-
Name
Trifluridine
- EINECS 200-722-8
- CAS No. 70-00-8
- Article Data32
- CAS DataBase
- Density 1.646 g/cm3
- Solubility
- Melting Point 190-193 °C(lit.)
- Formula C10H11F3N2O5
- Boiling Point
- Molecular Weight 296.203
- Flash Point
- Transport Information
- Appearance Almost white crystalline power
- Safety 22-36
- Risk Codes 20/21/22-40
-
Molecular Structure
-
Hazard Symbols
 Xn,
Xn,  Xi
Xi
- Synonyms Uridine, 2'-deoxy-5-(trifluoromethyl)-(7CI);2'-Deoxy-5-(trifluoromethyl)uridine;5-(Trifluoromethyl)-2'-deoxyuridine;5-(Trifluoromethyl)deoxyuridine;5-Trifluoromethyl-2'-deoxy-b-uridine;5-Trifluorothymidine;NSC 529182;NSC 75520;Trifluorothymidine;Viroptic;
- PSA 104.55000
- LogP -0.80390
Synthetic route

-
-
70-00-8
2'-deoxy-5-trifluoromethyluridine

| Conditions | Yield |
|---|---|
| With methanol; sodium methylate at 4 - 6℃; for 3.5h; | 97% |
-
-
65499-42-5
5-trifluoromethyl-2′-deoxyuridine-3′,5′-diacetate

-
-
70-00-8
2'-deoxy-5-trifluoromethyluridine

| Conditions | Yield |
|---|---|
| With ammonia In methanol at 20℃; | 95% |
| With ammonia In methanol at 0 - 20℃; for 16h; | 84% |
| With ammonia In methanol at 20℃; for 19h; | 76% |

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide In water at -3 - 60℃; for 2h; Temperature; Inert atmosphere; | 94.3% |
| With tert.-butylhydroperoxide In water at -5 - 65℃; for 3h; Inert atmosphere; Large scale; | 94.8% |
| With tert-butyl alcohol In water at 0 - 20℃; for 3h; | 59% |
| With mesoporous graphitic carbon nitride In dimethyl sulfoxide at 25℃; Irradiation; | 47% |
| With acetone In water at 20℃; for 40h; UV-irradiation; Inert atmosphere; | 44% |
-
-
21618-67-7
5-(trifluoromethyl)uridine

-
-
70-00-8
2'-deoxy-5-trifluoromethyluridine

| Conditions | Yield |
|---|---|
| Stage #1: 5-(trifluoromethyl)uridine With sodium hydrogencarbonate; carbonic acid dimethyl ester In N,N-dimethyl-formamide at 120℃; for 8h; Stage #2: With hydrogenchloride In water; N,N-dimethyl-formamide at 55℃; for 10h; Stage #3: With palladium on activated charcoal; hydrogen; sodium hydroxide In water; N,N-dimethyl-formamide at 15℃; under 4500.45 Torr; for 12h; pH=7 - 8; | 86.4% |
-
-
129664-47-7
1-<3,5-bis-O-(p-chlorobenzoyl)-2-deoxy-β-D-erythro-pentofuranosyl>-5-trifluoromethyluracil

-
-
70-00-8
2'-deoxy-5-trifluoromethyluridine

| Conditions | Yield |
|---|---|
| With sodium methylate In methanol at 20 - 30℃; for 4h; | 82.5% |
| With methanol; sodium methylate for 1h; Ambient temperature; | 67% |
-
-
65499-42-5
3',5'-di-O-acetyl-5-(trifluoromethyl)-2'-deoxyuridine

-
-
70-00-8
2'-deoxy-5-trifluoromethyluridine

| Conditions | Yield |
|---|---|
| With ammonia In methanol for 10h; Ambient temperature; | 82% |

| Conditions | Yield |
|---|---|
| With copper(l) iodide In N,N-dimethyl-formamide at 80℃; for 23h; Inert atmosphere; Sealed tube; regioselective reaction; | 76% |

| Conditions | Yield |
|---|---|
| With caesium carbonate In dimethyl sulfoxide for 24h; Inert atmosphere; Irradiation; | 71% |
| With caesium carbonate In dimethyl sulfoxide at 20℃; for 24h; Schlenk technique; Inert atmosphere; Irradiation; | 68% |
| With iron(III) sulfate; sulfuric acid; dihydrogen peroxide In water; dimethyl sulfoxide at 40 - 50℃; for 0.333333h; | 58% |
-
-
76513-99-0
5-(trifluoromethyl)-3',5'-di-O-trityl-2'-deoxyuridine

