyl ester

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid; orthoformic acid triethyl ester for 5h; | 97% |
-
-
1184873-21-9
N-(4-methoxyphenyl)-N-[1-(2-phenylethyl)-piperidin-4-yl]-hydrazine

-
-
539-88-8
4-oxopentanoic acid ethyl ester

-
-
1184873-27-5
C27H37N3O3

| Conditions | Yield |
|---|---|
| With acetic acid In toluene Reflux; | 96.1% |
-
-
1184873-19-5
N-[1-(2-phenylethyl)-piperidin-4-yl]-N-phenyl-hydrazine

-
-
539-88-8
4-oxopentanoic acid ethyl ester

-
-
1184873-24-2
C26H35N3O2

| Conditions | Yield |
|---|---|
| With acetic acid In toluene Reflux; | 95.2% |

| Conditions | Yield |
|---|---|
| With hydrogen; 5%-palladium/activated carbon at 25 - 150℃; under 4657.97 Torr; for 1h; Product distribution / selectivity; | 95.01% |

| Conditions | Yield |
|---|---|
| Stage #1: 4-oxopentanoic acid ethyl ester With cesium hydroxide; phenylsilane In 2-methyltetrahydrofuran at 25℃; for 6h; Green chemistry; Stage #2: With ethanol In 2-methyltetrahydrofuran at 80℃; for 2h; Kinetics; Green chemistry; chemoselective reaction; | 95% |
| With copper chromite at 250℃; under 147102 Torr; Hydrogenation; | |
| With lithium aluminium tetrahydride In diethyl ether |
-
-
6089-04-9
3-(tetrahydropyran-2'-yloxy)propyne

-
-
539-88-8
4-oxopentanoic acid ethyl ester

-
-
167169-59-7
(5R*S*) 5-methyl-5-<3-<(tetrahydro-2H-pyran-2-yl)oxy>prop-1-ynyl> dihydrofuran-2-(3H)-one

| Conditions | Yield |
|---|---|
| With hydrogenchloride; n-butyllithium In tetrahydrofuran; hexane 1.) RT, 30 min; 2.) -20 deg C, 15 h; 3.) 0 deg C; | 95% |
| With n-butyllithium; chloro-trimethyl-silane 1) THF, hexane, -78 deg C, 15 min then RT, 1 h, 2) THF, hexane, -78 deg C then -78 deg C to RT, 1 h, 3) THF, hexane, -78 deg C to RT, 20 h; Yield given. Multistep reaction; |

| Conditions | Yield |
|---|---|
| With ammonium acetate In neat (no solvent) at 55℃; for 2.5h; Green chemistry; | 95% |


| Conditions | Yield |
|---|---|
| With platinum doped titanium oxide; hydrogen In neat (no solvent) at 120℃; under 7500.75 Torr; for 2h; chemoselective reaction; | 94% |
| With palladium on activated charcoal; ethanol at 60℃; Hydrogenation.Beim 6-taegigen Hydrieren; | |
| Multi-step reaction with 2 steps 1: / neat (no solvent) / Green chemistry 2: hydrogen; / neat (no solvent) / 12 h / 85 °C / 760.05 Torr / Green chemistry View Scheme |
-
-
693-03-8
n-butyl magnesium bromide

-
-
539-88-8
4-oxopentanoic acid ethyl ester

-
-
3285-00-5
dihydro-5-butyl-5-methyl-2-(3H)-furanone

| Conditions | Yield |
|---|---|
| In diethyl ether; benzene at -5 - 0℃; for 0.25h; | 94% |
| With diethyl ether; benzene |
-
-
539-88-8
4-oxopentanoic acid ethyl ester

-
-
122-51-0
orthoformic acid triethyl ester

-
-
92557-39-6
ethyl 4,4-diethoxypentanoate

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In ethanol at 145℃; under 3750.38 Torr; for 2h; | 94% |
| With sulfuric acid |
-
-
539-88-8
4-oxopentanoic acid ethyl ester

-
-
122-51-0
orthoformic acid triethyl ester

-
A
-
92557-39-6
ethyl 4,4-diethoxypentanoate

-
B
-
109-94-4
formic acid ethyl ester

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In ethanol at 145℃; ketalization; | A 94% B n/a |


| Conditions | Yield |
|---|---|
| With ammonium acetate In neat (no solvent) at 55℃; for 3h; Green chemistry; | 94% |

