-
Name
Ethylbutylamine
- EINECS 236-415-0
- CAS No. 13360-63-9
- Article Data38
- CAS DataBase
- Density 0.74
- Solubility partly soluble
- Melting Point -77.8oC
- Formula C6H15 N
- Boiling Point 108 ºC
- Molecular Weight 101.192
- Flash Point 18 ºC
- Transport Information UN 2733
- Appearance A colorless liquid with an ammonia-like odor.
- Safety A poison by ingestion. When heated to decomposition it emits toxic vapors of NOx.
- Risk Codes R11;R22;R34
-
Molecular Structure
-
Hazard Symbols


- Synonyms Butylamine,N-ethyl- (6CI,7CI,8CI); Butylethylamine; Ethylbutylamine; N-Butyl-N-ethylamine;N-Butylethylamine; N-Ethyl-1-butanamine; N-Ethyl-N-butylamine;N-Ethylbutanamine; N-Ethylbutylamine
- PSA 12.03000
- LogP 1.78690
Synthetic route

-
-
13360-63-9
N-ethylbutylamine

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal In methanol at 20℃; for 16h; | 80% |
-
-
122-56-5
tributyl borane

-
-
81375-29-3
O-(p-toluenesulfonyl)-N-ethylhydroxylamine

-
-
13360-63-9
N-ethylbutylamine

| Conditions | Yield |
|---|---|
| In tetrahydrofuran for 2h; Ambient temperature; | 77% |
-
-
917-54-4
methyllithium

-
-
33704-65-3
Butyl-phenylsulfanylmethyl-amine; hydrochloride

-
-
13360-63-9
N-ethylbutylamine

| Conditions | Yield |
|---|---|
| In diethyl ether at -60℃; | 76% |
-
-
75-04-7
ethylamine

-
-
109-73-9
N-butylamine

-
A
-
102-82-9
tributyl-amine

-
B
-
13360-63-9
N-ethylbutylamine

-
C
-
4444-68-2
N,N-diethylbutylamine

-
D
-
4458-33-7
di-n-butylethylamine

-
E
-
111-92-2
dibutylamine

-
F
-
109-89-7
diethylamine

-
G
-
121-44-8
triethylamine

| Conditions | Yield |
|---|---|
| With hydrogen at 200℃; under 6000.6 Torr; Reagent/catalyst; Temperature; | A n/a B 60.7% C n/a D n/a E n/a F n/a G n/a |


| Conditions | Yield |
|---|---|
| With hydrogen at 150℃; under 3750.38 Torr; for 8h; Temperature; Pressure; Reagent/catalyst; | 55.3% |
| With hydrogen; nickel at 150℃; |
-
-
109-74-0
propyl cyanide

-
-
64-17-5
ethanol

-
A
-
13360-63-9
N-ethylbutylamine

-
B
-
4853-56-9
(butylidene)butylamine

-
C
-
109-73-9
N-butylamine

| Conditions | Yield |
|---|---|
| With Co(9.8%)/SiO2 catalyst; hydrogen at 129.84℃; under 9750.98 Torr; Reagent/catalyst; Temperature; | A 30.5% B 25.6% C 42.3% |

-
-
14925-83-8
N,N-dichloro-n-butylamine

-
-
557-20-0
diethylzinc

-
A
-
13360-63-9
N-ethylbutylamine

-
B
-
109-73-9
N-butylamine

| Conditions | Yield |
|---|---|
| With Petroleum ether |

-
-
1611-12-7
ethyl-butyliden-amine

-
-
13360-63-9
N-ethylbutylamine

| Conditions | Yield |
|---|---|
| With nickel; Petroleum ether at 75℃; under 154457 Torr; Hydrogenation; | |
| With ethanol; sodium | |
| With ethanol; platinum Hydrogenation; |
-
-
77228-69-4
N-benzyl-N-ethylbutan-1-amine

-
-
13360-63-9
N-ethylbutylamine

| Conditions | Yield |
|---|---|
| With platinum(IV) oxide; acetic acid at 65 - 75℃; Hydrogenolyse; |

| Conditions | Yield |
|---|---|
| With ethanol; platinum Hydrogenation; | |
| (i), (ii) H2, Pd-C, EtOH; Multistep reaction; | |
| With potassium hydroxide; dipotassium hydrogenphosphate; phosphoric acid at 10 - 12℃; for 4h; electrochemical reductive amination, lead cathode, current density 0.05 A/cm2, pH 12; Yield given; |
-
-
75-04-7
ethylamine