-
-
70-00-8
2'-deoxy-5-trifluoromethyluridine

| Conditions | Yield |
|---|---|
| With formic acid at 0℃; for 0.0166667h; | 40% |
-
-
89143-01-1
Hexanoic acid (2R,3S,5R)-5-(2,4-dioxo-5-trifluoromethyl-3,4-dihydro-2H-pyrimidin-1-yl)-2-hexanoyloxymethyl-tetrahydro-furan-3-yl ester

-
A
-
89143-05-5
5'-O-hexanoyl-2'-deoxy-5-trifluoromethyluridine

-
B
-
70-00-8
2'-deoxy-5-trifluoromethyluridine

| Conditions | Yield |
|---|---|
| With phosphate buffer In N,N-dimethyl-formamide at 25℃; for 18h; enzym: Pseudomonas fluorescens; | A 62 % Chromat. B 6 % Chromat. |
-
-
54-20-6
5-trifluoromethyluracil

-
-
50-89-5
thymidine

-
A
-
70-00-8
2'-deoxy-5-trifluoromethyluridine

-
B
-
65-71-4
thymin

| Conditions | Yield |
|---|---|
| With thymidine phosphorylase from Escherichia coli In phosphate buffer at 35℃; for 2h; pH=7.0; Title compound not separated from byproducts; | A 47 % Chromat. B n/a |

-
-
13030-62-1
3',5'-di-O-acetyl-2'-deoxyuridine

-
-
70-00-8
2'-deoxy-5-trifluoromethyluridine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 33 percent / XeF2 / 1 h / Ambient temperature 2: 82 percent / NH3 / methanol / 10 h / Ambient temperature View Scheme |
-
-
76513-98-9
3',5'-di-O-trityl-5-iodo-2'-deoxyuridine

-
-
70-00-8
2'-deoxy-5-trifluoromethyluridine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 1.) copper powder / 1.) HMPA, 120 deg C, 2.5 h, 2.) 45 deg C, 12 h 2: 40 percent / 98percent formic acid / 0.02 h / 0 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: hexamethyldisilazane / 1,2-dichloro-ethane / 0.75 h / Heating 2: 80 percent / zinc chloride / CHCl3 / 15 h / Ambient temperature 3: 67 percent / sodium methoxide, methanol / 1 h / Ambient temperature View Scheme |
-
-
7057-43-4
5-trifluoromethyl-2,4-bis(trimethylsilyloxy)pyrimidine

-
-
70-00-8
2'-deoxy-5-trifluoromethyluridine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 80 percent / zinc chloride / CHCl3 / 15 h / Ambient temperature 2: 67 percent / sodium methoxide, methanol / 1 h / Ambient temperature View Scheme |

| Conditions | Yield |
|---|---|
| With thymidine phosphorylase In aq. phosphate buffer at 37℃; for 1h; pH=6.8; Enzymatic reaction; |
-
-
951-78-0
2'-deoxyuridine

-
-
54-20-6
5-trifluoromethyluracil

-
A
-
70-00-8
2'-deoxy-5-trifluoromethyluridine

-
B
-
66-22-8
uracil

| Conditions | Yield |
|---|---|
| With pyrimidine nucleoside phosphorylase from Bacillus subtilis (E.C. 2.4.2.3) immobilized on Sepabeads EC-EP In aq. phosphate buffer at 20℃; for 10h; pH=7.5; Reagent/catalyst; Enzymatic reaction; |


| Conditions | Yield |
|---|---|
| With immobilized Bacillus psychrosaccharolyticus CECT 4074 nucleoside 2'-deoxyribosyltransferase In aq. phosphate buffer at 37℃; for 2h; pH=7.5; Concentration; Enzymatic reaction; | |
| With purine nucleoside phosphorylase; uridine phosphorylase In aq. phosphate buffer; dimethyl sulfoxide at 80℃; for 3h; pH=7; Enzymatic reaction; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 2 h / 20 - 125 °C 2: copper dichloride / chloroform / 24 h / 20 - 30 °C 3: sodium methylate / methanol / 4 h / 20 - 30 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: pyridine / 16 h / 20 °C 2.1: sodium hydride / N,N-dimethyl-formamide / 0.5 h / -60 °C 2.2: 16 h / 20 °C 3.1: copper(l) iodide; potassium fluoride / N,N-dimethyl-formamide / 16 h / 60 °C 3.2: 16 h / 20 °C 4.1: ammonia / methanol / 16 h / 0 - 20 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: sodium hydride / N,N-dimethyl-formamide / 0.5 h / -60 °C 1.2: 16 h / 20 °C 2.1: copper(l) iodide; potassium fluoride / N,N-dimethyl-formamide / 16 h / 60 °C 2.2: 16 h / 20 °C 3.1: ammonia / methanol / 16 h / 0 - 20 °C View Scheme |

