-
Name
Tetracycline
- EINECS 200-481-9
- CAS No. 60-54-8
- Article Data14
- CAS DataBase
- Density 1.644 g/cm3
- Solubility 1.7mg/mlL (28 °C) in water
- Melting Point 172-174 °C
- Formula C22H24N2O8
- Boiling Point 790.622 °C at 760 mmHg
- Molecular Weight 444.441
- Flash Point 431.953 °C
- Transport Information
- Appearance Light yellow crystal
- Safety 22-36-26
- Risk Codes 22-36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xn,
Xn,  Xi
Xi
- Synonyms 2-Naphthacenecarboxamide,4-(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,6,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-(7CI,8CI);Abramycin;Achromycin;Acromicina;Actisite;Agromicina;Ambramicina;Arcanacycline;Bio-Tetra;Biocycline;Ciclibion;Cyclomycin;Deschlorobiomycin;Florocycline;Gammatet;Ibicyn;Kinciclina;Latycin;Medocycline;Mericycline;Micycline;NSC 108579;Omegamycin;Orlycycline;Panmycin;Resteclin;Retet;Robitet Robicaps;Tetra-Co;Tetra-Proter;Tetracycline;Tetralan;Tetralen;Tetramig;
- PSA 181.62000
- LogP 0.48590
Synthetic route
-
-
102851-25-2
6-deoxy-6-hydroperoxy-5a,11a-dehydrotetracycline

-
-
60-54-8
TETRACYCLINE

| Conditions | Yield |
|---|---|
| With hydrogen; platinum In 1,4-dioxane under 2280 Torr; for 8h; Catalytic hydrogenation; | 62% |

| Conditions | Yield |
|---|---|
| With palladium on activated charcoal; 2-methoxy-ethanol; triethylamine Hydrogenation; | |
| With 1,4-dioxane; methanol; palladium on activated charcoal Hydrogenation; |

-
-
60-54-8
TETRACYCLINE

| Conditions | Yield |
|---|---|
| With water at 19.85℃; Kinetics; Further Variations:; Temperatures; |

-
-
60-54-8
TETRACYCLINE

| Conditions | Yield |
|---|---|
| With hydrogen; palladium In 1,4-dioxane at 23℃; under 760 Torr; for 2h; | 16.0 mg |

-
-
60-54-8
TETRACYCLINE

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: m-chloroperoxybenzoic acid / CH2Cl2 / -78 - 0 °C 1.2: air / CHCl3 2.1: 16.0 mg / H2 / Pd / dioxane / 2 h / 23 °C / 760 Torr View Scheme |

-
-
60-54-8
TETRACYCLINE

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: 77 percent / o-iodoxybenzoic acid / dimethylsulfoxide / 18 h / 35 °C 2.1: m-chloroperoxybenzoic acid / CH2Cl2 / -78 - 0 °C 2.2: air / CHCl3 3.1: 16.0 mg / H2 / Pd / dioxane / 2 h / 23 °C / 760 Torr View Scheme |

-
-
60-54-8
TETRACYCLINE

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: 76 percent / triethylamine trihydrofluoride / tetrahydrofuran / 12 h / 23 °C 2.1: 77 percent / o-iodoxybenzoic acid / dimethylsulfoxide / 18 h / 35 °C 3.1: m-chloroperoxybenzoic acid / CH2Cl2 / -78 - 0 °C 3.2: air / CHCl3 4.1: 16.0 mg / H2 / Pd / dioxane / 2 h / 23 °C / 760 Torr View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 16 steps 1.1: 80 percent / LDA / tetrahydrofuran / 0.25 h / -40 °C 2.1: 90 percent / SOCl2; TEA / CH2Cl2 / 0.17 h / -30 °C 3.1: BBr3 / CH2Cl2 / 0.25 h / -78 °C 4.1: H2; TEA / Pd-black / dioxane; H2O / 1 h 5.1: 72 percent / i-Pr2NEt / tetrahydrofuran; methanol / 2 h 6.1: 85 percent / Br2; (Bu3Sn)2O; MS-4A / CH2Cl2 / 0.25 h / -78 °C 7.1: 91 percent / Dess-Martin periodinane / acetonitrile; CH2Cl2 / 0.25 h 8.1: Zn / acetic acid / 0.03 h 9.1: Dess-Martin periodinane / acetonitrile; CH2Cl2 / 0.25 h 9.2: 60 percent / dimethyldioxirane; (R,R)-(PhCH-NTs)2BCl; TEA / CH2Cl2 / 0.5 h / -78 °C 10.1: NH2OH*HCl; TEA / methanol / 0.5 h 11.1: CDI / tetrahydrofuran / 0.75 h 12.1: 68 percent / polyposphoric acid / 0.75 h / 100 °C 13.1: 80 percent / formic acid / 1 h / 80 °C 14.1: 88 percent / BBr3 / CH2Cl2 / 15 h / 0 - 20 °C 15.1: 75 percent / O2; TPP / CHCl3 / 0.17 h / 20 - 40 °C / Irradiation 16.1: 62 percent / H2 / Pt-black / dioxane / 8 h / 2280 Torr View Scheme |
-
-
1665-56-1, 7518-17-4
anhydrotetracycline

