-
Name
tert-Butyl bromoacetate
- EINECS 226-133-6
- CAS No. 5292-43-3
- Article Data37
- CAS DataBase
- Density 1.352 g/cm3
- Solubility Insoluble in water, soluble in ethanol, diethyl ether
- Melting Point 44-47 °C
- Formula C6H11BrO2
- Boiling Point 163.999 °C at 760 mmHg
- Molecular Weight 195.056
- Flash Point 59.101 °C
- Transport Information UN 1993 3/PG 3
- Appearance slightly yellow transparent liquid
- Safety 26-24/25-16
- Risk Codes 10-36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi,
Xi, F
F
- Synonyms Aceticacid, bromo-, 1,1-dimethylethyl ester (9CI);Acetic acid, bromo-, tert-butylester (6CI,7CI,8CI);1,1-Dimethylethyl 2-bromoacetate;1,1-Dimethylethylbromoacetate;1,1-Dimethylethyl monobromoacetate;2-Bromoacetic acid tert-butylester;Bromoacetic acid 1,1-dimethylethyl ester;Bromoacetic acid tert-butylester;NSC 82470;tert-Butyl 2-bromoacetate;tert-Butyl a-bromoacetate;
- PSA 26.30000
- LogP 1.72300
Synthetic route

| Conditions | Yield |
|---|---|
| With Amberlyst-15 at -78 - 20℃; for 24h; Autoclave; | 97% |
| With perfluorosulfonic acid resin In tetrahydrofuran at 10 - 15℃; for 5h; Concentration; Temperature; Large scale; Green chemistry; | 97.6% |
| With amberlyst-15 In chloroform | 86% |
-
-
598-21-0
2-Bromoacetyl bromide

-
-
75-65-0
tert-butyl alcohol

-
-
5292-43-3
bromoacetic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane at 0℃; for 0.5h; | 96% |
| Stage #1: tert-butyl alcohol With triethylamine In dichloromethane at 0℃; for 0.166667h; Stage #2: 2-Bromoacetyl bromide In dichloromethane at 0 - 25℃; for 5h; | 71% |
| With diethyl ether; 2,3-Dimethylaniline |

| Conditions | Yield |
|---|---|
| With sulfuric acid In dichloromethane | 88.4% |

-
-
22118-09-8
2-bromoacetyl chloride

-
-
75-65-0
tert-butyl alcohol

-
-
5292-43-3
bromoacetic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With aluminum oxide In benzene for 15h; Ambient temperature; | 74% |
| With pyridine In diethyl ether | 50% |
| With triethylamine In dichloromethane at 0 - 20℃; for 3.75h; | 4.48 g |
-
-
98946-18-0
tert-Butyl 2,2,2-trichloroacetimidate

-
-
79-08-3
bromoacetic acid

-
-
5292-43-3
bromoacetic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| boron trifluoride diethyl etherate In dichloromethane; cyclohexane for 16h; Ambient temperature; | 71% |
-
-
125144-97-0
Diphenylphosphanyl-acetic acid phenyl ester

-
A
-
5292-43-3
bromoacetic acid tert-butyl ester

-
B
-
35948-16-4
2-diphenylphosphanyl-acetamide

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol; water for 2h; Heating; | A 5% B 44% |

| Conditions | Yield |
|---|---|
| With dmap; scandium tris(trifluoromethanesulfonate) In dichloromethane at -5 - 20℃; for 2h; | 35% |
| With toluene-4-sulfonic acid In toluene at 110.8℃; for 2h; Inert atmosphere; | |
| With sulfuric acid; magnesium sulfate In dichloromethane at 20℃; for 48h; |
-
-
13094-51-4
bromoacetic anhydride

-
-
75-65-0
tert-butyl alcohol

-
-
5292-43-3
bromoacetic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With diethyl ether |
-
-
5292-43-3
bromoacetic acid tert-butyl ester

