-
Name
(1S,4S)-2-BOC-2,5-DIAZABICYCLO[2.2.1]HEPTANE
- EINECS -0
- CAS No. 113451-59-5
- Article Data8
- CAS DataBase
- Density 1.105 g/cm3
- Solubility Insoluble in water.
- Melting Point 74-76 °C(lit.)
- Formula C10H18N2O2
- Boiling Point 276.372 °C at 760 mmHg
- Molecular Weight 294.372
- Flash Point 120.946 °C
- Transport Information
- Appearance White to slightly beige powder
- Safety 22-24/25
- Risk Codes 34
-
Molecular Structure
- Hazard Symbols
- Synonyms 1,1-Dimethylethyl(S,S)-2,5-diazabicyclo[2.2.1]heptane-5-carboxylate;N-Boc-(1S,4S)-2,5-Diazabicyclo[2.2.1]heptane;tert-Butyl(1S,4S)-(-)-2,5-diazabicyclo[2.2.1]heptane-2-carboxylate;tert-Butyl(1S,4S)-2,5-diazabicyclo[2.2.1]heptane-2-carboxylate;2,5-Diazabicyclo[2.2.1]heptane-2-carboxylicacid, 1,1-dimethylethyl ester, (1S)-;(1S,4S)-(-)-2-Boc-2,5-diazabicyclo[2.2.1]heptane;(1S,4S)-2,5-Diazabicyclo[2.2.1]heptane-2-carboxylic acid tert-butyl ester;(1S,4S)-N-Boc-2,5-diazabicyclo[2.2.1]heptane;(1S,4S)-tert-Butyl2,5-diazabicyclo[2.2.1]heptane-2-carboxylate;
- PSA 41.57000
- LogP 1.23430
Synthetic route
-
-
113451-54-0
(2S,4R)-2-(azidomethyl)-1-(tert-butoxycarbonyl)-4-<(methylsulfonyl)oxy>pyrrolidine

-
-
113451-59-5
(1S,4S)-2,5-Diaza-bicyclo[2.2.1]heptane-2-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal In methanol | 100% |
| Stage #1: (2S,4R)-2-(azidomethyl)-1-(tert-butoxycarbonyl)-4-<(methylsulfonyl)oxy>pyrrolidine With triphenylphosphine In tetrahydrofuran at 20℃; for 2h; Staudinger Azide Reduction; Stage #2: With water In tetrahydrofuran for 18h; | 71% |
-
-
132666-68-3
(1S,4S)-5-Benzyl-2-tert-butoxycarbonyl-2,5-diazabicyclo<2.2.1>heptane

-
-
113451-59-5
(1S,4S)-2,5-Diaza-bicyclo[2.2.1]heptane-2-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With palladium hydroxide 10 wt. % on activated carbon; hydrogen In methanol under 3102.97 Torr; | 90% |
| With palladium 10% on activated carbon; hydrogen In methanol at 50℃; under 22502.3 Torr; for 16h; | 85% |
| With 10% palladium on carbon; hydrogen | 76% |
| With palladium 10% on activated carbon; hydrogen In dichloromethane under 3102.97 Torr; | 10.4 g |
-
-
922139-40-0
tert-butyl (2S,4S)-4-amino-2-(hydroxymethyl)pyrrolidin-1-carboxylate

-
-
113451-59-5
(1S,4S)-2,5-Diaza-bicyclo[2.2.1]heptane-2-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With di-isopropyl azodicarboxylate; triphenylphosphine In tetrahydrofuran at 22 - 25℃; for 12h; Inert atmosphere; | 80.6% |
-
-
40216-83-9
L-4-hydroxyproline methyl ester hydrochloride

-
-
113451-59-5
(1S,4S)-2,5-Diaza-bicyclo[2.2.1]heptane-2-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1.1: triethylamine / dichloromethane / 17 h / 0 °C 2.1: lithium borohydride / tetrahydrofuran / 26 h / 0 - 20 °C 3.1: pyridine / dichloromethane / 18 h / 40 °C 4.1: trimethylsilylazide; tetrabutyl ammonium fluoride / tetrahydrofuran / 18 h / 70 °C 5.1: triethylamine / dichloromethane / 0.5 h / 0 °C 6.1: triphenylphosphine / tetrahydrofuran / 2 h / 20 °C 6.2: 18 h View Scheme |
-
-
51-35-4
4R-4-hydroxyproline