-
-
70-00-8
2'-deoxy-5-trifluoromethyluridine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: copper(l) iodide; potassium fluoride / N,N-dimethyl-formamide / 16 h / 60 °C 1.2: 16 h / 20 °C 2.1: ammonia / methanol / 16 h / 0 - 20 °C View Scheme |
-
-
36792-88-8
2-deoxy-D-ribose

-
-
70-00-8
2'-deoxy-5-trifluoromethyluridine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: acetyl chloride / 0.5 h / 5 - 10 °C 1.2: 5 h / 5 - 10 °C 2.1: dmap; triethylamine / toluene / 2 h / 25 - 30 °C 3.1: hydrogenchloride; acetyl chloride; acetic acid / 1,4-dioxane; chloroform / 3.25 h / 10 - 15 °C 4.1: 1,1,1,3,3,3-hexamethyl-disilazane; chloro-trimethyl-silane / 3 h / 120 - 125 °C 4.2: 3 h / 25 - 30 °C 5.1: sodium methylate; methanol / 1.5 h / 10 - 15 °C View Scheme |
-
-
51255-17-5, 51255-18-6, 60134-26-1, 89577-26-4, 96038-80-1, 96039-31-5, 144301-84-8, 144301-85-9
1-O-methyl-2-deoxy-D-ribofuranoside

-
-
70-00-8
2'-deoxy-5-trifluoromethyluridine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: dmap; triethylamine / toluene / 2 h / 25 - 30 °C 2.1: hydrogenchloride; acetyl chloride; acetic acid / 1,4-dioxane; chloroform / 3.25 h / 10 - 15 °C 3.1: 1,1,1,3,3,3-hexamethyl-disilazane; chloro-trimethyl-silane / 3 h / 120 - 125 °C 3.2: 3 h / 25 - 30 °C 4.1: sodium methylate; methanol / 1.5 h / 10 - 15 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: hydrogenchloride; acetyl chloride; acetic acid / 1,4-dioxane; chloroform / 3.25 h / 10 - 15 °C 2.1: 1,1,1,3,3,3-hexamethyl-disilazane; chloro-trimethyl-silane / 3 h / 120 - 125 °C 2.2: 3 h / 25 - 30 °C 3.1: sodium methylate; methanol / 1.5 h / 10 - 15 °C View Scheme |
-
-
4330-21-6
2-deoxy-3,5-di-O-p-toluoyl-α-D-erythro-pentofuranosyl chloride

-
-
54-20-6
5-trifluoromethyluracil

-
-
70-00-8
2'-deoxy-5-trifluoromethyluridine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: 1,1,1,3,3,3-hexamethyl-disilazane; chloro-trimethyl-silane / 3 h / 120 - 125 °C 1.2: 3 h / 25 - 30 °C 2.1: sodium methylate; methanol / 1.5 h / 10 - 15 °C View Scheme |
-
-
7057-46-7
(2R,3S,5R)-5-(2,4-dioxo-5-(trifluoromethyl)-3,4-dihydropyrimidin-1(2H)-yl)-2-(((4-methylbenzoyl)oxy)methyl)tetrahydrofuran-3-yl-4-methylbenzoate

-
-
70-00-8
2'-deoxy-5-trifluoromethyluridine

| Conditions | Yield |
|---|---|
| With methanol; sodium methylate at 10 - 15℃; for 1.5h; Temperature; | 39 g |
-
-
40554-98-1
[(2R,3R,4R)-3,4-diacetoxy-5-chlorotetrahydrofuran-2-yl]methyl acetate

-
-
70-00-8
2'-deoxy-5-trifluoromethyluridine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: acetonitrile / 3 h / 45 °C 2.1: acetyl chloride; methanol / 10 h / 0 °C 3.1: carbonic acid dimethyl ester; sodium hydrogencarbonate / N,N-dimethyl-formamide / 8 h / 120 °C 3.2: 10 h / 55 °C 3.3: 12 h / 15 °C / 4500.45 Torr / pH 7 - 8 View Scheme |
-
-
40615-36-9
4,4'-dimethoxytrityl chloride