-
-
123-73-9
crotonaldehyde

-
A
-
32280-46-9
N,N'-Diaethyl-1-methyl-1,3-propandiamin

-
B
-
13360-63-9
N-ethylbutylamine

| Conditions | Yield |
|---|---|
| With methanol; nickel at 125℃; Hydrogenation; |

-
-
75-04-7
ethylamine

-
-
123-73-9
crotonaldehyde

-
A
-
13360-63-9
N-ethylbutylamine

-
B
-
4444-68-2
N,N-diethylbutylamine

| Conditions | Yield |
|---|---|
| With methanol; nickel at 125℃; Hydrogenation; |


| Conditions | Yield |
|---|---|
| With nickel |

-
-
6898-74-4
Acetaldehyd-butylimin

-
-
13360-63-9
N-ethylbutylamine

| Conditions | Yield |
|---|---|
| With ethanol; platinum Hydrogenation; |

| Conditions | Yield |
|---|---|
| (i) aq. NaOH, HSO4, (ii) HCl; Multistep reaction; |
-
-
26683-07-8
butyl-ethyl-tetrahydropyran-2-yl-amine

-
-
13360-63-9
N-ethylbutylamine

| Conditions | Yield |
|---|---|
| (hydrolysis); |

| Conditions | Yield |
|---|---|
| With N,N,N,N,-tetramethylethylenediamine at 130 - 150℃; under 183877 Torr; |
-
-
54944-19-3
phenyl-trichloromethyl-phosphinic acid ethyl ester

-
-
109-73-9
N-butylamine

-
-
13360-63-9
N-ethylbutylamine

| Conditions | Yield |
|---|---|
| In xylene for 6h; Heating; | 72 % Chromat. |
| In xylene for 6h; Rate constant; Heating; | 72 % Chromat. |
-
-
102711-05-7
C24H29NP(1+)*I(1-)

-
-
13360-63-9
N-ethylbutylamine

| Conditions | Yield |
|---|---|
| With water; hydroxide |

-
-
13360-63-9
N-ethylbutylamine

| Conditions | Yield |
|---|---|
| With sodium hydroxide 1.) CH2Cl2, -20 deg C, 10 min, 2.) room temperature, 30 min, 3.) 70 deg C, 1 h; Yield given. Multistep reaction; |

-
-
13360-63-9
N-ethylbutylamine

| Conditions | Yield |
|---|---|
| With Li{AlH4} |
-
-
110-89-4
piperidine

-
-
885663-12-7
C14H21NO

-
A
-
13707-23-8
N-(4-methylbenzoyl)piperidine

-
B
-
13360-63-9
N-ethylbutylamine

| Conditions | Yield |
|---|---|
| With Al2(NMe2)6 In toluene at 90℃; for 16h; Equilibrium constant; |

-
-
885663-12-7
C14H21NO

-
-
103-67-3
benzyl-methyl-amine

-
A
-
13360-63-9
N-ethylbutylamine

-
B
-
57409-40-2
4-methyl-N-benzyl-N-methylbenzamide

| Conditions | Yield |
|---|---|
| With Al2(NMe2)6 In toluene at 90℃; for 16h; Equilibrium constant; |

-
-
13360-63-9
N-ethylbutylamine

-
-
287721-05-5
4-[phenyl(endo-8-methyl-8-azabicyclo[3.2.1]octan-3-yl)amino]benzoic acid

| Conditions | Yield |
|---|---|
| With N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate Acylation; | 100% |
-
-
13360-63-9
N-ethylbutylamine

-
-
1286315-70-5
N-[3-(trimethylamino)methyl-4-hydroxyphenyl]-1,8-naphthalimide iodide

-
-
1286315-76-1
N-[3-(butylethylamino)methyl-4-hydroxyphenyl]-1,8-naphthalimide

| Conditions | Yield |
|---|---|
| In water; acetonitrile at 25℃; for 0.166667h; Quantum yield; Inert atmosphere; Irradiation; | 100% |
-
-
13360-63-9
N-ethylbutylamine