-
-
60-54-8
TETRACYCLINE

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 75 percent / O2; TPP / CHCl3 / 0.17 h / 20 - 40 °C / Irradiation 2: 62 percent / H2 / Pt-black / dioxane / 8 h / 2280 Torr View Scheme |
-
-
286961-75-9
(4S,4aS,12aS)-4-Dimethylamino-3,11,12a-trihydroxy-10-methoxy-6-methyl-1,12-dioxo-1,4,4a,5,12,12a-hexahydro-naphthacene-2-carboxylic acid amide

-
-
60-54-8
TETRACYCLINE

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 88 percent / BBr3 / CH2Cl2 / 15 h / 0 - 20 °C 2: 75 percent / O2; TPP / CHCl3 / 0.17 h / 20 - 40 °C / Irradiation 3: 62 percent / H2 / Pt-black / dioxane / 8 h / 2280 Torr View Scheme |
-
-
286961-84-0
(4S,4aS,12aS)-4-Amino-3,11,12a-trihydroxy-10-methoxy-6-methyl-1,12-dioxo-1,4,4a,5,12,12a-hexahydro-naphthacene-2-carboxylic acid amide

-
-
60-54-8
TETRACYCLINE

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 80 percent / formic acid / 1 h / 80 °C 2: 88 percent / BBr3 / CH2Cl2 / 15 h / 0 - 20 °C 3: 75 percent / O2; TPP / CHCl3 / 0.17 h / 20 - 40 °C / Irradiation 4: 62 percent / H2 / Pt-black / dioxane / 8 h / 2280 Torr View Scheme |
-
-
286961-69-1
((1S,2S,3S,12aR)-2,6-Dihydroxy-3-hydroxymethyl-4,7-dimethoxy-11-methyl-5-oxo-1,2,3,5,12,12a-hexahydro-naphthacen-1-yl)-carbamic acid tert-butyl ester

-
-
60-54-8
TETRACYCLINE

| Conditions | Yield |
|---|---|
| Multi-step reaction with 11 steps 1.1: 85 percent / Br2; (Bu3Sn)2O; MS-4A / CH2Cl2 / 0.25 h / -78 °C 2.1: 91 percent / Dess-Martin periodinane / acetonitrile; CH2Cl2 / 0.25 h 3.1: Zn / acetic acid / 0.03 h 4.1: Dess-Martin periodinane / acetonitrile; CH2Cl2 / 0.25 h 4.2: 60 percent / dimethyldioxirane; (R,R)-(PhCH-NTs)2BCl; TEA / CH2Cl2 / 0.5 h / -78 °C 5.1: NH2OH*HCl; TEA / methanol / 0.5 h 6.1: CDI / tetrahydrofuran / 0.75 h 7.1: 68 percent / polyposphoric acid / 0.75 h / 100 °C 8.1: 80 percent / formic acid / 1 h / 80 °C 9.1: 88 percent / BBr3 / CH2Cl2 / 15 h / 0 - 20 °C 10.1: 75 percent / O2; TPP / CHCl3 / 0.17 h / 20 - 40 °C / Irradiation 11.1: 62 percent / H2 / Pt-black / dioxane / 8 h / 2280 Torr View Scheme |
-
-
286961-71-5
((1S,4aS,12aR)-6-Hydroxy-3-hydroxymethyl-4,7-dimethoxy-11-methyl-2,5-dioxo-1,2,4a,5,12,12a-hexahydro-naphthacen-1-yl)-carbamic acid tert-butyl ester

