-
Name
N-Methylacetamide
- EINECS 201-182-6
- CAS No. 79-16-3
- Article Data140
- CAS DataBase
- Density 0.87 g/cm3
- Solubility soluble in water
- Melting Point 26-28 °C(lit.)
- Formula C3H7NO
- Boiling Point 206 °C at 760 mmHg
- Molecular Weight 73.0947
- Flash Point 109.1 °C
- Transport Information
- Appearance colourless liquid or solid
- Safety 53-45
- Risk Codes 61
-
Molecular Structure
-
Hazard Symbols
 T
T
- Synonyms Methylacetamide;Monomethylacetamide;
- PSA 29.10000
- LogP 0.14320
Synthetic route

| Conditions | Yield |
|---|---|
| toluene-4-sulfonic acid at 50℃; for 1h; Beckmann rearrangement; | 100% |
| With bismuth(III) chloride for 0.166667h; Beckmann rearrangement; microwave irradiation; | 90% |
| With indium(III) triflate In acetonitrile for 2h; Beckmann rearrangement; Heating; | 90% |
-
-
3294-31-3
triethyl(methylamino)silane

-
-
2754-27-0
trimethylsilyl acetate

-
A
-
79-16-3
N-methyl-acetamide

-
B
-
107-46-0
Hexamethyldisiloxane

-
C
-
2652-41-7
1,1,1-triethyl-3,3,3-trimethyl-disiloxane

-
D
-
994-49-0
hexaethyl disiloxane

| Conditions | Yield |
|---|---|
| With N-Methylformamide at 50℃; for 14h; | A 100% B 21.9% C 33.9% D 36.2% |
| With N-Methylformamide at 50℃; for 14h; Product distribution; Mechanism; other amides as catalysts; | A 100% B 21.9% C 33.9% D 36.2% |

-
-
3294-31-3
triethyl(methylamino)silane

-
A
-
79-16-3
N-methyl-acetamide

-
B
-
107-46-0
Hexamethyldisiloxane

-
C
-
2652-41-7
1,1,1-triethyl-3,3,3-trimethyl-disiloxane

-
D
-
994-49-0
hexaethyl disiloxane

| Conditions | Yield |
|---|---|
| With N-Methylformamide; trimethylsilyl acetate at 50℃; for 14h; | A 100% B 21.9% C 33.9% D 36.2% |

-
-
2754-27-0
trimethylsilyl acetate

-
A
-
79-16-3
N-methyl-acetamide

-
B
-
107-46-0
Hexamethyldisiloxane

-
C
-
2652-41-7
1,1,1-triethyl-3,3,3-trimethyl-disiloxane

-
D
-
994-49-0
hexaethyl disiloxane

| Conditions | Yield |
|---|---|
| With N-Methylformamide; triethyl(methylamino)silane at 50℃; for 14h; | A 100% B 21.9% C 33.9% D 36.2% |


| Conditions | Yield |
|---|---|
| In methanol at 135℃; for 0.25h; Microwave irradiation; | 100% |

| Conditions | Yield |
|---|---|
| With aluminum oxide at 70℃; for 0.416667h; Neat (no solvent); | 98% |
| With magnesia In neat (no solvent) at 70℃; for 1h; Green chemistry; chemoselective reaction; | 98% |
| With 1-methyl-3-(4-sulfonylbutyl)-1H-imidazol-3-ium trifluoromethanesulfonate at 96 - 100℃; for 7h; Temperature; chemoselective reaction; | 90% |

| Conditions | Yield |
|---|---|
| With cyclohexene In dichloromethane at 15 - 20℃; Irradiation; | 97% |

| Conditions | Yield |
|---|---|
| With tetramethylammonium bromide In water at 80 - 110℃; for 10h; Reagent/catalyst; Autoclave; Large scale; | 96.3% |
| With water at 150℃; |
-
-
51094-87-2
N-bromo-N-methylacetamide

-
-
79-16-3
N-methyl-acetamide

| Conditions | Yield |
|---|---|
| With cyclohexene In dichloromethane at -70℃; Irradiation; | 95% |
-
-
130012-49-6
dimethyl N-thioethanoyl-N-methylphosphoramidate

