Nanjing Spring & Autumn Biological Engineering Co., Ltd.
Nanjing Spring & Autumn Biological Engineering Co., Ltd. Which was founded at 2008, has an R & D team composed very experienced natural products chemists. The company is a high-tech enterprise engaged in functional health care products raw ma
Cas:118525-40-9
Min.Order:1 Gram
Negotiable
Type:Lab/Research institutions
inquiryLonwin Chemical Group Limited
Why Choose Us 1. Quality Price We are the manufacturer, so we can provide the competitive price and high quality product. 2. Packing Products could be packaged according to customer's specializedrequirements. 3. Transport The products can be tr
Cas:118525-40-9
Min.Order:1000 Kilogram
FOB Price: $70.0 / 75.0
Type:Other
inquiryDayang Chem (Hangzhou) Co.,Ltd.
DayangChem exported this product to many countries and regions at best price. If you are looking for the material's manufacturer or supplier in China, DayangChem is your best choice. Pls contact with us freely for getting detailed product spe
Cas:118525-40-9
Min.Order:1 Kilogram
FOB Price: $3.0
Type:Lab/Research institutions
inquiryWuhan Fortuna Chemical Co.,Ltd
Unique advantages for Icaritin Cas 118525-40-9 Guaranteed purity High quality & competitive price Quality control Fast feedback Prompt shipment Appearance:Light yellow powder Storage:Store in 2-8℃ Package:10g,100g,1kg/foil bag Appli
Cas:118525-40-9
Min.Order:10 Gram
FOB Price: $15.0 / 45.0
Type:Trading Company
inquiryXi'an Xszo Chem Co., Ltd.
1. Factory price and high quality must be guaranteed, base on 8 years of production and R&D experience2. Free samples will be provided,ensure specifications and quality are right for customer3. Customers will receive the most professional technical s
Cas:118525-40-9
Min.Order:1 Gram
FOB Price: $0.1
Type:Manufacturers
inquiryShanghai Seasonsgreen Chemical Co.,Ltd
Shanghai Seasonsgreen Chemical is a high-tech research and development, production, sale and custom synthesis set in one high-tech chemical products enterprises. Our sales and marketing division is located in Shanghai, serving international pharmaceu
Cas:118525-40-9
Min.Order:1 Kilogram
FOB Price: $1.0
Type:Manufacturers
inquiryHenan Tianfu Chemical Co., Ltd.
Our company was built in 2009 with an ISO certificate.In the past 5 years, we have grown up as a famous fine chemicals supplier in China and we had established stable business relationships with Samsung,LG,Merck,Thermo Fisher Scientific and so on.O
Cas:118525-40-9
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryHebei Nengqian Chemical Import and Export Co., LTD
With our good experience, we offer detailed technical support and advice to assist customers. We communicate closely with customers to establish their quality requirements. Consistent Quality Our plant has strict quality control in each manufacturin
Cas:118525-40-9
Min.Order:1 Kilogram
FOB Price: $3.0 / 10.0
Type:Trading Company
inquiryZhuozhou Wenxi import and Export Co., Ltd
WITH US,YOUR MONEY IN SAFE,YOUR BUSINESS IN SAFE 1)Quick Response Within 12 hours; 2)Quality Guarantee: All products are strictly tested by our QC, confirmed by QA and approved by third party lab in China, USA, Canada, Germany, UK, Italy, France et
Cas:118525-40-9
Min.Order:1 Kilogram
FOB Price: $139.0 / 210.0
Type:Trading Company
inquiryShanghai Upbio Tech Co.,Ltd
1.No Less 8 years exporting experience. Clients can 100% received goods 2.Lower Price with higher quality 3,Free sample 4,We are sincerely responsible for the "product quality" and "After Service" Upbio is Specialized
Cas:118525-40-9
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryBaoji Guokang Healthchem co.,ltd
Our company has been in existence for 10 years since its establishment. We have our own unique team. The company integrates independent research and development, production and sales. We have established famous brands at home and abroad. At present,
Cas:118525-40-9
Min.Order:1 Gram
FOB Price: $42.0 / 45.0
Type:Trading Company
inquiryQingdao Beluga Import and Export Co., LTD
Icaritin CAS:118525-40-9 Qingdao Belugas Import and Export Co., Ltd. is a scientific and technological company integrating research and development, production and trade of chemical intermediates, specializing in high quality organic intermediates,
Cas:118525-40-9
Min.Order:1 Gram
Negotiable
Type:Lab/Research institutions
inquiryShandong Hanjiang Chemical Co., Ltd.
Hello, dear friend! I'm Hansen and Allen from China. Welcome to my lookchem mall! The following is a brief introduction of our company's products and services. If you are interested in our products, please contact us by emai
Changchun Artel lmport and Export trade company
best price,best quality and fast delivery assurance available in sample product and customization with years of export experience along with excellent quality, advanced services and competitive prices the quality of products conform u
Cas:118525-40-9
Min.Order:10 Gram
Negotiable
Type:Trading Company
inquiryHenan Wentao Chemical Product Co., Ltd.
