-
Name
o-Anisidine
- EINECS 201-963-1
- CAS No. 90-04-0
- Article Data321
- CAS DataBase
- Density 1.092 g/cm3
- Solubility water: 13g/L (20 °C)
- Melting Point 3-6 °C
- Formula C7H9NO
- Boiling Point 225 °C
- Molecular Weight 123.155
- Flash Point 107 °C
- Transport Information UN 2431 6.1/PG 3
- Appearance light red or light yellow oily liquid
- Safety 53-45
- Risk Codes 45-23/24/25-68
-
Molecular Structure
-
Hazard Symbols
 T,
T, Xi
Xi
- Synonyms o-Anisidine;o-Anisidine(8CI);1-Amino-2-methoxybenzene;2-Aminoanisole;2-Aminomethoxybenzene;2-Methoxy-1-aminobenzene;2-Methoxyaniline;2-Methoxybenzenamine;2-Methoxyphenylamine;NSC 3122;o-Aminoanisole;o-Aminomethoxybenzene;o-Methoxyaniline;o-Methoxyphenylamine;
- PSA 35.25000
- LogP 1.85860
Synthetic route

| Conditions | Yield |
|---|---|
| With bis(tri-ortho-tolylphosphine)palladium(0); (R)-(-)-1-[(S)-2-(dicyclohexylphosphino)ferrocenyl]ethyl-di-tert-butylphosphine; ammonia; sodium t-butanolate In 1,4-dioxane at 100℃; for 12h; Inert atmosphere; | 95% |
| With ammonium hydroxide In water at 20℃; for 9h; Green chemistry; | 94% |
| With [N,N'-bis(5-sulfonatosalicylidene)-1,2-diaminoethane]copper disodium salt; ammonia; sodium hydroxide In water at 120℃; for 12h; sealed tube; | 90% |

| Conditions | Yield |
|---|---|
| With borane-ammonia complex In methanol; water at 20℃; for 0.0833333h; | 99% |
| With hydrogen In neat (no solvent) at 59.84℃; under 30003 Torr; for 3.5h; Autoclave; | 99.1% |
| With sodium tetrahydroborate In methanol; water at 20℃; for 0.0833333h; Sealed tube; Green chemistry; | 99% |

| Conditions | Yield |
|---|---|
| With ammonium hydroxide In water at 20℃; for 9h; Green chemistry; | 95% |
| With ammonium hydroxide; caesium carbonate In acetonitrile for 7h; Reflux; Green chemistry; | 91% |
| With iron(III) oxide; sodium hydroxide; copper(l) iodide; ammonia In ethanol; water at 90℃; for 16h; | 90% |

| Conditions | Yield |
|---|---|
| With tris(dibenzylideneacetone)dipalladium (0); lithium hexamethyldisilazane; CyJohnPhos; 1,1,1-triphenylsilylamine In toluene at 100℃; for 17h; | 98% |
| With bis(tri-ortho-tolylphosphine)palladium(0); (R)-(-)-1-[(S)-2-(dicyclohexylphosphino)ferrocenyl]ethyl-di-tert-butylphosphine; ammonia; sodium t-butanolate In 1,4-dioxane at 100℃; for 24h; Inert atmosphere; | 89% |
| With bis[chloro(1,2,3-trihapto-allylbenzene)palladium(II)]; N-[2-(di(1-adamantyl)phosphino)phenyl]morpholine; ammonia; sodium t-butanolate In 1,4-dioxane at 20℃; for 14h; Inert atmosphere; chemoselective reaction; | 88% |
-
-
20442-97-1
2-methoxylphenyl azide