-
-
113451-59-5
(1S,4S)-2,5-Diaza-bicyclo[2.2.1]heptane-2-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1.1: thionyl chloride; hydrogenchloride / water / 18 h / 20 °C / Reflux 2.1: triethylamine / dichloromethane / 17 h / 0 °C 3.1: lithium borohydride / tetrahydrofuran / 26 h / 0 - 20 °C 4.1: pyridine / dichloromethane / 18 h / 40 °C 5.1: trimethylsilylazide; tetrabutyl ammonium fluoride / tetrahydrofuran / 18 h / 70 °C 6.1: triethylamine / dichloromethane / 0.5 h / 0 °C 7.1: triphenylphosphine / tetrahydrofuran / 2 h / 20 °C 7.2: 18 h View Scheme | |
| Multi-step reaction with 6 steps 1.1: sodium carbonate / water 1.3: 20 h 2.1: toluene / Reflux 3.1: hydrogen bromide 4.1: sodium methylate / methanol / 20 °C 5.1: triethylamine / dichloromethane / 0 - 20 °C 6.1: hydrogen; palladium hydroxide 10 wt. % on activated carbon / methanol / 3102.97 Torr View Scheme |
-
-
74844-91-0
1-tert-butyl 2-methyl (2S,4R)-4-hydroxy-1,2-pyrrolidinedicarboxylate

-
-
113451-59-5
(1S,4S)-2,5-Diaza-bicyclo[2.2.1]heptane-2-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: lithium borohydride / tetrahydrofuran / 26 h / 0 - 20 °C 2.1: pyridine / dichloromethane / 18 h / 40 °C 3.1: trimethylsilylazide; tetrabutyl ammonium fluoride / tetrahydrofuran / 18 h / 70 °C 4.1: triethylamine / dichloromethane / 0.5 h / 0 °C 5.1: triphenylphosphine / tetrahydrofuran / 2 h / 20 °C 5.2: 18 h View Scheme | |
| Multi-step reaction with 4 steps 1: triethylamine / dichloromethane / 2 h / 5 - 25 °C 2: sodium azide / N,N-dimethyl-formamide / 3 h / 90 °C 3: potassium borohydride; lithium chloride / tetrahydrofuran / 12 h / 25 °C 4: triphenylphosphine; di-isopropyl azodicarboxylate / tetrahydrofuran / 12 h / 22 - 25 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 4 steps 1: sodium tetrahydroborate / methanol / 18 h / 20 - 35 °C / Cooling with ice; Large scale 2: triethylamine / dichloromethane / 1 h / 20 - 35 °C / Cooling with liquid nitrogen; Large scale 3: 3 h / 80 °C / Large scale 4: palladium 10% on activated carbon; hydrogen / methanol / 16 h / 50 °C / 22502.3 Torr View Scheme |
-
-
61478-26-0
tert-butyl (2S,4R)-4-hydroxy-2-hydroxymethyl-pyrrolidine-1-carboxylate

-
-
113451-59-5
(1S,4S)-2,5-Diaza-bicyclo[2.2.1]heptane-2-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: pyridine / dichloromethane / 18 h / 40 °C 2.1: trimethylsilylazide; tetrabutyl ammonium fluoride / tetrahydrofuran / 18 h / 70 °C 3.1: triethylamine / dichloromethane / 0.5 h / 0 °C 4.1: triphenylphosphine / tetrahydrofuran / 2 h / 20 °C 4.2: 18 h View Scheme | |
| Multi-step reaction with 3 steps 1: triethylamine / dichloromethane / 1 h / 20 - 35 °C / Cooling with liquid nitrogen; Large scale 2: 3 h / 80 °C / Large scale 3: palladium 10% on activated carbon; hydrogen / methanol / 16 h / 50 °C / 22502.3 Torr View Scheme |
-
-
194089-91-3
tert-butyl (2S,4R)-4-hydroxy-2-({[(4-methylphenyl)sulfonyl]oxy}methyl)-pyrrolidine-1-carboxylate