-
A
-
79-16-3
N-methyl-acetamide

-
B
-
5310-10-1
N-methylthioacetamide

| Conditions | Yield |
|---|---|
| With water In tetrahydrofuran Ambient temperature; | A n/a B 94% |
-
-
100032-05-1
Acetic acid 2,4,4,6,6-pentamethoxy-2λ5,4λ5,6λ5-[1,3,5,2,4,6]triazatriphosphinin-2-yl ester

-
-
74-89-5
methylamine

-
A
-
79-16-3
N-methyl-acetamide

| Conditions | Yield |
|---|---|
| In tetrahydrofuran for 1h; Heating; | A 90% B 90% |

-
-
38100-40-2
N-acetyl-N-methyl-O-2,4-dinitrophenylhydroxylamine

-
-
79-16-3
N-methyl-acetamide

| Conditions | Yield |
|---|---|
| With cyclohexa-1,4-diene; potassium carbonate In acetone at 20℃; for 1.5h; Schlenk technique; Inert atmosphere; Irradiation; | 88% |
| With cyclohexa-1,4-diene; potassium carbonate; eosin y In acetone at 20℃; for 16h; Inert atmosphere; Irradiation; |
-
-
4381-85-5
2,5-diaza-3,4-dimethyl-2,4-hexadiene

-
A
-
79-16-3
N-methyl-acetamide

-
B
-
593-75-9, 685498-28-6
methyl isocyanate

| Conditions | Yield |
|---|---|
| With oxygen; rose bengal In acetonitrile at 13℃; for 9h; Irradiation; | A n/a B 87% |
| With oxygen; rose bengal In acetonitrile at 13℃; for 9h; Irradiation; | A 87% B n/a |

| Conditions | Yield |
|---|---|
| With sodium perchlorate Product distribution; Multistep reaction: 1.) acetonitrile, controlled potential electrolysis, 2.) H2O, acetonitrile, reflux, 1 h, var. N-hydroxy compounds,; | 82% |

| Conditions | Yield |
|---|---|
| With tetrabutylammonium periodite In dichloromethane at 20℃; for 1.08333h; | 82% |
| With 6H(1+)*Mo9O40PV3(6-); oxygen In acetonitrile at 60℃; under 760.051 Torr; for 2h; Inert atmosphere; Glovebox; | 54 %Chromat. |
-
-
79483-05-9
4,5-diethyl-2,5-dihydro-1,2,2,3-tetramethyl-1H-1,2,5-azasilaborole

-
A
-
79-16-3
N-methyl-acetamide

-
B
-
88636-30-0
4,5-diethyl-2,5-dihydro-2,2,3-trimethyl-1,2,5-oxasilaborole

| Conditions | Yield |
|---|---|
| With acetic acid In pentane Ar atmosphere, stirring (1 h, 20°C); cooling (-78°C), B-compound from pentane soln. (distn.); | A 82% B 81% |

| Conditions | Yield |
|---|---|
| In neat (no solvent) at 20℃; for 0.25h; Green chemistry; | 76% |
| With diethyl ether |
-
-
591-49-1
1-methylcyclohex-1-ene

-
-
5014-39-1
N-chloro-N-methylacetamide

-
A
-
79-16-3
N-methyl-acetamide

-
-
78162-71-7
N-(2-chloro-1-methylcyclohexyl)acetamide

| Conditions | Yield |
|---|---|
| In acetonitrile at 15 - 20℃; Irradiation; | A 76% B 16% |

-
-
15857-21-3
N,N'-diacetyl-N,N'-dimethyl-hydrazine

-
-
79-16-3
N-methyl-acetamide

| Conditions | Yield |
|---|---|
| With sodium In ammonium hydroxide for 1.5h; Heating; | 75% |

| Conditions | Yield |
|---|---|
| In chloroform for 1h; | 75% |

| Conditions | Yield |
|---|---|
| With dimethylbromosulphonium bromide at 80℃; for 2h; Beckmann rearrangement; Ionic liquid; | A 71% B n/a |
| With dimethylbromosulphonium bromide; zinc(II) chloride In acetonitrile for 2h; Beckmann rearrangement; Reflux; | A 60% B n/a |
| With phosphorus pentachloride; 1-butyl-3-methylimidazolium Tetrafluoroborate at 80℃; for 2h; Beckmann rearrangement; | A 66.8 % Chromat. B 33.2 % Chromat. |

| Conditions | Yield |
|---|---|
| With iodine at 20℃; for 0.0166667h; Neat (no solvent); | 70.87% |
-
-
5014-39-1
N-chloro-N-methylacetamide