-
-
90-04-0
2-methoxy-phenylamine

| Conditions | Yield |
|---|---|
| With zinc(II) tetrahydroborate In 1,2-dimethoxyethane for 3h; Ambient temperature; | 89% |
| Stage #1: 2-methoxylphenyl azide With hydrazine hydrate for 0.166667h; Inert atmosphere; Stage #2: for 12h; Irradiation; chemoselective reaction; | 87% |
| With water for 5h; Inert atmosphere; UV-irradiation; Sealed tube; chemoselective reaction; | 66% |

| Conditions | Yield |
|---|---|
| With ((+/-)-binap)Ni[P(OPh)3]2*2PhCH3; ammonia; 2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl; sodium t-butanolate In 1,4-dioxane at 120℃; for 18h; Inert atmosphere; Sealed tube; | A n/a B 70% |
| With C28H30Cl5N3Pd; ammonia; lithium isopropoxide; sodium t-butanolate In 1,4-dioxane at 100℃; for 2h; Reagent/catalyst; Inert atmosphere; Schlenk technique; | A n/a B 68% |
-
-
13513-82-1
1-(2-methoxyphenyl)ethanol

-
-
90-04-0
2-methoxy-phenylamine

| Conditions | Yield |
|---|---|
| With sodium azide; trifluoroacetic acid In hexane at 40℃; for 4h; Sealed tube; | 60% |
| With sodium azide; methanesulfonic acid; trifluoroacetic acid In hexane at 40℃; for 10h; | 60% |

| Conditions | Yield |
|---|---|
| With hydrogen In ethanol at 100℃; under 22502.3 Torr; for 10h; Autoclave; |

| Conditions | Yield |
|---|---|
| With bis{rhodium[3,3'-(1,3-phenylene)bis(2,2-dimethylpropanoic acid)]}; tert-butyl N-tosyloxycarbamate at 20℃; for 12h; chemoselective reaction; | A 37% B 37% |
| With titanium; sulfuric acid; hydroxylamine In water; acetonitrile at 40℃; Electrochemical reaction; | |
| With sulfuric acid; titanium(IV); hydroxylamine; acetic acid In water at 40℃; Electrochemical reaction; Inert atmosphere; |

| Conditions | Yield |
|---|---|
| With hydrogen In water at 130℃; under 3750.38 Torr; for 10h; Molecular sieve; Autoclave; |


-
-
90-04-0
2-methoxy-phenylamine

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid In dichloromethane at 20℃; for 16h; Polystyrene; | 52% |

| Conditions | Yield |
|---|---|
| With tetrabutylammomium bromide at 105℃; for 0.283333h; Reagent/catalyst; Temperature; | 93% |
-
-
3032-81-3
3,5-dichloroiodobenzene

-
B
-
90-04-0
2-methoxy-phenylamine

-
C
-
613-55-8, 18978-15-9, 19129-74-9
2,2'-dimethoxyazobenzene

| Conditions | Yield |
|---|---|
| With copper(l) iodide; caesium carbonate; N,N`-dimethylethylenediamine In 1,4-dioxane at 60℃; for 14h; Goldberg Reaction; Inert atmosphere; | A 25% B 30% C 26% |


| Conditions | Yield |
|---|---|
| With formic acid; 10-mesityl-10H-phenothiazine; ascorbic acid In water; acetonitrile at 20℃; for 4h; Inert atmosphere; Irradiation; Sealed tube; | A 76% B 51% |
-
-
613-55-8, 18978-15-9, 19129-74-9
2,2'-dimethoxyazobenzene

-
-
90-04-0
2-methoxy-phenylamine

| Conditions | Yield |
|---|---|
| With ammonium bromide; aluminium In methanol for 0.25h; sonication; | 90% |
| With 4,4'-di-tert-butylbiphenyl; lithium; nickel dichloride In tetrahydrofuran at 20℃; Reduction; | 74% |
| With SO2 In hydrogenchloride in the presence of I2, HI, or KI; |
-
-
23896-88-0
formic acid o-anisidide

-
-
90-04-0
2-methoxy-phenylamine

| Conditions | Yield |
|---|---|
| With water; sodium hydroxide In ethanol at 65 - 70℃; for 1h; | 96% |
| With sodium hydroxide In ethanol; water at 40℃; Kinetics; |

| Conditions | Yield |
|---|---|
| With formic acid; 10-mesityl-10H-phenothiazine; ascorbic acid In water; acetonitrile at 20℃; for 4h; Inert atmosphere; Irradiation; Sealed tube; | A 70% B 73% |
-
-
64-18-6
formic acid