-
-
60-54-8
TETRACYCLINE

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1.1: Dess-Martin periodinane / acetonitrile; CH2Cl2 / 0.25 h 1.2: 60 percent / dimethyldioxirane; (R,R)-(PhCH-NTs)2BCl; TEA / CH2Cl2 / 0.5 h / -78 °C 2.1: NH2OH*HCl; TEA / methanol / 0.5 h 3.1: CDI / tetrahydrofuran / 0.75 h 4.1: 68 percent / polyposphoric acid / 0.75 h / 100 °C 5.1: 80 percent / formic acid / 1 h / 80 °C 6.1: 88 percent / BBr3 / CH2Cl2 / 15 h / 0 - 20 °C 7.1: 75 percent / O2; TPP / CHCl3 / 0.17 h / 20 - 40 °C / Irradiation 8.1: 62 percent / H2 / Pt-black / dioxane / 8 h / 2280 Torr View Scheme |
-
-
286961-73-7
((1S,4aS,12aS)-3-Formyl-2,4a,6-trihydroxy-7-methoxy-11-methyl-4,5-dioxo-1,4,4a,5,12,12a-hexahydro-naphthacen-1-yl)-carbamic acid tert-butyl ester

-
-
60-54-8
TETRACYCLINE

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: NH2OH*HCl; TEA / methanol / 0.5 h 2: CDI / tetrahydrofuran / 0.75 h 3: 68 percent / polyposphoric acid / 0.75 h / 100 °C 4: 80 percent / formic acid / 1 h / 80 °C 5: 88 percent / BBr3 / CH2Cl2 / 15 h / 0 - 20 °C 6: 75 percent / O2; TPP / CHCl3 / 0.17 h / 20 - 40 °C / Irradiation 7: 62 percent / H2 / Pt-black / dioxane / 8 h / 2280 Torr View Scheme |
-
-
286961-82-8
((1S,2S,3S,12aR)-2,4,6-Trihydroxy-3-hydroxymethyl-7-methoxy-11-methyl-5-oxo-1,2,3,5,12,12a-hexahydro-naphthacen-1-yl)-carbamic acid tert-butyl ester

-
-
60-54-8
TETRACYCLINE

| Conditions | Yield |
|---|---|
| Multi-step reaction with 12 steps 1.1: 72 percent / i-Pr2NEt / tetrahydrofuran; methanol / 2 h 2.1: 85 percent / Br2; (Bu3Sn)2O; MS-4A / CH2Cl2 / 0.25 h / -78 °C 3.1: 91 percent / Dess-Martin periodinane / acetonitrile; CH2Cl2 / 0.25 h 4.1: Zn / acetic acid / 0.03 h 5.1: Dess-Martin periodinane / acetonitrile; CH2Cl2 / 0.25 h 5.2: 60 percent / dimethyldioxirane; (R,R)-(PhCH-NTs)2BCl; TEA / CH2Cl2 / 0.5 h / -78 °C 6.1: NH2OH*HCl; TEA / methanol / 0.5 h 7.1: CDI / tetrahydrofuran / 0.75 h 8.1: 68 percent / polyposphoric acid / 0.75 h / 100 °C 9.1: 80 percent / formic acid / 1 h / 80 °C 10.1: 88 percent / BBr3 / CH2Cl2 / 15 h / 0 - 20 °C 11.1: 75 percent / O2; TPP / CHCl3 / 0.17 h / 20 - 40 °C / Irradiation 12.1: 62 percent / H2 / Pt-black / dioxane / 8 h / 2280 Torr View Scheme |