Henan Wentao Chemical Product Co.,Ltd is Located in Zhengzhou High-tech Development Zone with import and export license, We passed ISO 9001:2008 as well, Henan Wentao has developed more than 1000 compounds, which are widely used in the fields of prod
Wuhan Han Sheng New Material Technology Co.,Ltd
Our Advantage: high quality with competitive price High quality standard: BP/USP/EP Enterprise standard All purity customized Fast and safe delivery We have reliable forwarder who can help us deliver our goods more fast and safe. We
Cas:118525-40-9
Min.Order:10 Kilogram
Negotiable
Type:Trading Company
inquiryTriumph International Development Limilted
Appearance:white powder/crystal Storage:Store in a cool,dry place and keep away from direct strong light Package:As customer request Application:Used for research and industrial manufacture. Transportation:Common products:Sea/A
Cas:118525-40-9
Min.Order:100 Metric Ton
Negotiable
Type:Lab/Research institutions
inquiryChengdu Biopurify Phytochemicals Ltd.
Chengdu Biopurify Phytochemicals Ltd. is a leading company in the research, development, manufacture and marketing of High Quality Phytochemicals and Extracts(especially Active Ingredients from Traditional Chinese Medicine,Traditional Chinese Medic
Hangzhou J&H Chemical Co., Ltd.
J&H CHEM R&D center can offer custom synthesis according to the contract research and development services for the fine chemicals, pharmaceutical, biotechnique and some of the other chemicals. J&H CHEM has some Manufacturing base in Jia
Wuhan Xinhao Biotechnology Co., Ltd.
1. We can provide customers with "one-stop"packaging service,from research,development,production,export and so on 2. Powerful R&D strength let our technology in a leading level,forever,in turn,to provide customers with better service .
Cas:118525-40-9
Min.Order:100 Gram
FOB Price: $10.0 / 100.0
Type:Trading Company
inquiryShaanxi Cuicheng Biomedical Technology Co., Ltd.
Why Choose Us 1. Quality Price We are the manufacturer, so we can provide the competitive price and high quality product base on 8 years of production and R&D experience. 2. Packing Products could be packaged according to customer&#
SHENGZHIKAI TECHNOLOGY INDUSTRY CO., LTD
Natural product : 100% natural product, without any synthetic ingredients. Manufacturer : China's leading manufacturer of plant extracts. Quality : Our products have undergone comprehensive inspection and all have passed strict testing. In s
Cas:118525-40-9
Min.Order:5 Gram
Negotiable
Type:Trading Company
inquiryZibo Hangyu Biotechnology Development Co., Ltd
Zibo Hangyu Biotechnology Development Co., Ltd is a leading manufacturer and supplier of chemicals in China. We develop produce and distribute high quality pharmaceuticals, intermediates, special chemicals and OLED intermediates and other fine chemi
Cas:118525-40-9
Min.Order:10 Gram
FOB Price: $100.0
Type:Lab/Research institutions
inquiryChanger pharmatech Co.,Ltd
Our products are strictly tested in quality, and the price is more favorable in the same industry. Capacity of production:Ranging from gram level to hundred-kilogram level Partnership: we have maintained long-term cooperation with many well-known p
SHANGHAI SYSTEAM BIOCHEM CO., LTD
We are one of a few suppliers that can offer custom synthesis service of this product We are specialized in custom synthesis, chemical/pharmaceutical/ pesticides outsourcing and contract research. We are committed to prov
Cas:118525-40-9
Min.Order:100 Gram
FOB Price: $100.0 / 2000.0
Type:Lab/Research institutions
inquiryJiangsu Qianyu Molecular Technology Co., LTD.
Our Advantages A. International Top level TechnologyOur company owned biomedicine experts are famous at home and abroad with rich experience in research and development in the field of efficient chiral functional molecules research and development an
Xiamen Jenny Chemical Technology Co., Ltd.
GMP standard, high purity, competitive price, in stock 1. Quick Response: within 6 hours after receiving your email. 2. Quality Guarantee: All products are strictly tested by our QC, confirmed by QA, and approved by a third-party lab in China, USA,
Henan Kanbei Chemical Co.,LTD
High quality, competitive price, fast delivery and first-class service we possesses have won the trust and praise of customers. Standard: BP/USP/EP The purity is equal or greater than 99%. As a supplier, we can provide high-quality products. Cle
Cas:118525-40-9
Min.Order:1 Metric Ton
Negotiable
Type:Lab/Research institutions
inquiryEAST CHEMSOURCES LIMITED
factory?direct?saleAppearance:White Powder Storage:Store In Dry, Cool And Ventilated Place Package:25kg/drum, also according to the clients requirement Application:It is widely used as a thickener, emulsifier and stabilizer Transportation:By Sea Or B
Cas:118525-40-9
Min.Order:1 Kilogram
FOB Price: $18.0 / 20.0
Type:Trading Company
inquiryShandong Mopai Biotechnology Co., LTD
Shandong Mopai Biotechnology Co., LTD is a leading manufacturer and supplier of chemicals in China. We develop produce and distribute high quality pharmaceuticals, intermediates, special chemicals and OLED intermediates and other fine chemicals. W
Synthetic route
-
-
489-32-7
icariin