-
-
110-83-8
cyclohexene

-
A
-
79-16-3
N-methyl-acetamide

-
-
24281-07-0, 33092-85-2, 35077-14-6, 53297-75-9
N-(trans-2-chlorocyclohexyl)acetamide

| Conditions | Yield |
|---|---|
| In acetonitrile at 15 - 20℃; Irradiation; | A 68% B 19% |

-
-
75288-12-9
N-(4-(dimethylamino)benzyl)-N-methylacetamide

-
-
79-16-3
N-methyl-acetamide

| Conditions | Yield |
|---|---|
| With water; trifluoroacetic acid In acetonitrile at 100℃; for 2h; Sealed tube; | 65% |
-
-
138610-12-5
3-(1-amino-2,2,2-trifluoroethylidene)pentane-2,4-dione

-
-
74-89-5
methylamine

-
A
-
79-16-3
N-methyl-acetamide

-
B
-
14092-14-9
4-(methylamino)-3-penten-2-one

| Conditions | Yield |
|---|---|
| In tetrahydrofuran for 0.5h; Cooling; | A 54% B 22% |

-
-
498-66-8
norborn-2-ene

-
-
5014-39-1
N-chloro-N-methylacetamide

-
A
-
79-16-3
N-methyl-acetamide

-
-
78174-14-8
N-((1R,2S,3R,4S)-3-Chloro-bicyclo[2.2.1]hept-2-yl)-N-methyl-acetamide

-
-
78174-14-8
N-((1R,2S,3S,4S)-3-Chloro-bicyclo[2.2.1]hept-2-yl)-N-methyl-acetamide

| Conditions | Yield |
|---|---|
| In dichloromethane at 15 - 20℃; Irradiation; Yield given. Yields of byproduct given; | A 52% B n/a C n/a |

-
-
105991-17-1
N-isopropyl-N'-methylacetamidinium chloride

-
A
-
79-16-3
N-methyl-acetamide

-
B
-
1118-69-0
N-isopropylacetamide

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water-d2 at 34℃; Product distribution; | A 52% B n/a |
-
-
127-19-5
N,N-dimethyl acetamide

-
-
2196-95-4, 17213-48-8
2,2'-diphenyl-[3,3']biindolylidene 1,1'-dioxide

-
A
-
79-16-3
N-methyl-acetamide

-
B
-
50-00-0
formaldehyd

-
C
-
5169-64-2
1,1'-dihydroxy-2,2'-diphenyl-3,3'-biindole

-
D
-
2415-33-0
2,2'-diphenyl-1H,1'H-3,3'-biindole

| Conditions | Yield |
|---|---|
| In benzene at 140℃; for 14h; | A 47% B n/a C n/a D 52% |
| In benzene at 140℃; for 14h; Product distribution; | A 47% B n/a C n/a D 52% |

-
-
2196-95-4, 17213-48-8
2,2'-diphenyl-[3,3']biindolylidene 1,1'-dioxide

-
A
-
79-16-3
N-methyl-acetamide

-
B
-
50-00-0
formaldehyd

-
C
-
5169-64-2
1,1'-dihydroxy-2,2'-diphenyl-3,3'-biindole

-
D
-
2415-33-0
2,2'-diphenyl-1H,1'H-3,3'-biindole

| Conditions | Yield |
|---|---|
| With N,N-dimethyl acetamide at 140℃; for 14h; | A 47% B n/a C n/a D 52% |


| Conditions | Yield |
|---|---|
| With chloro-trimethyl-silane for 2h; Heating; | 100% |
| With chloro-trimethyl-silane for 1h; Heating; | 64% |
| (i), (ii) PCl5, dioxane; Multistep reaction; |

| Conditions | Yield |
|---|---|
| With potassium carbonate In cyclohexane; water; ethyl acetate; toluene | 100% |
-
-
79-16-3
N-methyl-acetamide