-
-
60-54-8
TETRACYCLINE

| Conditions | Yield |
|---|---|
| Multi-step reaction with 9 steps 1.1: Zn / acetic acid / 0.03 h 2.1: Dess-Martin periodinane / acetonitrile; CH2Cl2 / 0.25 h 2.2: 60 percent / dimethyldioxirane; (R,R)-(PhCH-NTs)2BCl; TEA / CH2Cl2 / 0.5 h / -78 °C 3.1: NH2OH*HCl; TEA / methanol / 0.5 h 4.1: CDI / tetrahydrofuran / 0.75 h 5.1: 68 percent / polyposphoric acid / 0.75 h / 100 °C 6.1: 80 percent / formic acid / 1 h / 80 °C 7.1: 88 percent / BBr3 / CH2Cl2 / 15 h / 0 - 20 °C 8.1: 75 percent / O2; TPP / CHCl3 / 0.17 h / 20 - 40 °C / Irradiation 9.1: 62 percent / H2 / Pt-black / dioxane / 8 h / 2280 Torr View Scheme |
-
-
286961-74-8
((1S,4aS,12aS)-3-Cyano-2,4a,6-trihydroxy-7-methoxy-11-methyl-4,5-dioxo-1,4,4a,5,12,12a-hexahydro-naphthacen-1-yl)-carbamic acid tert-butyl ester

-
-
60-54-8
TETRACYCLINE

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 68 percent / polyposphoric acid / 0.75 h / 100 °C 2: 80 percent / formic acid / 1 h / 80 °C 3: 88 percent / BBr3 / CH2Cl2 / 15 h / 0 - 20 °C 4: 75 percent / O2; TPP / CHCl3 / 0.17 h / 20 - 40 °C / Irradiation 5: 62 percent / H2 / Pt-black / dioxane / 8 h / 2280 Torr View Scheme |

-
-
60-54-8
TETRACYCLINE

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: CDI / tetrahydrofuran / 0.75 h 2: 68 percent / polyposphoric acid / 0.75 h / 100 °C 3: 80 percent / formic acid / 1 h / 80 °C 4: 88 percent / BBr3 / CH2Cl2 / 15 h / 0 - 20 °C 5: 75 percent / O2; TPP / CHCl3 / 0.17 h / 20 - 40 °C / Irradiation 6: 62 percent / H2 / Pt-black / dioxane / 8 h / 2280 Torr View Scheme |
-
-
286961-70-4
C27H32BrNO8

-
-
60-54-8
TETRACYCLINE

| Conditions | Yield |
|---|---|
| Multi-step reaction with 10 steps 1.1: 91 percent / Dess-Martin periodinane / acetonitrile; CH2Cl2 / 0.25 h 2.1: Zn / acetic acid / 0.03 h 3.1: Dess-Martin periodinane / acetonitrile; CH2Cl2 / 0.25 h 3.2: 60 percent / dimethyldioxirane; (R,R)-(PhCH-NTs)2BCl; TEA / CH2Cl2 / 0.5 h / -78 °C 4.1: NH2OH*HCl; TEA / methanol / 0.5 h 5.1: CDI / tetrahydrofuran / 0.75 h 6.1: 68 percent / polyposphoric acid / 0.75 h / 100 °C 7.1: 80 percent / formic acid / 1 h / 80 °C 8.1: 88 percent / BBr3 / CH2Cl2 / 15 h / 0 - 20 °C 9.1: 75 percent / O2; TPP / CHCl3 / 0.17 h / 20 - 40 °C / Irradiation 10.1: 62 percent / H2 / Pt-black / dioxane / 8 h / 2280 Torr View Scheme |

-
-
60-54-8
TETRACYCLINE

| Conditions | Yield |
|---|---|
| Multi-step reaction with 13 steps 1.1: H2; TEA / Pd-black / dioxane; H2O / 1 h 2.1: 72 percent / i-Pr2NEt / tetrahydrofuran; methanol / 2 h 3.1: 85 percent / Br2; (Bu3Sn)2O; MS-4A / CH2Cl2 / 0.25 h / -78 °C 4.1: 91 percent / Dess-Martin periodinane / acetonitrile; CH2Cl2 / 0.25 h 5.1: Zn / acetic acid / 0.03 h 6.1: Dess-Martin periodinane / acetonitrile; CH2Cl2 / 0.25 h 6.2: 60 percent / dimethyldioxirane; (R,R)-(PhCH-NTs)2BCl; TEA / CH2Cl2 / 0.5 h / -78 °C 7.1: NH2OH*HCl; TEA / methanol / 0.5 h 8.1: CDI / tetrahydrofuran / 0.75 h 9.1: 68 percent / polyposphoric acid / 0.75 h / 100 °C 10.1: 80 percent / formic acid / 1 h / 80 °C 11.1: 88 percent / BBr3 / CH2Cl2 / 15 h / 0 - 20 °C 12.1: 75 percent / O2; TPP / CHCl3 / 0.17 h / 20 - 40 °C / Irradiation 13.1: 62 percent / H2 / Pt-black / dioxane / 8 h / 2280 Torr View Scheme |
-
-
286961-68-0
((1S,2S,3S,12aR)-2-Benzyloxy-3-benzyloxymethyl-4,6-dihydroxy-7-methoxy-11-methyl-5-oxo-1,2,3,5,12,12a-hexahydro-naphthacen-1-yl)-carbamic acid benzyl ester