-
-
113451-59-5
(1S,4S)-2,5-Diaza-bicyclo[2.2.1]heptane-2-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: trimethylsilylazide; tetrabutyl ammonium fluoride / tetrahydrofuran / 18 h / 70 °C 2.1: triethylamine / dichloromethane / 0.5 h / 0 °C 3.1: triphenylphosphine / tetrahydrofuran / 2 h / 20 °C 3.2: 18 h View Scheme |
-
-
114676-96-9
(2S,4R)-tert-butyl 2-(azidomethyl)-4-hydroxypyrrolidine-1-carboxylate

-
-
113451-59-5
(1S,4S)-2,5-Diaza-bicyclo[2.2.1]heptane-2-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: triethylamine / dichloromethane / 0.5 h / 0 °C 2.1: triphenylphosphine / tetrahydrofuran / 2 h / 20 °C 2.2: 18 h View Scheme |
-
-
84520-68-3
N-tert-butoxycarbonyl-cis-4-azido-L-proline methyl ester

-
-
113451-59-5
(1S,4S)-2,5-Diaza-bicyclo[2.2.1]heptane-2-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: potassium borohydride; lithium chloride / tetrahydrofuran / 12 h / 25 °C 2: triphenylphosphine; di-isopropyl azodicarboxylate / tetrahydrofuran / 12 h / 22 - 25 °C / Inert atmosphere View Scheme |
-
-
84520-67-2
(2S,4R)-4-(methylsulfonyloxy)pyrrolidine-1,2-dicarboxylic acid 1-tert-butylester-2-methylester

-
-
113451-59-5
(1S,4S)-2,5-Diaza-bicyclo[2.2.1]heptane-2-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: sodium azide / N,N-dimethyl-formamide / 3 h / 90 °C 2: potassium borohydride; lithium chloride / tetrahydrofuran / 12 h / 25 °C 3: triphenylphosphine; di-isopropyl azodicarboxylate / tetrahydrofuran / 12 h / 22 - 25 °C / Inert atmosphere View Scheme |
-
-
5234-75-3
(2S,4R)-1-(4-tolylsulfonyl)-4-<(4-tolylsulfonyl)oxy>-2-<<(4-tolylsulfonyl)oxy>methyl>pyrrolidine

-
-
113451-59-5
(1S,4S)-2,5-Diaza-bicyclo[2.2.1]heptane-2-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: toluene / Reflux 2: hydrogen bromide 3: sodium methylate / methanol / 20 °C 4: triethylamine / dichloromethane / 0 - 20 °C 5: hydrogen; palladium hydroxide 10 wt. % on activated carbon / methanol / 3102.97 Torr View Scheme |
-
-
5234-72-0
(1S,4S)-5-Benzyl-2-tosyl-2,5-diazabicyclo<2.2.1>heptane

-
-
113451-59-5
(1S,4S)-2,5-Diaza-bicyclo[2.2.1]heptane-2-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: hydrogen bromide 2: sodium methylate / methanol / 20 °C 3: triethylamine / dichloromethane / 0 - 20 °C 4: hydrogen; palladium hydroxide 10 wt. % on activated carbon / methanol / 3102.97 Torr View Scheme |
-
-
100944-15-8, 134003-82-0, 116258-17-4
(1S,4S)-2-Benzyl-2,5-diazabicyclo<2.2.1>heptane dihydrobromide

-
-
113451-59-5
(1S,4S)-2,5-Diaza-bicyclo[2.2.1]heptane-2-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: sodium methylate / methanol / 20 °C 2: triethylamine / dichloromethane / 0 - 20 °C 3: hydrogen; palladium hydroxide 10 wt. % on activated carbon / methanol / 3102.97 Torr View Scheme | |
| Multi-step reaction with 3 steps 1: sodium methylate / methanol / 20 °C 2: triethylamine / dichloromethane / 0 - 20 °C 3: palladium 10% on activated carbon; hydrogen / dichloromethane / 3102.97 Torr View Scheme |
-
-
127641-07-0
(1S,4S)-N-Benzyl-2,5-diazabicyclo-[2.2.1]heptane