-
-
1350349-87-9
4-(2,2,2-trifluoro-1-(3-(N-((2-(trimethylsilyl)ethoxy)methyl)propylsulfonamido)quinoxalin-2-yloxy)ethyl)pyridine 1-oxide

-
-
1350349-90-4
N-methyl-N-(4-(2,2,2-trifluoro-1-(3-(N-((2-(trimethylsilyl)ethoxy)methyl)propylsulfonamido)quinoxalin-2-yloxy)ethyl)pyridin-2-yl)acetamide

| Conditions | Yield |
|---|---|
| Stage #1: N-methyl-acetamide With 2,6-dimethylpyridine; oxalyl dichloride at 0℃; for 0.25h; Stage #2: 4-(2,2,2-trifluoro-1-(3-(N-((2-(trimethylsilyl)ethoxy)methyl)propylsulfonamido)quinoxalin-2-yloxy)ethyl)pyridine 1-oxide In dichloromethane at 20℃; for 18h; | 99% |

| Conditions | Yield |
|---|---|
| With cross-linked polyvinylpyrrolidone*N2O4; dinitrogen tetraoxide In dichloromethane at 20℃; for 3h; | 98% |
| With dinitrogen tetroxide impregnated on activated charcoal In dichloromethane at 20℃; for 3h; | 95% |
| With sodium perchlorate; cis-nitrous acid; acetic acid In water at 25℃; Kinetics; Mechanism; other catalysts, other solvent; influence of halides, basic catalysis, isotopic effect; |
-
-
79-16-3
N-methyl-acetamide

-
-
619-42-1
4-methoxycarbonylphenyl bromide

-
-
37619-13-9
N-(2-carbomethoxyphenyl)-N-methylacetamide

| Conditions | Yield |
|---|---|
| With palladium diacetate; caesium carbonate; 4,5-bis(diphenylphos4,5-bis(diphenylphosphino)-9,9-dimethylxanthenephino)-9,9-dimethylxanthene In 1,4-dioxane at 80℃; for 27h; Arylation; | 98% |
| With aluminium(III) triflate; caesium carbonate; 4,5-bis(diphenylphos4,5-bis(diphenylphosphino)-9,9-dimethylxanthenephino)-9,9-dimethylxanthene; bis(dibenzylideneacetone)-palladium(0) In toluene at 110℃; for 18h; Reagent/catalyst; | 89 %Spectr. |
-
-
79-16-3
N-methyl-acetamide

-
-
18157-20-5
chloro(diethoxy)(methyl)silane

| Conditions | Yield |
|---|---|
| With sodium methylate In toluene | 97.5% |


| Conditions | Yield |
|---|---|
| With aluminium trichloride In dichloromethane at 90℃; for 3.5h; | 97% |

| Conditions | Yield |
|---|---|
| With aluminium trichloride In dichloromethane at 90℃; for 3.5h; | 97% |
| With Nd2Na8(OCH2CF3)14(THF)6 at 120℃; for 10h; Inert atmosphere; Schlenk technique; | 90% |
| With 1-(3-sulfopropyl)pyridinium phosphotungstate In neat (no solvent) at 140℃; for 1.33333h; Microwave irradiation; | 77% |
| With water; boric acid at 150℃; for 24h; | 51% |
-
-
79-16-3
N-methyl-acetamide

-
-
623-00-7
4-bromobenzenecarbonitrile

-
-
99071-56-4
N-(4-cyanophenyl)-N-methylacetamide

| Conditions | Yield |
|---|---|
| With palladium diacetate; caesium carbonate; 4,5-bis(diphenylphos4,5-bis(diphenylphosphino)-9,9-dimethylxanthenephino)-9,9-dimethylxanthene In tetrahydrofuran at 45℃; for 42h; | 97% |
| With palladium diacetate; caesium carbonate; 4,5-bis(diphenylphos4,5-bis(diphenylphosphino)-9,9-dimethylxanthenephino)-9,9-dimethylxanthene In tetrahydrofuran at 45℃; for 42h; Arylation; | 97% |
-
-
79-16-3
N-methyl-acetamide