-
-
60-54-8
TETRACYCLINE

| Conditions | Yield |
|---|---|
| Multi-step reaction with 14 steps 1.1: BBr3 / CH2Cl2 / 0.25 h / -78 °C 2.1: H2; TEA / Pd-black / dioxane; H2O / 1 h 3.1: 72 percent / i-Pr2NEt / tetrahydrofuran; methanol / 2 h 4.1: 85 percent / Br2; (Bu3Sn)2O; MS-4A / CH2Cl2 / 0.25 h / -78 °C 5.1: 91 percent / Dess-Martin periodinane / acetonitrile; CH2Cl2 / 0.25 h 6.1: Zn / acetic acid / 0.03 h 7.1: Dess-Martin periodinane / acetonitrile; CH2Cl2 / 0.25 h 7.2: 60 percent / dimethyldioxirane; (R,R)-(PhCH-NTs)2BCl; TEA / CH2Cl2 / 0.5 h / -78 °C 8.1: NH2OH*HCl; TEA / methanol / 0.5 h 9.1: CDI / tetrahydrofuran / 0.75 h 10.1: 68 percent / polyposphoric acid / 0.75 h / 100 °C 11.1: 80 percent / formic acid / 1 h / 80 °C 12.1: 88 percent / BBr3 / CH2Cl2 / 15 h / 0 - 20 °C 13.1: 75 percent / O2; TPP / CHCl3 / 0.17 h / 20 - 40 °C / Irradiation 14.1: 62 percent / H2 / Pt-black / dioxane / 8 h / 2280 Torr View Scheme |
-
-
286961-67-9
((1S,2S,3S,12aR)-2-Benzyloxy-3-benzyloxymethyl-4,6,11-trihydroxy-7-methoxy-11-methyl-5-oxo-1,2,3,5,11,11a,12,12a-octahydro-naphthacen-1-yl)-carbamic acid benzyl ester

-
-
60-54-8
TETRACYCLINE

| Conditions | Yield |
|---|---|
| Multi-step reaction with 15 steps 1.1: 90 percent / SOCl2; TEA / CH2Cl2 / 0.17 h / -30 °C 2.1: BBr3 / CH2Cl2 / 0.25 h / -78 °C 3.1: H2; TEA / Pd-black / dioxane; H2O / 1 h 4.1: 72 percent / i-Pr2NEt / tetrahydrofuran; methanol / 2 h 5.1: 85 percent / Br2; (Bu3Sn)2O; MS-4A / CH2Cl2 / 0.25 h / -78 °C 6.1: 91 percent / Dess-Martin periodinane / acetonitrile; CH2Cl2 / 0.25 h 7.1: Zn / acetic acid / 0.03 h 8.1: Dess-Martin periodinane / acetonitrile; CH2Cl2 / 0.25 h 8.2: 60 percent / dimethyldioxirane; (R,R)-(PhCH-NTs)2BCl; TEA / CH2Cl2 / 0.5 h / -78 °C 9.1: NH2OH*HCl; TEA / methanol / 0.5 h 10.1: CDI / tetrahydrofuran / 0.75 h 11.1: 68 percent / polyposphoric acid / 0.75 h / 100 °C 12.1: 80 percent / formic acid / 1 h / 80 °C 13.1: 88 percent / BBr3 / CH2Cl2 / 15 h / 0 - 20 °C 14.1: 75 percent / O2; TPP / CHCl3 / 0.17 h / 20 - 40 °C / Irradiation 15.1: 62 percent / H2 / Pt-black / dioxane / 8 h / 2280 Torr View Scheme |

-
-
60-54-8
TETRACYCLINE

| Conditions | Yield |
|---|---|
| With hydrogen; palladium In 1,4-dioxane at 23℃; under 760.051 Torr; for 2.08333h; |
-
-
73590-58-6
omeprazole