-
-
113451-59-5
(1S,4S)-2,5-Diaza-bicyclo[2.2.1]heptane-2-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: triethylamine / dichloromethane / 0 - 20 °C 2: hydrogen; palladium hydroxide 10 wt. % on activated carbon / methanol / 3102.97 Torr View Scheme | |
| Multi-step reaction with 2 steps 1: triethylamine / dichloromethane / 0 - 20 °C 2: palladium 10% on activated carbon; hydrogen / dichloromethane / 3102.97 Torr View Scheme |
-
-
1257295-06-9
2-(2-ethylbenzoimidazol-1-yl)-9-methyl-6-morpholin-4-yl-9H-purine-8-carbaldehyde

-
-
113451-59-5
(1S,4S)-2,5-Diaza-bicyclo[2.2.1]heptane-2-carboxylic acid tert-butyl ester

-
-
1257382-14-1
C30H39N9O3

| Conditions | Yield |
|---|---|
| Stage #1: 2-(2-ethylbenzoimidazol-1-yl)-9-methyl-6-morpholin-4-yl-9H-purine-8-carbaldehyde; (1S,4S)-2,5-Diaza-bicyclo[2.2.1]heptane-2-carboxylic acid tert-butyl ester In 1,2-dichloro-ethane at 20℃; for 3h; Molecular sieve; Stage #2: With sodium tris(acetoxy)borohydride In 1,2-dichloro-ethane at 20℃; for 17h; Molecular sieve; | 100% |
-
-
113451-59-5
(1S,4S)-2,5-Diaza-bicyclo[2.2.1]heptane-2-carboxylic acid tert-butyl ester

-
-
5260-20-8, 89487-38-7
(1S,4S)-2,5-Diazabicyclo<2.2.1>heptane dihydrochloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride In 1,4-dioxane; methanol at 20℃; for 0.5h; | 100% |
-
-
124-63-0
methanesulfonyl chloride

-
-
113451-59-5
(1S,4S)-2,5-Diaza-bicyclo[2.2.1]heptane-2-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran at 0 - 20℃; for 20h; | 100% |
| With triethylamine In tetrahydrofuran at 0 - 30℃; for 12h; Inert atmosphere; | 100% |
-
-
79-30-1
isobutyryl chloride

-
-
113451-59-5
(1S,4S)-2,5-Diaza-bicyclo[2.2.1]heptane-2-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; Inert atmosphere; | 100% |
-
-
4595-59-9
5-bromopyrimidine

-
-
113451-59-5
(1S,4S)-2,5-Diaza-bicyclo[2.2.1]heptane-2-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| 99% | |
| With sodium t-butanolate; tris-(dibenzylideneacetone)dipalladium(0); 2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl In toluene at 80 - 85℃; for 5h; | 91% |
| With tris-(dibenzylideneacetone)dipalladium(0); sodium t-butanolate In toluene at 80 - 85℃; for 5h; | 91% |
-
-
626-55-1
3-Bromopyridine

-
-
113451-59-5
(1S,4S)-2,5-Diaza-bicyclo[2.2.1]heptane-2-carboxylic acid tert-butyl ester

-
-
286946-36-9
(1S,4S)-tert-butyl 5-(pyridin-3-yl)-2,5-diazabicyclo[2.2.1]heptane-2-carboxylate

| Conditions | Yield |
|---|---|
| 99% | |
| With tris-(dibenzylideneacetone)dipalladium(0); 2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl; sodium t-butanolate In toluene at 85℃; for 64h; Inert atmosphere; | 99% |
| With sodium t-butanolate; tris-(dibenzylideneacetone)dipalladium(0); 2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl In toluene at 80 - 85℃; for 5h; |
-
-
16110-09-1
2,5 dichloropyridine

-
-
113451-59-5
(1S,4S)-2,5-Diaza-bicyclo[2.2.1]heptane-2-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| 99% |
-
-
64119-42-2
ethyl 6-chloro-5-cyano-2-methylnicotinate

-
-
113451-59-5
(1S,4S)-2,5-Diaza-bicyclo[2.2.1]heptane-2-carboxylic acid tert-butyl ester

-
-
898229-94-2
(1S,4S)-tert-butyl 5-(3-cyano-5-(ethoxycarbonyl)-6-methylpyridin-2-yl)-2,5-diazabicyclo[2.2.1]heptane-2-carboxylate

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 20℃; for 1h; | 99% |
| With N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 20℃; for 1h; | 99% |
-
-
15084-51-2
4-tert-butylbenzenesulfonyl chloride