-
-
70-00-8
2'-deoxy-5-trifluoromethyluridine

-
-
133128-06-0
5'-O-(4,4'-dimethoxytrityl)-α,α,α-trifluorothymidine

| Conditions | Yield |
|---|---|
| With pyridine; dmap at 0 - 20℃; for 21h; | 96% |
| With pyridine at 20℃; for 41.5h; Inert atmosphere; | 72% |
| With pyridine |
-
-
854751-16-9
chloro-bis-(2-methoxy-phenyl)-phenyl-methane

-
-
70-00-8
2'-deoxy-5-trifluoromethyluridine

| Conditions | Yield |
|---|---|
| With dmap In pyridine; dichloromethane | 96% |
-
-
18162-48-6
tert-butyldimethylsilyl chloride

-
-
70-00-8
2'-deoxy-5-trifluoromethyluridine

-
-
127978-84-1
5'-O-(tert-butyldimethylsilyl)-2'-deoxy-5-(trifluoromethyl)uridine

| Conditions | Yield |
|---|---|
| With 1H-imidazole; dmap In N,N-dimethyl-formamide at 25℃; for 1h; | 93% |
| With pyridine for 12h; Ambient temperature; | 88% |
-
-
108-24-7
acetic anhydride

-
-
70-00-8
2'-deoxy-5-trifluoromethyluridine

-
-
65499-42-5
3',5'-di-O-acetyl-5-(trifluoromethyl)-2'-deoxyuridine

| Conditions | Yield |
|---|---|
| dmap In acetonitrile | 92% |
-
-
70-00-8
2'-deoxy-5-trifluoromethyluridine

-
-
14599-46-3
5-carboxy-2'-deoxyuridine

| Conditions | Yield |
|---|---|
| With sodium hydroxide at 37℃; for 36h; pH=10; | 90% |

-
-
70-00-8
2'-deoxy-5-trifluoromethyluridine

-
-
133128-06-0
5'-O-(4,4'-dimethoxytrityl)-α,α,α-trifluorothymidine

| Conditions | Yield |
|---|---|
| With lithium carbonate; N-ethyl-N,N-diisopropylamine In tetrahydrofuran at 20℃; Heating; | 87% |

| Conditions | Yield |
|---|---|
| With water at 60℃; for 24h; | 84% |
-
-
55726-22-2
arachidic anhydride

-
-
70-00-8
2'-deoxy-5-trifluoromethyluridine

-
-
89143-03-3
Icosanoic acid (2R,3S,5R)-5-(2,4-dioxo-5-trifluoromethyl-3,4-dihydro-2H-pyrimidin-1-yl)-2-icosanoyloxymethyl-tetrahydro-furan-3-yl ester

| Conditions | Yield |
|---|---|
| dmap In acetonitrile | 80% |
-
-
56666-54-7
3-fluorobenzoic acid anhydride

-
-
70-00-8
2'-deoxy-5-trifluoromethyluridine

-
-
99502-68-8
3',5'-di-O-(m-fluorobenzoyl)-2'-deoxy-5-trifluoromethyluridine

| Conditions | Yield |
|---|---|
| dmap In acetonitrile | 72% |
-
-
70-00-8
2'-deoxy-5-trifluoromethyluridine

-
-
76068-79-6
chloromethyl n-heptanoate

-
-
161632-25-3
3-n-heptanoyloxymethyl-2'-deoxy-5-trifluoromethyluridine

| Conditions | Yield |
|---|---|
| With sodium iodide; potassium carbonate | 71.3% |
-
-
69304-37-6
1,3-Dichloro-1,1,3,3-tetraisopropyldisiloxane

-
-
70-00-8
2'-deoxy-5-trifluoromethyluridine

-
-
133100-91-1
3',5'-O-TPDS-α,α,α-trifluorothymidine

| Conditions | Yield |
|---|---|
| With pyridine for 3h; Ambient temperature; | 70% |
-
-
13222-85-0
p-methylbenzoic anhydride

-
-
70-00-8
2'-deoxy-5-trifluoromethyluridine

-
-
7057-46-7
(2R,3S,5R)-5-(2,4-dioxo-5-(trifluoromethyl)-3,4-dihydropyrimidin-1(2H)-yl)-2-(((4-methylbenzoyl)oxy)methyl)tetrahydrofuran-3-yl-4-methylbenzoate

| Conditions | Yield |
|---|---|
| dmap In acetonitrile | 68% |
-
-
1191-99-7
2,3-dihydro-2H-furan