-
-
60-54-8
TETRACYCLINE

| Conditions | Yield |
|---|---|
| In water pH=9; pH-value; Cooling; |
-
-
50-00-0
formaldehyd

-
-
60-54-8
TETRACYCLINE

-
-
70458-96-7
1-ethyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-3-quinoline carboxylic acid

| Conditions | Yield |
|---|---|
| In ethanol for 0.05h; Mannich reaction; microwave irradiation; | 78% |

-
-
60-54-8
TETRACYCLINE

| Conditions | Yield |
|---|---|
| With hydrogenchloride; N-chloro-succinimide In water at 25℃; for 0.5h; | 74.4% |

| Conditions | Yield |
|---|---|
| In ethanol for 0.05h; Mannich reaction; microwave irradiation; | 69% |


| Conditions | Yield |
|---|---|
| In ethanol for 0.05h; Mannich reaction; microwave irradiation; | 61% |

-
-
60-54-8
TETRACYCLINE

-
-
74-88-4
methyl iodide

-
-
6602-90-0
1,4,4a,5,5a,6,11,12a-octahydro-2-aminocarbonyl-3,6,10,12,12a-hexahydroxy-6-methyl-1,11-dioxonaphthacene-4-trimethylammonium iodide

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 20℃; for 96h; | 57% |
| In tetrahydrofuran at 20℃; for 144h; |

| Conditions | Yield |
|---|---|
| In ethanol for 0.05h; Mannich reaction; microwave irradiation; | 54% |


-
-
60-54-8
TETRACYCLINE

| Conditions | Yield |
|---|---|
| In further solvent(s) the soln. of salt in ethanol/triethyl orthoformate was heated at 40-50°C, added tetracycline, refluxed for 24-48 h, concn. by heating under reduced prdssure, cooled to room temp.; filtered, washed with cold EtOH, dried in vac. over anhyd. CaCl2; | 42% |

| Conditions | Yield |
|---|---|
| Stage #1: TETRACYCLINE With ethylenediaminetetraacetic acid; silver carbonate In N,N-dimethyl-formamide at 37℃; for 48h; Molecular sieve; Stage #2: With dipotassium hydrogenphosphate; water; edetate disodium In N,N-dimethyl-formamide; acetonitrile at 0 - 20℃; for 8h; pH=5.5; Stage #3: In chloroform at 0 - 5℃; for 168h; | A n/a B 42% |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran for 4h; Inert atmosphere; Reflux; | 36% |
| In tetrahydrofuran for 4h; Reflux; Inert atmosphere; | 36% |


| Conditions | Yield |
|---|---|
| With acetic acid In ethanol at 20℃; for 12h; Mannich Aminomethylation; | 31% |

-
-
60-54-8
TETRACYCLINE


| Conditions | Yield |
|---|---|
| With mercury(II) diacetate; acetic acid at 50℃; for 48h; | A 30% B 10% |

-
-
60-54-8
TETRACYCLINE

| Conditions | Yield |
|---|---|
| In further solvent(s) the soln. of salt in ethanol/triethyl orthoformate was heated 40-50°C, added tetracycline, refluxed for 24-48 h, concn. by heating underreduced pressure, cooled to room temp.; filtered, washed with cold EtOH, dried in vac. over anhyd. CaCl2; | 28% |
-
-
60-54-8
TETRACYCLINE

| Conditions | Yield |
|---|---|
| With 3-chloro-benzenecarboperoxoic acid In methanol for 0.0833333h; Solvent; Inert atmosphere; Cooling with ice; | 27% |
-
-
60-54-8
TETRACYCLINE

| Conditions | Yield |
|---|---|
| In dimethyl sulfoxide at 30℃; for 48h; | 26% |

| Conditions | Yield |
|---|---|
| With acetic acid In ethanol at 20℃; for 12h; Mannich Aminomethylation; | 24% |