-
-
113451-59-5
(1S,4S)-2,5-Diaza-bicyclo[2.2.1]heptane-2-carboxylic acid tert-butyl ester

-
-
1116571-82-4
C20H30N2O4S

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In dichloromethane at 20℃; for 48h; | 99% |
| With N-ethyl-N,N-diisopropylamine In dichloromethane | |
| With N-ethyl-N,N-diisopropylamine In dichloromethane at 20℃; for 16h; Inert atmosphere; |
-
-
153947-34-3
1-iodo-2-diphenylmethoxy-ethane

-
-
113451-59-5
(1S,4S)-2,5-Diaza-bicyclo[2.2.1]heptane-2-carboxylic acid tert-butyl ester

-
-
321365-86-0
(1S,4S)-2-[2-(diphenylmethoxy)ethyl]-5-t-Boc-2,5-diazabicyclo[2.2.1]heptane

| Conditions | Yield |
|---|---|
| With potassium carbonate In tetrahydrofuran Heating; | 98% |

-
-
113451-59-5
(1S,4S)-2,5-Diaza-bicyclo[2.2.1]heptane-2-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In 1-methyl-pyrrolidin-2-one at 50℃; | 98% |
-
-
108-86-1
bromobenzene

-
-
113451-59-5
(1S,4S)-2,5-Diaza-bicyclo[2.2.1]heptane-2-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With sodium t-butanolate; tris-(dibenzylideneacetone)dipalladium(0); 2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl In toluene at 80 - 85℃; for 5h; | 97% |
-
-
761440-64-6
1-(3-methoxy-4 nitrophenyl)piperidin-4-one

-
-
113451-59-5
(1S,4S)-2,5-Diaza-bicyclo[2.2.1]heptane-2-carboxylic acid tert-butyl ester

-
-
1089280-04-5
1,1-dimethylethyl (1S,4S)-5-{1-[3-(methyloxy)-4-nitrophenyl]-4-piperidinyl}-2,5-diazabicyclo[2.2.1]heptane-2-carboxylate

| Conditions | Yield |
|---|---|
| Stage #1: 1-(3-methoxy-4 nitrophenyl)piperidin-4-one; (1S,4S)-2,5-Diaza-bicyclo[2.2.1]heptane-2-carboxylic acid tert-butyl ester With sodium tris(acetoxy)borohydride; acetic acid; triethylamine In dichloromethane for 1h; Stage #2: With sodium hydrogencarbonate In dichloromethane; water | 96% |
-
-
113451-59-5
(1S,4S)-2,5-Diaza-bicyclo[2.2.1]heptane-2-carboxylic acid tert-butyl ester

-
-
13831-31-7
Acetoxyacetyl chloride

-
-
1146691-54-4
5-(2-acetoxy-acetyl)-2,5-diaza-bicyclo[2.2.1]heptane-2-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| Stage #1: (1S,4S)-2,5-Diaza-bicyclo[2.2.1]heptane-2-carboxylic acid tert-butyl ester With triethylamine In dichloromethane at 5℃; for 0.833333h; Stage #2: Acetoxyacetyl chloride In dichloromethane for 16h; | 96% |
-
-
51788-77-3
1-(2,4,6-trifluorophenyl)ethan-1-one

-
-
113451-59-5
(1S,4S)-2,5-Diaza-bicyclo[2.2.1]heptane-2-carboxylic acid tert-butyl ester

-
-
1196958-94-7
tert-butyl (1S,4S)-5-(2-acetyl-3,5-difluorophenyl)-2,5-diazabicyclo[2.2.1]heptane-2-carboxylate

| Conditions | Yield |
|---|---|
| With potassium carbonate In 1,4-dioxane at 100℃; for 24h; Inert atmosphere; regioselective reaction; | 96% |
-
-
851916-39-7
(1S,4S)-4-(2,5-dimethyl-1H-pyrrol-1-yl)-1-isopropylcyclopent-2-ene-1-carboxylic acid

-
-
113451-59-5
(1S,4S)-2,5-Diaza-bicyclo[2.2.1]heptane-2-carboxylic acid tert-butyl ester

-
-
1294006-20-4
C25H37N3O3

| Conditions | Yield |
|---|---|
| With O-(benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium tetrafluoroborate; triethylamine In N,N-dimethyl-formamide at 0℃; | 95% |
-
-
73291-09-5
4-chloromethylbenzaldehyde