-
-
70-00-8
2'-deoxy-5-trifluoromethyluridine

-
-
93298-69-2
2'-deoxy-3',5'-di-O-(tetrahydro-2-furyl)-5-trifluoromethyluridine

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In 1,4-dioxane for 0.5h; Ambient temperature; | 67% |
-
-
70-00-8
2'-deoxy-5-trifluoromethyluridine

-
-
123822-62-8
C10H9F3N2O4

| Conditions | Yield |
|---|---|
| With di-isopropyl azodicarboxylate; triphenylphosphine In acetonitrile at -15 - 0℃; for 3.16667h; | 67% |
-
-
874-60-2
4-methyl-benzoyl chloride

-
-
142-61-0
Hexanoyl chloride

-
-
70-00-8
2'-deoxy-5-trifluoromethyluridine

-
-
110073-57-9
4-Methyl-benzoic acid (2R,3S,5R)-5-(2,4-dioxo-5-trifluoromethyl-3,4-dihydro-2H-pyrimidin-1-yl)-2-hexanoyloxymethyl-tetrahydro-furan-3-yl ester

| Conditions | Yield |
|---|---|
| With pyridine | 66% |

-
-
2051-49-2
n-hexanoic anhydride

-
-
70-00-8
2'-deoxy-5-trifluoromethyluridine

-
-
89143-01-1
Hexanoic acid (2R,3S,5R)-5-(2,4-dioxo-5-trifluoromethyl-3,4-dihydro-2H-pyrimidin-1-yl)-2-hexanoyloxymethyl-tetrahydro-furan-3-yl ester

| Conditions | Yield |
|---|---|
| dmap In acetonitrile | 64% |
-
-
874-60-2
4-methyl-benzoyl chloride

-
-
70-00-8
2'-deoxy-5-trifluoromethyluridine

-
-
99502-67-7
4-Methyl-benzoic acid (2R,3S,5R)-5-(2,4-dioxo-5-trifluoromethyl-3,4-dihydro-2H-pyrimidin-1-yl)-3-hydroxy-tetrahydro-furan-2-ylmethyl ester

| Conditions | Yield |
|---|---|
| With pyridine | 62% |
-
-
2082-76-0
capric anhydride

-
-
70-00-8
2'-deoxy-5-trifluoromethyluridine

-
-
89142-99-4
Decanoic acid (2R,3S,5R)-2-decanoyloxymethyl-5-(2,4-dioxo-5-trifluoromethyl-3,4-dihydro-2H-pyrimidin-1-yl)-tetrahydro-furan-3-yl ester

| Conditions | Yield |
|---|---|
| dmap In acetonitrile | 59% |
-
-
874-60-2
4-methyl-benzoyl chloride

-
-
142-61-0
Hexanoyl chloride

-
-
70-00-8
2'-deoxy-5-trifluoromethyluridine

-
-
110073-58-0
4-Methyl-benzoic acid (2R,3S,5R)-5-(2,4-dioxo-5-trifluoromethyl-3,4-dihydro-2H-pyrimidin-1-yl)-3-hexanoyloxy-tetrahydro-furan-2-ylmethyl ester

| Conditions | Yield |
|---|---|
| With pyridine | 57% |

-
-
98-88-4
benzoyl chloride

-
-
70-00-8
2'-deoxy-5-trifluoromethyluridine

-
-
95969-47-4
N3-benzoyl-2'-deoxy-5-(trifluoromethyl)uridine

| Conditions | Yield |
|---|---|
| With triethylamine In N,N-dimethyl acetamide for 12h; Ambient temperature; | 56% |
-
-
623-65-4
palmitic anhydride

-
-
70-00-8
2'-deoxy-5-trifluoromethyluridine

-
-
89143-02-2
Hexadecanoic acid (2R,3S,5R)-5-(2,4-dioxo-5-trifluoromethyl-3,4-dihydro-2H-pyrimidin-1-yl)-2-hexadecanoyloxymethyl-tetrahydro-furan-3-yl ester

| Conditions | Yield |
|---|---|
| dmap In acetonitrile | 53% |
-
-
70-00-8
2'-deoxy-5-trifluoromethyluridine