-
-
175140-70-2
4-N-(2,4,6-trimethylphenyl)-6-chloro-2-methyl-5-nitropyrimidin-4-amine

-
-
175139-13-6
4-N-(2,4,6-trimethylphenyl)-6-(N-n-butylethylamino)-2-methyl-5-nitropyrimidin-4-amine

| Conditions | Yield |
|---|---|
| With triethylamine In acetone Ambient temperature; | 98% |
-
-
75-15-0
carbon disulfide

-
-
13360-63-9
N-ethylbutylamine

-
-
163594-66-9
(N-ethyl-N'-n-butyldithiocarbamato) sodium

| Conditions | Yield |
|---|---|
| Stage #1: N-ethylbutylamine With sodium hydroxide In methanol; water at 0℃; for 0.333333h; Stage #2: carbon disulfide In methanol; water at 20℃; | 98% |
| Stage #1: N-ethylbutylamine With sodium hydroxide In methanol; water at 0℃; for 0.333333h; Stage #2: carbon disulfide In methanol; water at 0 - 20℃; | 98% |
| With sodium hydroxide In water | 98% |
| With sodium hydroxide In water for 2h; Cooling with ice; |
-
-
1945-84-2
2-ethynylpyridine

-
-
13360-63-9
N-ethylbutylamine

| Conditions | Yield |
|---|---|
| With ethylene glycol at 150℃; for 8h; Inert atmosphere; Sealed tube; Green chemistry; regioselective reaction; | 98% |
-
-
13360-63-9
N-ethylbutylamine

-
-
169882-29-5
3-[2-bromo-4-(1-methylethyl)phenyl]-7-chloro-5-methyl-3H-1,2,3-triazolo[4,5-d]pyrimidine

| Conditions | Yield |
|---|---|
| With triethylamine In ethanol Heating; | 97% |
-
-
13360-63-9
N-ethylbutylamine


| Conditions | Yield |
|---|---|
| With dmap In dichloromethane at 20℃; for 16h; | 91% |
-
-
13360-63-9
N-ethylbutylamine

-
-
672953-00-3
[bis-(ethylbutylamino)-dimethylguanidinium] chloride

| Conditions | Yield |
|---|---|
| Stage #1: N-ethylbutylamine; dichloromethylenedimethyliminium chloride With triethylamine In dichloromethane at 0 - 20℃; for 4h; Stage #2: With sodium hydroxide In water Stage #3: With hydrogenchloride In diethyl ether; water pH=7; | 91% |

-
-
13360-63-9
N-ethylbutylamine

| Conditions | Yield |
|---|---|
| Stage #1: N,N'-bis[3-(trimethylammonio)methyl-4-hydroxyphenyl]-1,4,5,8-naphthalenetetracarboxylic diimide diiodide; N-ethylbutylamine With sodium dithionite In water; acetonitrile at 25℃; for 0.5h; Stage #2: With air In water; acetonitrile for 0.166667h; cooling; Further stages.; | 90% |
-
-
2579-22-8
Phenylpropargyl aldehyde

-
-
13360-63-9
N-ethylbutylamine

| Conditions | Yield |
|---|---|
| In ethanol at 60℃; for 0.25h; regioselective reaction; | 90% |
-
-
13360-63-9
N-ethylbutylamine

-
-
157286-81-2
4-chloro-2,5-dimethyl-7-(2,4,6-trimethylphenyl)-7H-pyrrolo-[2,3-d]pyrimidine

| Conditions | Yield |
|---|---|
| 89% | |
| In dimethyl sulfoxide at 130℃; for 3h; | 88% |
-
-
75-15-0
carbon disulfide

-
-
13360-63-9
N-ethylbutylamine

-
-
10025-82-8
indium(III) chloride

-
-
878385-08-1
tris(N,N-ethylbutyldithiocarbamato)indium(III)

| Conditions | Yield |
|---|---|
| With sodium hydroxide In methanol NaOH and N,N-ethylbutylamine were dissplved in MeOH, CS2 was dropped slowly at 0°C for 1 h, InCl3 was added; ppt. was filtered, dtied in vacuo, and recrystd.; elem. anal.; | 89% |