-
-
113451-59-5
(1S,4S)-2,5-Diaza-bicyclo[2.2.1]heptane-2-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile at 50℃; | 93.1% |
-
-
113451-59-5
(1S,4S)-2,5-Diaza-bicyclo[2.2.1]heptane-2-carboxylic acid tert-butyl ester

-
-
407-25-0
trifluoroacetic anhydride

-
-
878805-66-4
(1S,4S)-5-(2,2,2-trifluoroacetyl)-2,5-diazabicyclo[2.2.1]heptane-2-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran at 0℃; for 5h; Inert atmosphere; | 93% |
| With triethylamine In tetrahydrofuran at 20℃; for 1h; |

-
-
113451-59-5
(1S,4S)-2,5-Diaza-bicyclo[2.2.1]heptane-2-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile at 80℃; for 24h; | 93% |
| With potassium carbonate In acetonitrile at 80℃; for 24h; | 93% |
-
-
1138-80-3
N-(Benzyloxycarbonyl)glycine

-
-
113451-59-5
(1S,4S)-2,5-Diaza-bicyclo[2.2.1]heptane-2-carboxylic acid tert-butyl ester

-
-
774610-01-4
1,1-dimethylethyl(1S,4S)-5-(N-{[benzyloxy]carbonyl}glycyl)-2,5-diazabicyclo[2.2.1]heptane-2-carboxylate

| Conditions | Yield |
|---|---|
| With 3-methyl-N-(3-methylbutyl)-1-butanamine; benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 20℃; | 92% |
| With 4-methyl-morpholine; benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane | |
| With 4-methyl-morpholine; benzotriazol-1-ol; N-(3-dimethylaminopropyl)-N-ethylcarbodiimide In dichloromethane at 20℃; for 24h; Product distribution / selectivity; |
-
-
841200-43-9
2-[4-(2-bromoethoxy)phenoxy]benzothiazole

-
-
113451-59-5
(1S,4S)-2,5-Diaza-bicyclo[2.2.1]heptane-2-carboxylic acid tert-butyl ester

-
-
1146691-49-7
(S,S)-5-(2-[4-(benzothiazol-2-yloxy)-phenoxy]-ethyl)-2,5-diaza-bicyclo[2.2.1]heptane-2-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In acetonitrile at 20 - 60℃; for 69h; | 92% |
-
-
113451-59-5
(1S,4S)-2,5-Diaza-bicyclo[2.2.1]heptane-2-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With nitrogen(II) oxide; calcium hydroxide In water at 20℃; under 12929 Torr; for 120h; Green chemistry; | 92% |
-
-
113451-59-5
(1S,4S)-2,5-Diaza-bicyclo[2.2.1]heptane-2-carboxylic acid tert-butyl ester

-
-
321365-84-8
2-[bis(4-fluorophenyl)methoxy]ethyl iodide

-
-
321365-88-2
3-[2-[bis(4-fluorophenyl)methoxy]ethyl]-8-(1S,4S)-N-t-Boc-2,5-diazabicyclo[2.2.1]heptane

| Conditions | Yield |
|---|---|
| With potassium carbonate In tetrahydrofuran Heating; | 91% |

-
-
113451-59-5
(1S,4S)-2,5-Diaza-bicyclo[2.2.1]heptane-2-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In dimethyl sulfoxide at 90℃; | 90% |
-
-
65873-72-5
6-methoxy-3-pyridinecarboxaldehyde

-
-
113451-59-5
(1S,4S)-2,5-Diaza-bicyclo[2.2.1]heptane-2-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With sodium tris(acetoxy)borohydride In 1,2-dichloro-ethane at 20℃; | 90% |
-
-
27996-87-8
2-fluoro-5-nitrobenzaldehye

-
-
113451-59-5
(1S,4S)-2,5-Diaza-bicyclo[2.2.1]heptane-2-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 50℃; Inert atmosphere; | 89% |
-
-
35661-60-0
Fmoc-Leu-OH

-
-
113451-59-5
(1S,4S)-2,5-Diaza-bicyclo[2.2.1]heptane-2-carboxylic acid tert-butyl ester