-
-
91-23-6
2-Nitroanisole

-
A
-
23896-88-0
formic acid o-anisidide

-
B
-
90-04-0
2-methoxy-phenylamine

| Conditions | Yield |
|---|---|
| platinum on charcoal; sulfided; vanadia In water at 90 - 95℃; for 2.5h; | A 24.8% B 62.8% |

-
-
110-71-4
1,2-dimethoxyethane

-
-
74-87-3
methylene chloride

-
-
95-55-6
2-amino-phenol

-
-
90-04-0
2-methoxy-phenylamine

| Conditions | Yield |
|---|---|
| With sodium methylate In methanol | 67.8% |


| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide; CYANAMID; bis-[(trifluoroacetoxy)iodo]benzene In acetonitrile at 20℃; for 1h; chemoselective reaction; | 88% |
| With sodium hydroxide; hydroxylamine-O-sulfonic acid In acetonitrile at 20℃; for 16h; | 80% |
| With N-Bromosuccinimide; N-methoxylamine hydrochloride; bis-[(trifluoroacetoxy)iodo]benzene In acetonitrile at 20℃; | 79% |

| Conditions | Yield |
|---|---|
| With ethene; 5%-palladium/activated carbon; ammonium acetate; potassium carbonate In acetonitrile at 90℃; under 760.051 Torr; for 15h; Reagent/catalyst; Schlenk technique; | 84% |
| With styrene; ammonium hydroxide In 1-methyl-pyrrolidin-2-one at 130℃; for 20h; Sealed tube; Inert atmosphere; | 72% |

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; Inert atmosphere; | 100% |
| With cadmium(II) oxide at 80℃; for 0.166667h; Neat (no solvent); Microwave irradiation; | 98% |
| With tris(pentafluorophenyl)borate In neat (no solvent) at 20℃; for 0.0166667h; Green chemistry; | 95% |

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane for 2h; | 100% |
| With hydroxyapatite supported copper(I) oxide In acetonitrile at 50℃; for 0.0833333h; | 91% |
| With triethylamine at 0 - 20℃; for 3h; | 90% |
-
-
90-04-0
2-methoxy-phenylamine

-
-
98-09-9
benzenesulfonyl chloride

-
-
21226-32-4
N-(2-methoxyphenyl)benzenesulfonamide

| Conditions | Yield |
|---|---|
| With pyridine for 12h; Ambient temperature; | 100% |
| With sodium hydroxide at 20℃; for 1h; Addition; | 85% |
| 51% |
-
-
90-04-0
2-methoxy-phenylamine

-
-
107-07-3
2-chloro-ethanol

-
-
28005-76-7
2-[2-hydroxyethyl-(2-methoxyphenyl)amino]ethanol

| Conditions | Yield |
|---|---|
| With calcium carbonate In water at 110℃; for 72h; | 100% |
| With potassium carbonate at 95℃; for 22h; | 98% |
| With potassium carbonate | 88% |
-
-
90-04-0
2-methoxy-phenylamine

-
-
87-13-8
diethyl 2-ethoxymethylenemalonate

-
-
104007-09-2
2-[(2-methoxyphenylamino)-methylene]-malonic acid diethyl ester

| Conditions | Yield |
|---|---|
| In ethanol at 90℃; for 18h; | 100% |
| at 130℃; | 99% |
| In neat (no solvent) at 120℃; for 0.75h; | 98% |

| Conditions | Yield |
|---|---|
| With Oxone; potassium hydroxide; disodium hydrogenphosphate; tetra(n-butyl)ammonium hydrogensulfate In dichloromethane; water; acetone at 0℃; for 0.75h; pH=7.5-8.5; | 100% |
| With tert.-butylhydroperoxide; 3 A molecular sieve; zirconium(IV) tert-butoxide In dichloromethane for 2h; Ambient temperature; | 85% |
| With dihydrogen peroxide; acetonitrile In aq. buffer at 20℃; for 1h; pH=11; Green chemistry; | 84% |
-
-
90-04-0
2-methoxy-phenylamine