-
-
603-35-0
triphenylphosphine

-
-
86302-43-4
(tert-Butoxycarbonylmethylene)triphenylphosphorane

| Conditions | Yield |
|---|---|
| Stage #1: bromoacetic acid tert-butyl ester; triphenylphosphine Stage #2: With sodium hydroxide | 100% |
| In toluene at 0 - 20℃; | 95% |
| Stage #1: bromoacetic acid tert-butyl ester; triphenylphosphine In toluene at 0 - 20℃; for 8h; Inert atmosphere; Schlenk technique; Stage #2: With sodium hydroxide In dichloromethane; water at 20℃; for 1h; Inert atmosphere; Schlenk technique; | 80% |
-
-
5292-43-3
bromoacetic acid tert-butyl ester

-
-
122-52-1
triethyl phosphite

-
-
27784-76-5
tert-butyl diethylphosphonoacetate

| Conditions | Yield |
|---|---|
| at 65℃; for 2h; Substitution; Michaelis-Arbuzov reaction; | 100% |
| at 50℃; for 2h; | 100% |
| at 90℃; for 6h; Inert atmosphere; | 97% |
-
-
5292-43-3
bromoacetic acid tert-butyl ester

-
-
17277-58-6
methyl (phenylsulfanyl)acetate

-
-
115391-98-5
1-methyl-4-t-butyl (+/-)-2-phenylthiosuccinate

| Conditions | Yield |
|---|---|
| With lithium hexamethyldisilazane In tetrahydrofuran at 0℃; for 1h; | 100% |
-
-
5292-43-3
bromoacetic acid tert-butyl ester

-
-
96035-98-2
(3S,4S)-4-<(1R)-1-benzyloxycarbonylethyl>-3-<(1R)-1-tert-butyldimethylsilyloxyethyl>-2-azetidinone

-
-
105318-04-5
(3S,4S)-4-<(1R)-1-benzyloxycarbonylethyl>-1-(tert-butoxycarbonylmethyl)-3-<(1R)-1-tert-butyldimethylsilyloxy>-2-azetidinone

| Conditions | Yield |
|---|---|
| With sodium hydride In tetrahydrofuran at 0℃; for 2h; | 100% |
-
-
5292-43-3
bromoacetic acid tert-butyl ester

-
-
137743-86-3
12-Benzyloxymethyl-2,5,8,11,14,17,20,23-octaoxa-bicyclo[22.3.1]octacosa-1(27),24(28),25-trien-28-ol

-
-
137743-87-4
(12-Benzyloxymethyl-2,5,8,11,14,17,20,23-octaoxa-bicyclo[22.3.1]octacosa-1(27),24(28),25-trien-28-yloxy)-acetic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone for 48h; Heating; | 100% |
-
-
5292-43-3
bromoacetic acid tert-butyl ester

-
-
110267-58-8
(S)-2-((3R,6R)-5-Oxo-3-phenyl-[1,4]thiazepan-6-ylamino)-4-phenyl-butyric acid ethyl ester

-
-
102208-40-2
t-butyl α-{6(R)-[1(S)-ethoxycarbonyl-3-phenylpropylamino]-5-oxo-3(R)-phenylperhydro-1,4-thiazepin-4-yl}acetate

| Conditions | Yield |
|---|---|
| With sodium hydride In N,N-dimethyl-formamide for 5h; Ambient temperature; | 100% |
-
-
5292-43-3
bromoacetic acid tert-butyl ester

-
-
110143-59-4
(S)-2-((3S,6R)-5-Oxo-3-phenyl-[1,4]thiazepan-6-ylamino)-4-phenyl-butyric acid ethyl ester

-
-
110221-41-5
(S)-2-((3S,6R)-4-tert-Butoxycarbonylmethyl-5-oxo-3-phenyl-[1,4]thiazepan-6-ylamino)-4-phenyl-butyric acid ethyl ester

| Conditions | Yield |
|---|---|
| With sodium hydride In N,N-dimethyl-formamide for 5h; Ambient temperature; | 100% |
-
-
5292-43-3
bromoacetic acid tert-butyl ester

-
-
108-91-8
cyclohexylamine

-
-
66937-55-1
N-cyclohexylglycine t-butyl ester

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In ethanol at 20℃; for 12h; | 100% |
| With triethylamine In ethanol Ambient temperature; | |
| With sodium hydrogencarbonate In ethanol | 31.9 g (97%) |
-
-
5292-43-3
bromoacetic acid tert-butyl ester