-
-
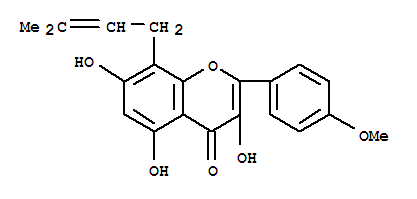
118525-40-9
icaritin

| Conditions | Yield |
|---|---|
| With water; sodium acetate; cellulase In ethanol; water; dimethyl sulfoxide at 37℃; pH=5.0; | 100% |
| With cellulase | |
| Multi-step reaction with 2 steps 1: β-glucanase; sodium acetate / aq. buffer / 5 h / 50 °C / Enzymatic reaction 2: sulfuric acid / ethanol; water / 5 h / 50 °C View Scheme |

-
-
118525-40-9
icaritin

| Conditions | Yield |
|---|---|
| With hydrogenchloride In methanol for 2h; Reflux; Inert atmosphere; | 96% |

-
-
118525-40-9
icaritin

| Conditions | Yield |
|---|---|
| With hydrogenchloride In methanol; water at 65℃; for 1h; Inert atmosphere; | 95% |

-
-
118525-40-9
icaritin

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol at 60℃; | 87% |
| With sodium hydroxide In ethanol; water at 60℃; | 87% |

-
-
118525-40-9
icaritin

| Conditions | Yield |
|---|---|
| With cyclohexa-1,4-diene; palladium 10% on activated carbon In methanol at 20℃; for 2h; | 86% |
| With cyclohexa-1,4-diene; palladium 10% on activated carbon In methanol at 20℃; for 2h; | 84% |
-
-
113558-15-9
icariside II

-
-
118525-40-9
icaritin

| Conditions | Yield |
|---|---|
| With sulfuric acid In ethanol; water at 50℃; for 5h; | 82% |
| With sulfuric acid In ethanol; water at 50℃; for 5h; | 82% |
| With sulfuric acid In ethanol; water at 50℃; for 5h; | 82% |

-
-
118525-40-9
icaritin

| Conditions | Yield |
|---|---|
| With hydrogenchloride In 1,4-dioxane; isopropyl alcohol for 6.16667h; Solvent; Reagent/catalyst; Inert atmosphere; Reflux; | 70.6% |
-
-
56725-99-6
3-hydroxy-7-O-β-glucose-8-prenyl-4'-methoxychrysin

-
-
118525-40-9
icaritin

| Conditions | Yield |
|---|---|
| With cellulase In dimethyl sulfoxide at 37℃; pH=5.7; | 68% |

| Conditions | Yield |
|---|---|
| With sulfuric acid In methanol |


-
-
118525-40-9
icaritin

| Conditions | Yield |
|---|---|
| With sulfuric acid | |
| With potassium hydroxide |

| Conditions | Yield |
|---|---|
| With potassium hydroxide |
-
-
110623-72-8
4'-methoxy-5-hydroxy-8-3,3-dimethylallylflavone 3-O-β-D-glucopyranosyl(1<*>2)α-L-rhamnopyranoside-7-O-β-D-glucopyranoside