-
-
407-25-0
trifluoroacetic anhydride

-
-
14815-12-4
N-(2-methoxyphenyl)-2,2,2-trifluoro-acetamide

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran at 0 - 20℃; for 0.75h; | 100% |
| With pyridine In dichloromethane at 0 - 20℃; for 72h; Inert atmosphere; | 99% |
| In diethyl ether | |
| Stage #1: 2-methoxy-phenylamine With pyridine In dichloromethane at 0℃; for 0.5h; Stage #2: trifluoroacetic anhydride In dichloromethane at 0 - 30℃; for 1.25h; | |
| With pyridine In dichloromethane at 0 - 20℃; for 1.25h; |
-
-
90-04-0
2-methoxy-phenylamine

-
-
20442-97-1
2-methoxylphenyl azide

| Conditions | Yield |
|---|---|
| With tert.-butylnitrite; trimethylsilylazide In acetonitrile at 20℃; for 1h; Inert atmosphere; | 100% |
| Stage #1: 2-methoxy-phenylamine With sulfuric acid; sodium nitrite In water; acetic acid at 0 - 5℃; for 0.166667h; Stage #2: With sodium azide In water; acetic acid at 0 - 5℃; for 3h; | 99% |
| Stage #1: 2-methoxy-phenylamine With hydrogenchloride; sodium nitrite In water at 0℃; Inert atmosphere; Stage #2: With sodium azide; sodium carbonate In water at 0 - 20℃; pH=7 -Ca. 8; Inert atmosphere; | 96.9% |
-
-
5271-67-0
2-Thiophenecarbonyl chloride

-
-
90-04-0
2-methoxy-phenylamine

-
-
136340-86-8
N-(2-methoxyphenyl)-2-thiophenecarboxamide

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0 - 20℃; | 100% |
| In toluene |

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In water; ethyl acetate at 20℃; for 6h; | 100% |
| With sodium carbonate In dichloromethane Ambient temperature; | 98% |
| With triethylamine In diethyl ether for 16h; Ambient temperature; | 98% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide; disodium hydrogenphosphate; tetra(n-butyl)ammonium hydrogensulfate In dichloromethane; acetone other substituted anilines; | 100% |
-
-
90-04-0
2-methoxy-phenylamine

-
-
120-14-9
3,4-dimethoxy-benzaldehyde

-
-
82363-28-8
N-m,p-dimethoxybenzylidene-o-anisidine

| Conditions | Yield |
|---|---|
| In toluene Heating; | 100% |
| In neat (no solvent) Time; Irradiation; Green chemistry; | 72.6% |
| With 5A molecular sieve In toluene for 4h; |
-
-
90-04-0
2-methoxy-phenylamine

-
-
623-47-2
propynoic acid ethyl ester

-
-
115607-78-8
ethyl 3-<(2-methoxyphenyl)amino>acrylate

| Conditions | Yield |
|---|---|
| In ethanol for 3h; Heating; | 100% |
-
-
59565-09-2
2-(vinyloxy)ethyl isothiocyanate

-
-
90-04-0
2-methoxy-phenylamine

| Conditions | Yield |
|---|---|
| at 38℃; | 100% |
-
-
90-04-0
2-methoxy-phenylamine

-
-
131019-88-0
N-(2-methoxyphenyl)-4,5,6,7-tetrahydro-1H-benzimidazole-5-carboxamide

| Conditions | Yield |
|---|---|
| In 1,2-dichloro-ethane for 2h; Ambient temperature; | 100% |
| In 1,2-dichloro-ethane for 2h; Ambient temperature; Yield given; |
-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
90-04-0
2-methoxy-phenylamine