-
-
103-49-1
dibenzylamine

-
-
94226-56-9
tert-butyl 2-(dibenzylamino)ethanoate

| Conditions | Yield |
|---|---|
| In 1,4-dioxane; ethanol for 4h; Heating; | 100% |
| In 1,4-dioxane; ethanol for 8h; Reflux; | 94% |
| In ethanol at 20℃; for 4h; | 90% |
| In 1,4-dioxane; ethanol for 5h; Heating; | 89% |
-
-
110-91-8
morpholine

-
-
5292-43-3
bromoacetic acid tert-butyl ester

-
-
88217-68-9
(4-morpholinyl)acetic acid 1,1-dimethylethyl ester

| Conditions | Yield |
|---|---|
| In tetrahydrofuran for 1.5h; | 100% |
| With triethylamine In tetrahydrofuran at 60℃; for 2h; | 100% |
| With potassium carbonate In tetrahydrofuran at 0 - 20℃; for 12.5h; | 81% |
-
-
5292-43-3
bromoacetic acid tert-butyl ester

-
-
498-02-2
1-(3-methoxy-4-hydroxyphenyl)ethanone

-
-
188891-12-5
(4-acetyl-2-methoxy-phenoxy)acetic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 48h; | 100% |
| Stage #1: 1-(3-methoxy-4-hydroxyphenyl)ethanone With sodium hydroxide In methanol at 20℃; for 15h; Stage #2: bromoacetic acid tert-butyl ester In methanol for 15h; Reflux; | 100% |
| With potassium carbonate In water; N,N-dimethyl-formamide at 0℃; | 99% |
-
-
5292-43-3
bromoacetic acid tert-butyl ester

-
-
49827-15-8
t-butyl iodoacetate

| Conditions | Yield |
|---|---|
| With sodium iodide In acetone | 100% |
| With sodium iodide In acetone at 65℃; for 4h; Inert atmosphere; | 98% |
| With sodium iodide In acetone at 20℃; for 2h; | 90% |
-
-
5292-43-3
bromoacetic acid tert-butyl ester

-
-
5978-22-3
N(ε)-benzoyloxycarbonyl-L-lysine tert-butyl ester hydrochloride

-
-
205379-07-3
Nα,Nα-bis[(tert-butyloxycarbonyl)methyl]-Nε-benzyloxycarbonyl-L-lysine tert-butyl ester

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 55℃; Inert atmosphere; | 100% |
| With N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 55℃; Inert atmosphere; | 97% |
| With N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 55℃; for 48h; | 89% |
-
-
5292-43-3
bromoacetic acid tert-butyl ester

-
-
175777-31-8
(αR,4S)-N-[2-hydroxy-1-phenylethyl]-4-methyl-1,4-dihydro-2H-isoquinolin-3-one

| Conditions | Yield |
|---|---|
| With N,N,N,N,N,N-hexamethylphosphoric triamide; n-butyllithium In tetrahydrofuran at -78 - 20℃; for 18h; Substitution; | 100% |
-
-
5292-43-3
bromoacetic acid tert-butyl ester

-
-
178445-83-5
3-(3,4-dimethoxyphenyl)-1-(3-hydroxyphenyl)-1-propanone

-
-
178445-86-8
2[[3-(3,4-dimethyoxyphenyl)-1-oxopropyl]phenoxy]acetic acid 1,1-dimethylester

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone at 20℃; for 16h; Inert atmosphere; | 100% |
| With potassium carbonate In acetone at 20℃; for 20h; Alkylation; | 99% |
| With potassium carbonate In acetone for 16h; Inert atmosphere; | 97% |
-
-
5292-43-3
bromoacetic acid tert-butyl ester