-
-
112-67-4
n-hexadecanoyl chloride

-
-
89144-54-7
Hexadecanoic acid (2R,3S,5R)-5-(2,4-dioxo-5-trifluoromethyl-3,4-dihydro-2H-pyrimidin-1-yl)-3-hydroxy-tetrahydro-furan-2-ylmethyl ester

| Conditions | Yield |
|---|---|
| With pyridine | 52% |
Trifluridine Chemical Properties
IUPAC Name: 1-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-5-(trifluoromethyl) pyrimidine-2,4-dione
Empirical Formula: C10H11F3N2O5
Molecular Weight: 296.1999g/mol
EINECS: 200-722-8
Structure of Trifluridine (CAS NO.70-00-8):
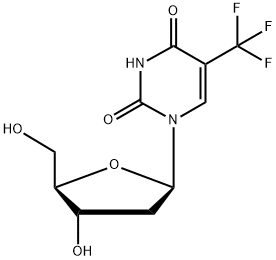
Index of Refraction: 1.534
Molar Refractivity: 55.92 cm3
Molar Volume: 179.8 cm3
Polarizability: 22.17×10-24cm3
Surface Tension: 56.1 dyne/cm
Density: 1.646 g/cm3
Melting Point: 190-193 °C(lit.)
Product Categories: Pharmaceutical Raw Materials;Pyrimidine series;API intermediates;Nucleotides and Nucleosides;Antivirals for Research and Experimental Use;Biochemistry;Chemical Reagents for Pharmacology Research;Nucleosides and their analogs;Nucleosides, Nucleotides & Related Reagents;Bases & Related Reagents;Nucleotides
Canonical SMILES: C1C(C(OC1N2C=C(C(=O)NC2=O)C(F)(F)F)CO)O
Isomeric SMILES: C1[C@@H]([C@H](O[C@H]1N2C=C(C(=O)NC2=O)C(F)(F)F)CO)O
InChI: InChI=1S/C10H11F3N2O5/c11-10(12,13)4-2-15(9(19)14-8(4)18)7-1-5(17)6(3-16)20-7/h2,5-7,16-17H,1,3H2,(H,14,18,19)/t5-,6+,7+/m0/s1
InChIKey: VSQQQLOSPVPRAZ-RRKCRQDMSA-N
Trifluridine Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| monkey | LDLo | intravenous | 400mg/kg (400mg/kg) | Government Reports Announcements. Vol. 74(9), Pg. 60, 1974. | |
| mouse | LD50 | intraperitoneal | 1931mg/kg (1931mg/kg) | National Cancer Institute Screening Program Data Summary, Developmental Therapeutics Program. Vol. JAN1986, | |
| mouse | LD50 | intravenous | 3381mg/kg (3381mg/kg) | Progress Report Submitted to the National Cancer Institute by Arthur D. Little, Inc. Vol. -, Pg. 260, 1967. | |
| rat | LD50 | intravenous | 2946mg/kg (2946mg/kg) | LIVER: OTHER CHANGES | Progress Report Submitted to the National Cancer Institute by Arthur D. Little, Inc. Vol. -, Pg. 260, 1967. |
Trifluridine Safety Profile
Hazard Codes:  Xn,
Xn, Xi
Xi
Risk Statements: 20/21/22-40
R20/21/22:Harmful by inhalation, in contact with skin and if swallowed.
R40:Limited evidence of a carcinogenic effect.
Safety Statements: 22-36
S22:Do not breathe dust.
S36:Wear suitable protective clothing.
WGK Germany: 3
RTECS: XP2087500
Hazard Note: Keep Cold
Trifluridine Specification
Trifluridine , its cas register number is 70-00-8. It also can be called 2'-Deoxy-5-(trifluoromethyl)uridine ; 2,4(1H,3H)-Pyrimidinedione, 1-(2-deoxy-beta-D-ribofuranosyl)-5-(trifluoromethyl)- ; 5-(Trifluoromethyl)deoxyuridine ; 5-Trifluoro-2'-deoxythymidine ; 5-Trifluoromethyl-2-deoxyuridine ; F3DThd ; F3T ; F3TDR ; NSC 529182 ; NSC 75520 ; TFDU ;
Trifluoromethyldeoxyuridine ; Trifluorothymidine ; Trifluridina ; Trifluridine ; Trifluridinum ; UNII-RMW9V5RW38 ; Uridine, 2'-deoxy-5-(trifluoromethyl)- ; Viroptic .
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View