-
-
110-91-8
morpholine

-
-
60-54-8
TETRACYCLINE

-
-
298-12-4
Glyoxilic acid

-
-
13729-37-8
(4-dimethylamino-3,6,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-1,4,4a,5,5a,6,11,12a-octahydro-naphthacene-2-carbonylamino)-morpholin-4-yl-acetic acid

| Conditions | Yield |
|---|---|
| In tert-butyl alcohol Heating; |


| Conditions | Yield |
|---|---|
| With 1,1,1,3,3,3-hexamethyl-disilazane In pyridine at 20℃; for 0.0166667h; |

| Conditions | Yield |
|---|---|
| With 1,1,1,3,3,3-hexamethyl-disilazane In pyridine at 20℃; for 12h; |
-
-
60-54-8
TETRACYCLINE

| Conditions | Yield |
|---|---|
| (i) DMF, (ii) piperidine; Multistep reaction; |

| Conditions | Yield |
|---|---|
| In water at 23℃; formation constant; further solvents;; |

| Conditions | Yield |
|---|---|
| In water at 23℃; formation constant; further solvents;; |

| Conditions | Yield |
|---|---|
| With 2-hydroxyethanethiol at 4℃; Irradiation; Title compound not separated from byproducts; |

| Conditions | Yield |
|---|---|
| In water at 23℃; formation constant; further solvents;; |

| Conditions | Yield |
|---|---|
| In water at 23℃; formation constant; further solvents;; |

| Conditions | Yield |
|---|---|
| In water at 23℃; formation constant; further solvents;; |

| Conditions | Yield |
|---|---|
| In water at 23℃; formation constant; further solvents;; |

| Conditions | Yield |
|---|---|
| In water at 23℃; formation constant; further solvents;; |