-
-
246158-07-6
(2S,3S)-2-azido-1-(4-tert-butoxyphenyl)hex-5-en-3-ol

-
-
246158-10-1
{1-[1-azido-2-(4-tert-butoxy-phenyl)-ethyl]-but-3-enyloxy}-acetic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With sodium hydroxide; tetra(n-butyl)ammonium hydrogensulfate In benzene at 20℃; for 1h; Alkylation; | 100% |
| With sodium hydroxide; tetra(n-butyl)ammonium hydrogensulfate In water; benzene for 1h; |
-
-
5292-43-3
bromoacetic acid tert-butyl ester

-
-
244219-73-6
(1,4,7,10-tetraaza-cyclododec-1-yl)-acetic acid benzyl ester

-
-
192635-89-5
phenylmethyl 2-(1,4,7,10-Tetraaza-4,7,10-tris(((tert-butyl)oxycarbonyl)methyl)cyclododecyl)-acetate

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile at 20℃; for 2.5h; Alkylation; | 100% |
| With potassium carbonate In acetonitrile at 20℃; | 67% |
-
-
675-20-7
piperidin-2-one

-
-
5292-43-3
bromoacetic acid tert-butyl ester

-
-
216252-69-6
1-t-Butoxycarbonylmethyl-2-piperidone

| Conditions | Yield |
|---|---|
| Stage #1: piperidin-2-one With sodium hydride In toluene at 0℃; for 1h; Metallation; Stage #2: bromoacetic acid tert-butyl ester at 20℃; for 10h; Alkylation; | 100% |
| With P-tris(dimethylamino)-C-dimethylphosphonium ylide In tetrahydrofuran at -78 - 20℃; for 3h; Alkylation; | 71% |
-
-
53031-80-4
(E)-3-phenylmethylene-2-piperidinone

-
-
5292-43-3
bromoacetic acid tert-butyl ester

-
-
250248-74-9
{2-Oxo-3-[1-phenyl-meth-(E)-ylidene]-piperidin-1-yl}-acetic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With lithium hexamethyldisilazane In tetrahydrofuran at 0 - 20℃; Alkylation; | 100% |
-
-
5292-43-3
bromoacetic acid tert-butyl ester

-
-
58404-02-7
dimethyl(2-chloro-2-propenyl)malonate

-
-
192516-45-3
2-(2-Chloro-2-propenyl)-2-(methoxycarbonyl)-1,4-butanedioic Acid 4-(1,1-Dimethylethyl) 1-Methyl Ester

| Conditions | Yield |
|---|---|
| With sodium hydroxide; N-benzyl-N,N,N-triethylammonium chloride | 100% |
| With sodium hydroxide; N-benzyl-N,N,N-triethylammonium chloride In water at 20℃; Alkylation; |
-
-
524-38-9
N-hydroxyphthalimide

-
-
5292-43-3
bromoacetic acid tert-butyl ester

-
-
54224-25-8
t-butyl (1,3-dioxo-1,3-dihydroisoindol-2-yloxy)acetate

| Conditions | Yield |
|---|---|
| With potassium carbonate In 1-methyl-pyrrolidin-2-one at 20 - 50℃; Inert atmosphere; | 100% |
| In N,N-dimethyl-formamide | 97% |
| With triethylamine In tetrahydrofuran at 0 - 20℃; | 90% |
-
-
5292-43-3
bromoacetic acid tert-butyl ester

-
-
106-53-6
para-bromobenzenethiol

-
-
283153-83-3
2-(4-bromophenylsulfanyl)acetic acid t-butyl ester

| Conditions | Yield |
|---|---|
| With potassium carbonate In DMF (N,N-dimethyl-formamide) at 20℃; for 1h; | 100% |
| With potassium hydroxide In ethanol | 98.6% |
| With pyridine In dimethyl sulfoxide at 20℃; |
-
-
5292-43-3
bromoacetic acid tert-butyl ester

-
-
824-79-3
sodium 4-methylbenzenesulfinate

-
-
98317-43-2
tert-butyl [(4-methylphenyl)sulfonyl]acetate

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 20℃; for 24h; | 100% |
| In 1,4-dioxane; water for 10h; Heating; |
-
-
38788-38-4
dibutyl telluride

-
-
5292-43-3
bromoacetic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 20℃; | 100% |
-
-
5292-43-3
bromoacetic acid tert-butyl ester