-
-
154150-18-2
tert-butyl 2-methoxyphenylcarbamate

| Conditions | Yield |
|---|---|
| In tetrahydrofuran Heating; | 100% |
| In tetrahydrofuran Inert atmosphere; Reflux; | 100% |
| In tetrahydrofuran for 17h; Ambient temperature; | 99% |

| Conditions | Yield |
|---|---|
| With zirconium(IV) chloride at 20℃; for 0.25h; | 100% |
| With niobium pentachloride In dichloromethane at 20℃; for 3h; | 91% |
| cadmium(II) chloride In dichloromethane at 20℃; for 3h; | 91% |
-
-
90-04-0
2-methoxy-phenylamine

| Conditions | Yield |
|---|---|
| Stage #1: trifluoromethyl dihydro-1,4-dioxin-3-carbonyl chloride With pyridine; polystyrene-bound 4-hydroxy-3-nitrobenzophenone In dichloromethane at 20℃; for 24h; Acylation; Stage #2: 2-methoxy-phenylamine With triethylamine In acetonitrile for 2.5h; Acylation; Heating; | 100% |
-
-
98-01-1
furfural

-
-
90-04-0
2-methoxy-phenylamine

-
-
14744-30-0
N-[(furan-2-yl)methylene]-2-methoxybenzenamine

| Conditions | Yield |
|---|---|
| In methanol at 20℃; for 24h; | 100% |
| In water at 20℃; for 2h; | 58% |
| In dichloromethane for 0.5h; |
-
-
32315-10-9
bis(trichloromethyl) carbonate

-
-
90-04-0
2-methoxy-phenylamine

-
-
700-87-8
1-Isocyanato-2-methoxy-benzene

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane | 100% |
| In dichloromethane at 20℃; for 1.5h; | |
| In dichloromethane at 0℃; for 0.5h; | |
| With triethylamine In 1,2-dichloro-ethane at 0 - 85℃; for 8.5h; Inert atmosphere; | |
| With triethylamine In 1,2-dichloro-ethane at 0 - 85℃; for 8.5h; Inert atmosphere; |

| Conditions | Yield |
|---|---|
| In methanol | 100% |

| Conditions | Yield |
|---|---|
| With 1,1'-bis(diphenylphosphino)ferrocene; tris(dibenzylideneacetone)dipalladium (0); sodium t-butanolate In toluene at 110℃; for 12h; | 100% |
| With palladium diacetate; sodium t-butanolate; tri tert-butylphosphoniumtetrafluoroborate In toluene at 100℃; for 24h; Schlenk technique; Inert atmosphere; | 100% |
| With potassium hydroxide; copper(II) oxide In dimethyl sulfoxide at 110℃; for 1.7h; | 98% |

| Conditions | Yield |
|---|---|
| With zirconium(IV) chloride at 20℃; for 0.25h; | 100% |
| With activated-[Zr6O4(OH)4(BDC-C5H4NOS)6]*4.5H2O*3.5DMF In neat (no solvent) at 20℃; for 12h; regioselective reaction; | 95% |
| With niobium pentachloride In dichloromethane at 20℃; for 1.5h; | 91% |
| With tungstophosphoric acid In dichloromethane at 20℃; for 3h; | 85% |
| With zirconyl triflate In acetonitrile at 20℃; for 1h; regioselective reaction; | 82% |

| Conditions | Yield |
|---|---|
| With ammonium formate; palladium on activated charcoal In methanol; water at 20℃; for 3.5h; | 100% |
-
-
90-04-0
2-methoxy-phenylamine

-
-
107-12-0
propiononitrile

-
A
-
107411-34-7
2-methoxy-N,N-dipropyl aniline

-
B
-
139944-56-2
2-methoxy-N-propylaniline

| Conditions | Yield |
|---|---|
| With ammonium formate; palladium on activated charcoal In methanol; water at 20℃; for 3.1h; | A n/a B 100% |


| Conditions | Yield |
|---|---|
| With 4 A molecular sieve In toluene at 20℃; for 24h; Condensation; | 100% |
| In dichloromethane at 20℃; for 18h; Molecular sieve; |

| Conditions | Yield |
|---|---|
| at 20℃; for 0.133333h; Milling; Inert atmosphere; | 100% |
| In methanol for 5h; Heating / reflux; | 85% |
| In methanol at 5 - 20℃; |

| Conditions | Yield |
|---|---|
| With magnesium sulfate In dichloromethane at 20℃; for 12h; | 100% |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 20℃; | 100% |
-
-
90-04-0
2-methoxy-phenylamine