| Conditions | Yield |
|---|---|
| In water at 23℃; formation constant; further solvents;; |
Tetracycline History
The Tetracycline (CAS NO.60-54-8) are a large family of antibiotics that were discovered as natural products by Benjamin Minge Duggar and first described in 1948. Tetracycline was then discovered by Lloyd Conover in the research departments of Pfizer. The patent for tetracycline, U.S. Patent 2,699,054, was first issued in 1950. However, Nubian mummies have been studied in the 1990s and were found to contain significant levels of tetracycline; there is evidence that the beer brewed at the time could have been the source. Tetracycline sparked the development of many chemically altered antibiotics and in doing so has proved to be one of the most important discoveries made in the field of antibiotics.
Tetracycline Consensus Reports
EPA Genetic Toxicology Program.
Tetracycline Specification
The Tetracycline with CAS registry number of 60-54-8 is also known as Abramycin. The IUPAC name is (2Z,4S,4aS,5aS,6S,12aS)-2-[amino(hydroxy)methylidene]-4-(dimethylamino)-6,10,11,12a-tetrahydroxy-6-methyl-4,4a,5,5a-tetrahydrotetracene-1,3,12-trione. It belongs to product categories of Antibiotic Explorer; Intermediates & Fine Chemicals; Pharmaceuticals; Antibacterial; Antibiotics; Antibiotics A to; Antibiotics by Application; Antibiotics T-ZAntibiotics; Chemical Structure Class; Genetic Marker SelectionAntibiotics; Interferes with Protein SynthesisSpectrum of Activity; L-ZAntibiotics; Mechanism of Action; Tetracyclines; Peptide Synthesis/Antibiotics. Its EINECS registry number is 200-481-9. In addition, the formula is C22H24N2O8 and the molecular weight is 444.43. This chemical is a light yellow crystal and should be sealed in dry, dark place under 0 °C.
Physical properties about Tetracycline are: (1)ACD/LogP: 0.62; (2)# of Rule of 5 Violations: 2 ; (3)ACD/BCF (pH 5.5): 1; (4)ACD/BCF (pH 7.4): 1; (5)ACD/KOC (pH 5.5): 1; (6)ACD/KOC (pH 7.4): 1; (7)#H bond acceptors: 10; (8)#H bond donors: 7; (9)#Freely Rotating Bonds: 7; (10)Index of Refraction: 1.741; (11)Molar Refractivity: 109.088 cm3; (12)Molar Volume: 270.304 cm3; (13)Surface Tension: 100.608 dyne/cm; (14)Density: 1.644 g/cm3; (15)Flash Point: 431.953 °C; (16)Enthalpy of Vaporization: 120.61 kJ/mol; (17)Boiling Point: 790.622 °C at 760 mmHg.
Preparation of Tetracycline: it is prepared by reaction of 6-deoxy-6-hydroperoxy-5a,11a-dehydrotetracycline. The reaction needs reagent H2, catalyst Pt-black and solvent dioxane at the pressure of 2280 for 8 hours. The yield is about 62%.
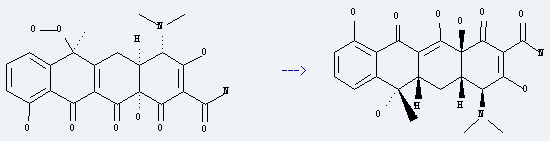
Uses of Tetracycline: it is first-line therapy for Rocky Mountain spotted fever, Q fever, psittacosis and lymphogranuloma venereum, and to eradicate nasal carriage of meningococci. What's more, it is used as a marker of bone growth for biopsies in humans. It is also used as a biomarker in wildlife and in transcriptional activation.
When you are using this chemical, please be cautious about it. As a chemical, it is irritating to eyes, respiratory system and skin. And it is harmful if swallowed. During using it, wear suitable protective clothing. Do not breathe dust. If contact with eyes accidently, rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
1. Canonical SMILES: CC1(C2CC3C(C(=O)C(=C(N)O)C(=O)C3(C(=O)C2=C(C4=C1C=CC=C4O)O)O)N(C)C)O
2. Isomeric SMILES: C[C@@]1([C@H]2C[C@H]3[C@@H](C(=O)/C(=C(\N)/O)/C(=O)[C@]3(C(=O)C2=C(C4=C1C=CC=C4O)O)O)N(C)C)O
3. InChI: InChI=1S/C22H24N2O8/c1-21(31)8-5-4-6-11(25)12(8)16(26)13-9(21)7-10-15(24(2)3)17(27)14(20(23)30)19(29)22(10,32)18(13)28/h4-6,9-10,15,25-26,30-32H,7,23H2,1-3H3/b20-14-/t9-,10-,15-,21+,22-/m0/s1
4. InChIKey: JYHCQVWYCGHXGP-BPPSBWQWSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| guinea pig | LD50 | oral | 1875mg/kg (1875mg/kg) | "Toxicometric Parameters of Industrial Toxic Chemicals Under Single Exposure," Izmerov, N.F., et al., Moscow, Centre of International Projects, GKNT, 1982Vol. -, Pg. 109, 1982. | |
| mouse | LD50 | intraperitoneal | 120mg/kg (120mg/kg) | "Index of Antibiotics from Actinomycetes," Umezawa, H. et al., eds., Tokyo, Univ. of Tokyo Press, 1967Vol. -, Pg. 134, 1967. | |
| mouse | LD50 | intravenous | 157mg/kg (157mg/kg) | Farmaco, Edizione Scientifica. Vol. 10, Pg. 346, 1955. | |
| mouse | LD50 | oral | 678mg/kg (678mg/kg) | American Journal of Tropical Medicine and Hygiene. Vol. 2, Pg. 254, 1953. | |
| mouse | LD50 | subcutaneous | 400mg/kg (400mg/kg) | "CRC Handbook of Antibiotic Compounds," Vols.1- , Berdy, J., Boca Raton, FL, CRC Press, 1980Vol. 3, Pg. 42, 1980. | |
| rat | LD50 | intraperitoneal | 310mg/kg (310mg/kg) | Drugs in Japan Vol. 6, Pg. 493, 1982. | |
| rat | LD50 | intravenous | 129mg/kg (129mg/kg) | Antibiotics and Chemotherapy Vol. 4, Pg. 411, 1954. | |
| rat | LD50 | oral | 807mg/kg (807mg/kg) | Toxicology and Applied Pharmacology. Vol. 18, Pg. 185, 1971. | |
| women | LDLo | multiple routes | 310mg/kg (310mg/kg) | GASTROINTESTINAL: NAUSEA OR VOMITING LIVER: "JAUNDICE, OTHER OR UNCLASSIFIED" | New England Journal of Medicine. Vol. 270, Pg. 157, 1964. |
| women | TDLo | oral | 600mg/kg/15D (600mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) GASTROINTESTINAL: DECREASED MOTILITY OR CONSTIPATION KIDNEY, URETER, AND BLADDER: URINE VOLUME DECREASED | Southern Medical Journal. Vol. 71, Pg. 961, 1978. |
Related Products
- Tetracycline
- Tetracycline hydrochloride
- 60548-09-6
- 60548-42-7
- 6054-97-3
- 6054-98-4
- 6054-99-5
- 605-50-5
- 6055-19-2
- 605-55-0
- 6055-52-3
- 60555-59-1
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View