-
-
723294-34-6
1-[2-(2-{3-[2-(2-butyltellanyl-ethoxy)-ethoxy]-2,2-bis-[2-(2-butyltellanyl-ethoxy)-ethoxymethyl]-propoxy}-ethoxy)-ethyltellanyl]-butane

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 20℃; | 100% |
-
-
494799-19-8
methyl 2-bromo-3-cyclohexyl-1H-indole-6-carboxylate

-
-
5292-43-3
bromoacetic acid tert-butyl ester

-
-
774213-79-5
methyl 2-bromo-1-(2-tert-butoxy-2-oxoethyl)-3-cyclohexyl-1H-indole-6-carboxylate

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 4h; | 100% |
| Stage #1: methyl 2-bromo-3-cyclohexyl-1H-indole-6-carboxylate With sodium hydride In N,N-dimethyl-formamide at 0 - 20℃; Stage #2: bromoacetic acid tert-butyl ester In N,N-dimethyl-formamide at 60℃; for 3h; | 95% |
| Stage #1: methyl 2-bromo-3-cyclohexyl-1H-indole-6-carboxylate With sodium hydride In N,N-dimethyl-formamide at 20℃; for 1h; Stage #2: bromoacetic acid tert-butyl ester In N,N-dimethyl-formamide at 20℃; for 0.666667h; | 90% |
-
-
5292-43-3
bromoacetic acid tert-butyl ester

-
-
63628-63-7
Nε-benzyloxycarbonyl-L-lysine tert-butyl ester

-
-
205379-07-3
Nα,Nα-bis[(tert-butyloxycarbonyl)methyl]-Nε-benzyloxycarbonyl-L-lysine tert-butyl ester

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 65℃; for 18h; | 100% |
| With N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 55℃; for 24h; Inert atmosphere; | 99% |
| With N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 55℃; | 98% |
-
-
5292-43-3
bromoacetic acid tert-butyl ester

-
-
107-18-6
allyl alcohol

-
-
85428-58-6
tert-butyl 2-(allyloxy)acetate

| Conditions | Yield |
|---|---|
| Stage #1: allyl alcohol With tetra(n-butyl)ammonium hydrogensulfate; sodium hydroxide In water; toluene at 20℃; for 1h; Industry scale; Stage #2: bromoacetic acid tert-butyl ester In water; toluene at 5 - 20℃; | 100% |
| With tetra(n-butyl)ammonium hydrogensulfate; sodium hydroxide In water; toluene at 0 - 20℃; | 99% |
| With sodium hydroxide; tetra(n-butyl)ammonium hydrogensulfate In water; benzene at 0 - 20℃; |
-
-
5292-43-3
bromoacetic acid tert-butyl ester

-
-
22204-53-1
(2S)-2-(6-methoxy(2-naphthyl))propanoic acid

-
-
646509-90-2
((tert-butyl)oxycarbonyl)methyl (2S)-2-(6-methoxy(2-naphthyl))propanoate

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In N,N-dimethyl-formamide at 20℃; for 48h; | 100% |
| With sodium hydrogencarbonate In N,N-dimethyl-formamide at 20℃; for 48h; |
-
-
5292-43-3
bromoacetic acid tert-butyl ester