-
-
118525-40-9
icaritin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: β-glucosidase 2: 1 N H2SO4 / methanol View Scheme |

-
A
-
118525-40-9
icaritin

-
B
-
113558-15-9
icariside II

-
C
-
56725-99-6
3-hydroxy-7-O-β-glucose-8-prenyl-4'-methoxychrysin

| Conditions | Yield |
|---|---|
| With hydrogenchloride; methanol; water at 80℃; for 8h; Product distribution / selectivity; Heating / reflux; | |
| With sodium methylate In pyridine at 80℃; for 8h; Product distribution / selectivity; Heating / reflux; | |
| With acetic acid; β-glucuronidase; naringinase; hesperidinase; β-galactosidase; cellulase; amyloglucosidase at 37℃; for 48h; pH=4.5; Product distribution / selectivity; | |
| Stage #1: epimedium koreanum Nakai, leaves; 1-butanol-ether-hexane-methanol extract of With water at 121℃; for 0.5h; Stage #2: at 30℃; for 120h; Product distribution / selectivity; |


-
A
-
118525-40-9
icaritin

-
B
-
56725-99-6
3-hydroxy-7-O-β-glucose-8-prenyl-4'-methoxychrysin

| Conditions | Yield |
|---|---|
| With sulfuric acid; water In ethanol for 1.5h; Reflux; |
-
-
100-09-4
4-methoxybenzoic acid

-
-
118525-40-9
icaritin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1.1: thionyl chloride / dichloromethane / 4 h / Reflux 2.1: potassium carbonate / acetone / 24.5 h / 65 °C 3.1: sodium carbonate; sodium hydrogencarbonate; Oxone / acetone; water; dichloromethane / 29 h / 0 °C / pH 9 3.2: 2 h / 20 °C 4.1: 5%-palladium/activated carbon; hydrogen / methanol; ethyl acetate / 24 h / 20 °C 5.1: potassium carbonate / acetone / 6 h / 20 °C 6.1: potassium carbonate / acetone / 12 h / 50 °C 7.1: N,N-diethylaniline / 0.5 h / 190 °C / Microwave irradiation 8.1: hydrogenchloride / methanol / 2 h / Reflux; Inert atmosphere View Scheme |
-
-
99-96-7
4-hydroxy-benzoic acid

-
-
118525-40-9
icaritin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 9 steps 1.1: sodium hydroxide / water / 4 h / 40 °C 2.1: thionyl chloride / dichloromethane / 4 h / Reflux 3.1: potassium carbonate / acetone / 24.5 h / 65 °C 4.1: sodium carbonate; sodium hydrogencarbonate; Oxone / acetone; water; dichloromethane / 29 h / 0 °C / pH 9 4.2: 2 h / 20 °C 5.1: 5%-palladium/activated carbon; hydrogen / methanol; ethyl acetate / 24 h / 20 °C 6.1: potassium carbonate / acetone / 6 h / 20 °C 7.1: potassium carbonate / acetone / 12 h / 50 °C 8.1: N,N-diethylaniline / 0.5 h / 190 °C / Microwave irradiation 9.1: hydrogenchloride / methanol / 2 h / Reflux; Inert atmosphere View Scheme |
-
-
100-07-2
4-methoxy-benzoyl chloride

-
-
118525-40-9
icaritin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1.1: potassium carbonate / acetone / 24.5 h / 65 °C 2.1: sodium carbonate; sodium hydrogencarbonate; Oxone / acetone; water; dichloromethane / 29 h / 0 °C / pH 9 2.2: 2 h / 20 °C 3.1: 5%-palladium/activated carbon; hydrogen / methanol; ethyl acetate / 24 h / 20 °C 4.1: potassium carbonate / acetone / 6 h / 20 °C 5.1: potassium carbonate / acetone / 12 h / 50 °C 6.1: N,N-diethylaniline / 0.5 h / 190 °C / Microwave irradiation 7.1: hydrogenchloride / methanol / 2 h / Reflux; Inert atmosphere View Scheme |