-
-
623-47-2
propynoic acid ethyl ester

-
-
142781-90-6
ethyl 3-(2-methoxyphenylamino)acrylate

| Conditions | Yield |
|---|---|
| In ethanol | 100% |
o-Anisidine Specification
The o-Anisidine with CAS registry number of 90-04-0 is also known as 1-Amino-2-methoxybenzene. The IUPAC name is 2-Methoxyaniline. It belongs to product categories of Intermediates of Dyes and Pigments; Intermediates. Its EINECS registry number is 201-963-1. In addition, the formula is C7H9NO and the molecular weight is 123.15. This chemical is a light red or light yellow oily liquid and should be stored in dry, ventilated place without sunlight, moisture and heat.
Physical properties about o-Anisidine are: (1)XLogP3: 1.2; (2)H-Bond Donor: 1; (3)H-Bond Acceptor: 2; (4)Rotatable Bond Count: 1; (5)Exact Mass: 123.068414; (6)MonoIsotopic Mass: 123.068414; (7)Topological Polar Surface Area: 35.2; (8)Heavy Atom Count: 9; (9)Complexity: 85; (10)Covalently-Bonded Unit Count: 1.
Preparation of o-Anisidine: it is prepared by reduction of o-nitrophenyl ether. The o-nitrophenyl ether can be obtained by oxy reaction of o-chlorotoluene. Sodium sulfide or iron can be used as reducing agent. The process is as follows. The first step at 70-78 °C and the second step at 118-120 °C.
(1) C6H4NClO2+CH3OH+NaOH→C7H7NO3+NaCl+H2O (2) 4C7H7NO3+6Na2S+7H2O→C7H6NO+3Na2S3O3+6NaOH
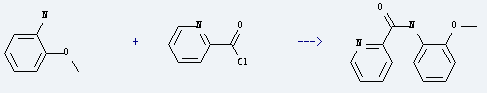
Uses of o-Anisidine: it can be used for making dyes, azoic dyes and naphthol as-ol and other dyes. It also can be used as dyes intermediate. Besides, it is used as reagent for testing codeine and alkaloid. What's more, this chemical is used to prepare pyridine-2-carboxylic acid o-anisidide by reaction with pyridine-2-carbonyl chloride. The reation needs reagent pyridine and the yield is about 40%.
When you are using this chemical, please be cautious about it. As a chemical, it is toxic by inhalation, in contact with skin and if swallowed. It has possible risk of irreversible effects and may cause cancer. During using it, obtain special instructions before use. In case of accident or if you feel unwell seek medical advice immediately.
You can still convert the following datas into molecular structure:
1. Canonical SMILES: COC1=CC=CC=C1N
2. InChI: InChI=1S/C7H9NO/c1-9-7-5-3-2-4-6(7)8/h2-5H,8H2,1H3
3. InChIKey: VMPITZXILSNTON-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| bird - wild | LD50 | oral | 422mg/kg (422mg/kg) | Archives of Environmental Contamination and Toxicology. Vol. 12, Pg. 355, 1983. | |
| mouse | LD50 | oral | 1400mg/kg (1400mg/kg) | KIDNEY, URETER, AND BLADDER: OTHER CHANGES BLOOD: NORMOCYTIC ANEMIA BLOOD: OTHER CHANGES | IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man. Vol. 27, Pg. 63, 1982. |
| rabbit | LD50 | oral | 870mg/kg (870mg/kg) | KIDNEY, URETER, AND BLADDER: OTHER CHANGES BLOOD: OTHER CHANGES BLOOD: NORMOCYTIC ANEMIA | IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man. Vol. 27, Pg. 63, 1982. |
| rat | LD50 | oral | 1150mg/kg (1150mg/kg) | Trudy Leningradskogo Sanitarno-Gigienicheskogo Meditsinskogo Instituta. Vol. 128, Pg. 14, 1979. |
Related Products
- o-Anisidine
- o-Anisidine antimonyl tartrate
- o-Anisidine hydrochloride
- 9004-06-2
- 9004-07-3
- 9004-10-8
- 9004-14-2
- 9004-32-4
- 9004-34-6
- 9004-35-7
- 9004-36-8
- 9004-38-0
- 9004-41-5
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View