-
-
1186643-72-0
1,1-dimethylethyl (1S,4S)-5-(N-{[(9H-fluoren-9-ylmethyl)oxy]carbonyl}-L-leucyl)-2,5-diazabicyclo[2.2.1]heptane-2-carboxylate

| Conditions | Yield |
|---|---|
| With 4-methyl-morpholine; benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 20℃; | 88.3% |

-
-
113451-59-5
(1S,4S)-2,5-Diaza-bicyclo[2.2.1]heptane-2-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile at 80℃; for 12h; | 87.7% |
tert-Butyl (1S,4S)-2,5-diazabicyclo[2.2.1]heptan-2-carboxylate Chemical Properties
Systematic Name: tert-Butyl 2,5-diazabicyclo[2.2.1]heptane-2-carboxylate
Synonyms of (1S,4S)-(-)-2-Boc-2,5-Diazabicyclo[2.2.1]heptane (CAS NO.113451-59-5): (1S,4S)-N-(tert-Butoxycarbonyl)-2,5-diazabicyclo[2.2.1]heptane ; tert-butyl 2,5-diazabicyclo[2.2.1]heptane-2-carboxylate
CAS NO: 113451-59-5
Molecular Formula: C10H18N2O2
Molecular Weight: 198.26
Molecular Structure: 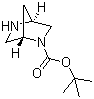
H bond acceptors: 4
H bond donors: 1
Freely Rotating Bonds: 2
Polar Surface Area: 32.78 Å2
Index of Refraction: 1.5
Molar Refractivity: 52.84 cm3
Molar Volume: 179.4 cm3
Surface Tension: 37.3 dyne/cm
Density: 1.104 g/cm3
Flash Point: 120.9 °C
Enthalpy of Vaporization: 51.49 kJ/mol
Boiling Point: 276.4 °C at 760 mmHg
Vapour Pressure: 0.00482 mmHg at 25°C
Melting point: 74-76 ºC
Alpha : -44 º (c=1 in chloroform)
Appearance: (1S,4S)-(-)-2-Boc-2,5-Diazabicyclo[2.2.1]heptane (CAS NO.113451-59-5) is white to slightly beige powder .
SMILES: O=C(OC(C)(C)C)N1CC2NCC1C2
InChI: InChI=1/C10H18N2O2/c1-10(2,3)14-9(13)12-6-7-4-8(12)5-11-7/h7-8,11H,4-6H2,1-3H3
InChIKey: UXAWXZDXVOYLII-UHFFFAOYAY
Std. InChI: InChI=1S/C10H18N2O2/c1-10(2,3)14-9(13)12-6-7-4-8(12)5-11-7/h7-8,11H,4-6H2,1-3H3
Std. InChIKey: UXAWXZDXVOYLII-UHFFFAOYSA-N
tert-Butyl (1S,4S)-2,5-diazabicyclo[2.2.1]heptan-2-carboxylate Safety Profile
Safety Statements: 22-24/25
S22: Do not breathe dust.
S24/25: When (1S,4S)-(-)-2-Boc-2,5-Diazabicyclo[2.2.1]heptane (CAS NO.113451-59-5) is used,avoid contact with skin and eyes.
WGK Germany: 3
Related Products
- tert-Butyl (1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)acetate
- tert-Butyl (1S,4S)-2,5-diazabicyclo[2.2.1]heptan-2-carboxylate
- tert-Butyl (3-methylazetidin-3-yl)methylcarbamate
- tert-Butyl (3S,4R)-4-(2-methoxyphenyl)pyrrolidin-3-ylcarbamate
- tert-Butyl (3S,4R)-4-phenylpyrrolidin-3-ylcarbamate
- tert-Butyl (4-chloropyridin-2-yl)carbamate
- tert-Butyl (4-fluoro-3-pyrrolidinyl)carbamate
- tert-Butyl (4-iodopyridin-2-yl)carbamate
- tert-Butyl (4-methylpiperidin-4-yl)carbamate
- tert-Butyl (4R,6R)-2-[[[6-(2-4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)pyrrol-1-yl]ethyl]-2,2-dimethyl-1,3-dioxan-4-yl]acetate
- 113472-97-2
- 1134-76-5
- 113479-65-5
- 113-48-4
- 113484-74-5
- 113492-03-8
- 1134-94-7
- 113504-93-1
- 113507-06-5
- 113507-82-7
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View