-
-
13360-63-9
N-ethylbutylamine

-
-
197803-09-1
7-chloro-5-methyl-3-(2,4,6-trimethyl-pyridin-3-yl)-3H-[1,2,3]triazolo[4,5-d]pyrimidine

| Conditions | Yield |
|---|---|
| With triethylamine In ethanol Heating; | 86% |
-
-
110-52-1
1,4-dibromo-butane

-
-
13360-63-9
N-ethylbutylamine

-
-
90076-65-6
bis(trifluoromethane)sulfonimide lithium

-
-
1119616-46-4
N-butyl-N-ethylpyrrolidinium bis(trifluoromethanesulfonyl)imide

| Conditions | Yield |
|---|---|
| Stage #1: 1,4-dibromo-butane; N-ethylbutylamine With potassium carbonate In water at 120℃; for 0.333333h; Autoclave; Microwave irradiation; Green chemistry; Stage #2: bis(trifluoromethane)sulfonimide lithium In water at 20℃; for 1h; Green chemistry; | 86% |

-
-
111-24-0
1,5-dibromo-pentane

-
-
13360-63-9
N-ethylbutylamine

-
-
90076-65-6
bis(trifluoromethane)sulfonimide lithium

-
-
1174233-40-9
N-butyl-N-ethylpiperidinium bis(trifluoromethanesulfonyl)imide

| Conditions | Yield |
|---|---|
| Stage #1: 1,5-dibromo-pentane; N-ethylbutylamine With potassium carbonate In water at 120℃; for 0.666667h; Autoclave; Microwave irradiation; Green chemistry; Stage #2: bis(trifluoromethane)sulfonimide lithium In water at 20℃; for 1h; Green chemistry; | 86% |

-
-
13360-63-9
N-ethylbutylamine

| Conditions | Yield |
|---|---|
| In acetonitrile Reflux; | 86% |

| Conditions | Yield |
|---|---|
| 85% |

| Conditions | Yield |
|---|---|
| With tetrakis(actonitrile)copper(I) hexafluorophosphate In tetrahydrofuran at 20℃; for 18h; Inert atmosphere; diastereoselective reaction; | 85% |
-
-
13360-63-9
N-ethylbutylamine

-
-
40307-11-7
1-ethyl-4-ethynylbenzene

| Conditions | Yield |
|---|---|
| With ethylene glycol at 150℃; for 8h; Inert atmosphere; Sealed tube; Green chemistry; regioselective reaction; | 85% |
-
-
13360-63-9
N-ethylbutylamine

-
-
557099-37-3
2-chloro-1,3-dinitro-5-(trifluoromethylthio)benzene

| Conditions | Yield |
|---|---|
| In ethanol for 0.5h; | 84% |
| In methanol for 0.5h; Heating; | 84% |
-
-
13360-63-9
N-ethylbutylamine

-
-
842158-34-3
1-chloro-2,6-dinitrobenzene-4-(pentafluorosulfanyl)benzene

| Conditions | Yield |
|---|---|
| In ethanol for 0.5h; | 84% |
Ethylbutylamine Chemical Properties
The chemical synonyms of are 1-Butanamine,N-ethyl-;butyl(ethyl)amine;Butylamine, N-ethyl-;Butylamine,N-ethyl-;Butylethylamine;N-Butylethylamine;N-Butyl-N-ethylamine;n-ethyl-1-butanamin .Its product categories is Amines;C2 to C6 and Nitrogen Compounds.The molecular structure of N-ETHYLBUTYLAMINE(13360-63-9) is
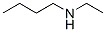 .
.Ethylbutylamine Uses
Ethylbutylamine Production
Ethylbutylamine Toxicity Data With Reference
| 1. | orl-rat LD50:310 mg/kg | GISAAA Gigiena i Sanitariya. 39 (3)(1974),106. | ||
| 2. | orl-mus LD50:418 mg/kg | GISAAA Gigiena i Sanitariya. 39 (3)(1974),106. |
Ethylbutylamine Consensus Reports
Ethylbutylamine Safety Profile
A poison by ingestion. When heated to decomposition it emits toxic vapors of NOx. Risk Codes:R11;R22;R34
Hazard codes is  F,
F,  C .
C .
Risk statements is 11-22-34 and safety statements is 16-26-36/37/39-45-33.
HazardClass is 3.
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View