-
-
6940-80-3
(S)-N-benzylalaninol

-
-
873197-15-0
(S)-[N-benzyl-(2-hydroxy-1-methylethyl)-amino]acetic acid

| Conditions | Yield |
|---|---|
| With potassium carbonate In water; toluene at 20 - 65℃; for 21h; Inert atmosphere; | 100% |
| With potassium carbonate In N,N-dimethyl-formamide at 0 - 20℃; | 89% |
| With potassium hydrogencarbonate; sodium iodide In N,N-dimethyl-formamide at 20℃; for 12h; | 76% |
tert-Butyl bromoacetate Specification
The tert-Butyl bromoacetate, with the CAS registry number 5292-43-3, is also known as Bromoacetic acid tert-butyl ester. It belongs to the product categories of Acid based bromo compounds; C6 to C7; Carbonyl Compounds; Esters; Organic chemical; Building Blocks; C6 to C7; Carbonyl Compounds; Chemical Synthesis; Organic Building Blocks. Its EINECS number is 226-133-6. This chemical's molecular formula is C6H11BrO2 and molecular weight is 195.05. What's more, its systematic name is 2-Methyl-2-propanyl bromoacetate. This chemical should be sealed and stored in a cool and ventilated place. Moreover, it should be protected from oxides, heat and fire. It is used in organic synthesis.
Physical properties of tert-Butyl bromoacetate are: (1)ACD/LogP: 2.003; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 2.00; (4)ACD/LogD (pH 7.4): 2.00; (5)ACD/BCF (pH 5.5): 19.61; (6)ACD/BCF (pH 7.4): 19.61; (7)ACD/KOC (pH 5.5): 292.94; (8)ACD/KOC (pH 7.4): 292.94; (9)#H bond acceptors: 2; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 3; (12)Polar Surface Area: 26.3 Å2; (13)Index of Refraction: 1.457; (14)Molar Refractivity: 39.318 cm3; (15)Molar Volume: 144.288 cm3; (16)Polarizability: 15.587×10-24cm3; (17)Surface Tension: 31.9 dyne/cm; (18)Density: 1.352 g/cm3; (19)Flash Point: 59.101 °C; (20)Enthalpy of Vaporization: 40.053 kJ/mol; (21)Boiling Point: 163.999 °C at 760 mmHg; (22)Vapour Pressure: 2.0 mmHg at 25°C.
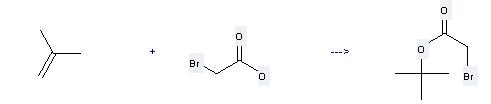
Preparation of tert-Butyl bromoacetate: this chemical can be prepared by bromoacetic acid and 2-methyl-propene. This reaction will need reagent Amberlyst-15 and solvent CHCl3. The yield is about 86%.
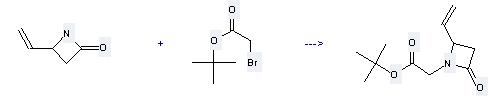
Uses of tert-Butyl bromoacetate: it can be used to produce (2-oxo-4-vinyl-azetidin-1-yl)-acetic acid tert-butyl ester. It will need reagent KOH and solvents tetrahydrofuran, dimethylformamide with the reaction time of 16 hours. The yield is about 80%.
When you are using this chemical, please be cautious about it as the following:
This chemical is irritating to eyes, respiratory system and skin. It is flammable, so you should keep it away from sources of ignition - No smoking. In case of contact with eyes, you should rinse immediately with plenty of water and seek medical advice. When using it, you must avoid contact with skin and eyes.
You can still convert the following datas into molecular structure:
(1)SMILES: BrCC(=O)OC(C)(C)C
(2)Std. InChI: InChI=1S/C6H11BrO2/c1-6(2,3)9-5(8)4-7/h4H2,1-3H3
(3)Std. InChIKey: BNWCETAHAJSBFG-UHFFFAOYSA-N
Related Products
- tert-Butyl (1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)acetate
- tert-Butyl (3S,4R)-4-(2-methoxyphenyl)pyrrolidin-3-ylcarbamate
- tert-Butyl (3S,4R)-4-phenylpyrrolidin-3-ylcarbamate
- tert-Butyl (4-aminophenyl)carbamate
- tert-Butyl (4-bromothiazol-2-yl)methylcarbamate
- tert-Butyl (4-chloropyridin-2-yl)carbamate
- tert-Butyl (4-fluoro-3-pyrrolidinyl)carbamate
- tert-Butyl (4-iodopyridin-2-yl)carbamate
- tert-Butyl (4-methylpiperidin-4-yl)carbamate
- tert-Butyl (4R,6R)-2-[[[6-(2-4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)pyrrol-1-yl]ethyl]-2,2-dimethyl-1,3-dioxan-4-yl]acetate
- 5292-45-5
- 5292-47-7
- 52927-22-7
- 529-28-2
- 52931-63-2
- 52932-74-8
- 529-32-8
- 52933-01-4
- 529-34-0
- 529-36-2
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View