-
-
367967-44-0
2-[(E)-1-methyl-1-buten-1-yl]-4-methylaniline

| Conditions | Yield |
|---|---|
| With phosphorus pentachloride In chloroform at 80℃; for 2.5h; | 97% |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran | 97% |
-
-
79-16-3
N-methyl-acetamide

-
-
459-46-1
4-Fluorobenzyl bromide

-
-
86010-70-0
N-(4-fluorobenzyl)-N-methylacetamide

| Conditions | Yield |
|---|---|
| With sodium hydride In tetrahydrofuran; mineral oil at 19 - 40℃; | 97% |
| Stage #1: N-methyl-acetamide With sodium hydride In tetrahydrofuran Stage #2: 4-Fluorobenzyl bromide at 40℃; for 3h; |
-
-
79-16-3
N-methyl-acetamide

-
-
648893-53-2
2-formyl-4,6-dimethyl-1-[(1R)-1,2,3,4-tetrahydronaphthalen-1-yl]-1H-pyrrolo[2,3-b]pyridine-3-carbonitrile

| Conditions | Yield |
|---|---|
| Stage #1: 4,6-dimethyl-1-[(1R)-1,2,3,4-tetrahydronaphthalen-1-yl]-1H-pyrrolo[2,3-b]pyridine-3-carbonitrile With n-butyllithium; diisopropylamine In tetrahydrofuran; hexane at -78℃; for 0.75h; Stage #2: N-methyl-acetamide In tetrahydrofuran; hexane at 20℃; for 1.5h; | 96.3% |

| Conditions | Yield |
|---|---|
| With trichloroisocyanuric acid In dichloromethane at 20℃; for 1h; | 96% |
| With trichloroisocyanuric acid at 0 - 25℃; | 95% |
| With sodium hypochlorite; disodium hydrogenphosphate; potassium dihydrogenphosphate; sodium chloride In water at 24.9℃; Rate constant; Mechanism; Thermodynamic data; var. of conc., pH, temp., Ea, ΔH(excit.), ΔS(excit.); |
-
-
79-16-3
N-methyl-acetamide

-
-
54256-37-0
2-(ethylthio)acetyl chloride

-
-
221444-63-9
N-acetyl-2-ethylsulfenyl-N-methylacetamide

| Conditions | Yield |
|---|---|
| In benzene for 12h; Heating; | 96% |
-
-
79-16-3
N-methyl-acetamide

-
-
10403-47-1
2-bromo 5-nitroaniline

| Conditions | Yield |
|---|---|
| With triethylamine; trichlorophosphate In toluene for 18h; Heating; | 96% |
-
-
79-16-3
N-methyl-acetamide

-
-
648893-68-9
1-[(4-fluorophenyl)(phenyl)methyl]-2-formyl-4,6-dimethyl-1H-pyrrolo[2,3-b]pyridine-3-carbonitrile

| Conditions | Yield |
|---|---|
| Stage #1: 1-[(4-fluorophenyl)-(phenyl)methyl]-4,6-dimethyl-1H-pyrrolo[2,3-b]pyridine-3-carbonitrile With n-butyllithium; diisopropylamine In tetrahydrofuran; hexane; N,N-dimethyl-formamide at -78℃; for 0.75h; Stage #2: N-methyl-acetamide In tetrahydrofuran; hexane at 20℃; for 1.5h; | 95.4% |
-
-
79-16-3
N-methyl-acetamide

-
-
91-21-4
1,2,3,4-tetrahydroisoquinoline

-
-
14028-67-2
N-acetyl-1,2,3,4-tetrahydroisoquinoline

| Conditions | Yield |
|---|---|
| With aluminium trichloride In dichloromethane at 90℃; for 3h; | 95% |
-
-
79-16-3
N-methyl-acetamide

-
-
235779-09-6
6-(cyclopent-1-en-1-yl)-2-methyl-aniline

| Conditions | Yield |
|---|---|
| With phosphorus pentachloride In chloroform at 80℃; for 2.5h; | 94% |
-
-
79-16-3
N-methyl-acetamide