Ethyl levulinate Specification
The Ethyl levulinate, with the CAS registry number 539-88-8, is also known as Levulinic acid, ethyl ester. It belongs to the product category of Pharmaceutical Intermediates. Its EINECS number is 208-728-2. This chemical's molecular formula is C7H12O3 and molecular weight is 144.17. What's more, its systematic name is Ethyl 4-oxopentanoate. Its classification codes are: (1)Natural Product; (2)Skin / Eye Irritant. This chemical should be sealed and stored in a cool, ventilated and dry place. Moreover, it should be protected from acids, heat and fire.
Physical properties of Ethyl levulinate are: (1)ACD/LogP: 0.4; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 0.40; (4)ACD/LogD (pH 7.4): 0.40; (5)ACD/BCF (pH 5.5): 1.19; (6)ACD/BCF (pH 7.4): 1.19; (7)ACD/KOC (pH 5.5): 39.34; (8)ACD/KOC (pH 7.4): 39.34; (9)#H bond acceptors: 3; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 5; (12)Polar Surface Area: 43.37 Å2; (13)Index of Refraction: 1.415; (14)Molar Refractivity: 36.283 cm3; (15)Molar Volume: 144.685 cm3; (16)Polarizability: 14.384×10-24cm3; (17)Surface Tension: 30.7 dyne/cm; (18)Density: 0.996 g/cm3; (19)Flash Point: 77.919 °C; (20)Enthalpy of Vaporization: 44.174 kJ/mol; (21)Boiling Point: 205.499 °C at 760 mmHg; (22)Vapour Pressure: 0.2 mmHg at 25°C.
Preparation: this chemical can be prepared by 2-methoxy-2-methyl-cyclopropanecarboxylic acid ethyl ester at the ambient temperature. This reaction will need reagent conc. sulfuric acid and solvents acetone, H2O with the reaction time of 2 hours. The yield is about 93%.

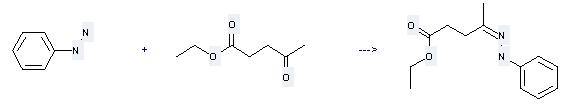
Uses of Ethyl levulinate: it can be used to produce 4-phenylhydrazono-valeric acid ethyl ester by heating. The reaction time is 2 hours. The yield is about 89%.
When you are using this chemical, please be cautious about it as the following:
This chemical is irritating to eyes, respiratory system and skin. When using it, you must avoid contact with skin and eyes.
You can still convert the following datas into molecular structure:
(1)SMILES: O=C(C)CCC(=O)OCC
(2)Std. InChI: InChI=1S/C7H12O3/c1-3-10-7(9)5-4-6(2)8/h3-5H2,1-2H3
(3)Std. InChIKey: GMEONFUTDYJSNV-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| rabbit | LD50 | skin | > 5gm/kg (5000mg/kg) | Food and Chemical Toxicology. Vol. 20, Pg. 679, 1982. | |
| rat | LD50 | oral | > 5gm/kg (5000mg/kg) | Food and Chemical Toxicology. Vol. 20, Pg. 679, 1982. |
Related Products
- Ethyl (13-cis)-9-(4-methoxy-2,3,6-trimethylphenyl)-3,7-dimethyl-2,4,6,8-nonatetraenoate
- ethyl (1R,2R)-1-phenyl-2-(trideuteriomethylamino)cyclohex-3-ene-1-carboxylate,hydrochloride
- Ethyl (1S,2R)-2-(dimethylamino)-1-phenylcyclohex-3-ene-1-carboxylate hydrochloride
- Ethyl (2,4,6-trimethylbenzoyl) phenylphosphinate
- Ethyl (2-amino-4-hydroxy-6-methyl-5-pyrimidinyl)acetate
- Ethyl (2-bromopropionamido)acetate
- Ethyl (2-cyanoimino-5,6-dichloro-1,2,3,4-tetrahydroquinazolin-3-yl)acetate
- ETHYL (2E,4Z)-DECADIENOATE
- Ethyl (2-hydroxyethyl)dimethyl-ammonium benzilate chloride
- Ethyl (2-mercaptoethyl) carbamate S-ester with O,O-dimethyl phosphorodithioate
- 539-90-2
- 5399-03-1
- 53990-33-3
- 5399-18-8
- 5399-20-2
- 5399-21-3
- 5399-22-4
- 539-92-4
- 5399-27-9
- 539-93-5
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View