-
-
5356-71-8
1,3-diphenyl-1H-pyrazol-5-amine

-
-
1257533-49-5
N'-(1,3-diphenyl-1H-pyrazol-5-yl)-N-methylethanimidamide

| Conditions | Yield |
|---|---|
| With trichlorophosphate at 30 - 40℃; Microwave irradiation; | 94% |
N-Methylacetamide Chemical Properties
Molecule structure of N-Methylacetamide (CAS NO.79-16-3):
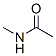
EINECS: 201-182-6
IUPAC Name: N-Methylacetamide
Molecular Weight: 73.09378 g/mol
Molecular Formula: C3H7NO
Surface Tension: 23.2 dyne/cm
Density: 0.87 g/cm3
Melting Point: 26-28 °C(lit.)
Flash Point: 109.1 °C
Water Solubility: soluble
Enthalpy of Vaporization: 44.22 kJ/mol
Boiling Point: 206 °C at 760 mmHg
Vapour Pressure: 0.243 mmHg at 25 °C
XLogP3: -1.1
H-Bond Donor: 1
H-Bond Acceptor: 1
Tautomer Count: 2
Exact Mass: 73.052764
MonoIsotopic Mass: 73.052764
Canonical SMILES: CC(=O)NC
InChI: InChI=1S/C3H7NO/c1-3(5)4-2/h1-2H3,(H,4,5)
InChIKey: OHLUUHNLEMFGTQ-UHFFFAOYSA-N
Classification Code: Mutation data; Reproductive Effect
N-Methylacetamide Uses
The N-Methylacetamide(79-16-3) is used as pesticide, medicine and other organic synthesis intermediates.
N-Methylacetamide Toxicity Data With Reference
| 1. | mmo-esc 10 g/L | CRSUBM Cancer Research Supplement 3 (1955),69. | ||
| 2. | orl-rat LD50:5000 mg/kg | JRPFA4 Journal of Reproduction and Fertility. 4 (1962),219. | ||
| 3. | ipr-rat LD50:2750 mg/kg | JRPFA4 Journal of Reproduction and Fertility. 4 (1962),219. | ||
| 4. | scu-rat LD50:3600 mg/kg | COREAF Comptes Rendus Hebdomadaires des Seances de lAcademie des Sciences. 251 (1960),1937. | ||
| 5. | ipr-mus LD50:4380 mg/kg | JPPMAB Journal of Pharmacy and Pharmacology. 16 (1964),472. | ||
| 6. | ivn-mus LD50:4015 mg/kg | JPPMAB Journal of Pharmacy and Pharmacology. 16 (1964),472. | ||
| 7. | ivn-rbt LDLo:16,940 mg/kg | ARZNAD Arzneimittel-Forschung. Drug Research. 20 (1970),1242. |
N-Methylacetamide Consensus Reports
Reported in EPA TSCA Inventory.
N-Methylacetamide Safety Profile
Moderately toxic by intraperitoneal and subcutaneous routes. Mildly toxic by ingestion and intravenous routes. An experimental teratogen. Experimental reproductive effects. Mutation data reported. When heated to decomposition it emits toxic fumes of NOx.
Hazard Codes:  T
T
Risk Statements: 61
R61:May cause harm to the unborn child ;
Safety Statements: 53-45
S53:Avoid exposure - obtain special instruction before use ;
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) ;
WGK Germany: 2
RTECS: AC5960000
HS Code: 29241900
Hazardous Substances Data: 79-16-3(Hazardous Substances Data)
N-Methylacetamide Specification
N-Methylacetamide (CAS NO.79-16-3) is also named as AI3-18019 ; Acetamide, N-methyl- ; Acetamide, methyl- ; Acetic acid, amide, N-methyl ; HSDB 94 ; Methylacetamide ; Monomethylacetamide ; N-Methylacetamide ; N-Monomethylacetamide ; NSC 747 ; X 44 . N-Methylacetamide (CAS NO.79-16-3) is colourless liquid or solid. It is stable and combustible but incompatible with strong oxidizing agents. It is toxic. It is flammable. It will produce toxic nitrogen oxide fumes when buring. So the storage environment should be ventilate, low-temperature and dry.
Related Products
- N-Methylacetamide
- 791642-68-7
- 791644-48-9
- 791644-59-2
- 791644-60-5
- 79165-06-3
- 79173-09-4
- 79173-38-9
- 79173-62-9
- 79-17-4
- 79175-35-2
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View