-
-
118525-40-9
icaritin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1.1: sodium carbonate; sodium hydrogencarbonate; Oxone / acetone; water; dichloromethane / 29 h / 0 °C / pH 9 1.2: 2 h / 20 °C 2.1: 5%-palladium/activated carbon; hydrogen / methanol; ethyl acetate / 24 h / 20 °C 3.1: potassium carbonate / acetone / 6 h / 20 °C 4.1: potassium carbonate / acetone / 12 h / 50 °C 5.1: N,N-diethylaniline / 0.5 h / 190 °C / Microwave irradiation 6.1: hydrogenchloride / methanol / 2 h / Reflux; Inert atmosphere View Scheme |

-
-
118525-40-9
icaritin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 5%-palladium/activated carbon; hydrogen / methanol; ethyl acetate / 24 h / 20 °C 2: potassium carbonate / acetone / 6 h / 20 °C 3: potassium carbonate / acetone / 12 h / 50 °C 4: N,N-diethylaniline / 0.5 h / 190 °C / Microwave irradiation 5: hydrogenchloride / methanol / 2 h / Reflux; Inert atmosphere View Scheme |
-
-
491-54-3
4'-O-methylkaempferol

-
-
118525-40-9
icaritin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: potassium carbonate / acetone / 6 h / 20 °C 2: potassium carbonate / acetone / 12 h / 50 °C 3: N,N-diethylaniline / 0.5 h / 190 °C / Microwave irradiation 4: hydrogenchloride / methanol / 2 h / Reflux; Inert atmosphere View Scheme | |
| Multi-step reaction with 4 steps 1: N-ethyl-N,N-diisopropylamine / tetrahydrofuran / 20 h / 0 - 20 °C / Inert atmosphere 2: caesium carbonate; tetrabutylammomium bromide / N,N-dimethyl-formamide / 18 h / 10 - 20 °C / Inert atmosphere 3: europium(III) trifluoromethanesulfonate / dichloromethane / 2 h / -5 - 0 °C / Inert atmosphere 4: hydrogenchloride / 1,4-dioxane; isopropyl alcohol / 6.17 h / Inert atmosphere; Reflux View Scheme | |
| Multi-step reaction with 4 steps 1: N-ethyl-N,N-diisopropylamine / tetrahydrofuran / 20 h / 0 - 20 °C / Inert atmosphere 2: caesium carbonate; tetrabutylammomium bromide / tetrahydrofuran / 40 h / 30 °C / Inert atmosphere 3: europium(III) trifluoromethanesulfonate / dichloromethane / 2 h / -5 - 0 °C / Inert atmosphere 4: hydrogenchloride / 1,4-dioxane; isopropyl alcohol / 6.17 h / Inert atmosphere; Reflux View Scheme |

-
-
118525-40-9
icaritin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: potassium carbonate / acetone / 12 h / 50 °C 2: N,N-diethylaniline / 0.5 h / 190 °C / Microwave irradiation 3: hydrogenchloride / methanol / 2 h / Reflux; Inert atmosphere View Scheme | |
| Multi-step reaction with 3 steps 1: caesium carbonate; tetrabutylammomium bromide / tetrahydrofuran / 40 h / 30 °C / Inert atmosphere 2: europium(III) trifluoromethanesulfonate / dichloromethane / 2 h / -5 - 0 °C / Inert atmosphere 3: hydrogenchloride / 1,4-dioxane; isopropyl alcohol / 6.17 h / Inert atmosphere; Reflux View Scheme | |
| Multi-step reaction with 3 steps 1: caesium carbonate; tetrabutylammomium bromide / N,N-dimethyl-formamide / 18 h / 10 - 20 °C / Inert atmosphere 2: europium(III) trifluoromethanesulfonate / dichloromethane / 2 h / -5 - 0 °C / Inert atmosphere 3: hydrogenchloride / 1,4-dioxane; isopropyl alcohol / 6.17 h / Inert atmosphere; Reflux View Scheme |

-
-
118525-40-9
icaritin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: N,N-diethylaniline / 0.5 h / 190 °C / Microwave irradiation 2: hydrogenchloride / methanol / 2 h / Reflux; Inert atmosphere View Scheme | |
| Multi-step reaction with 2 steps 1: europium(III) trifluoromethanesulfonate / dichloromethane / 2 h / -5 - 0 °C / Inert atmosphere 2: hydrogenchloride / 1,4-dioxane; isopropyl alcohol / 6.17 h / Inert atmosphere; Reflux View Scheme |

-
-
118525-40-9
icaritin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: hydrogenchloride / methanol; water / 2.5 h / Reflux; Inert atmosphere 2: palladium 10% on activated carbon; cyclohexa-1,4-diene / methanol / 2 h / 20 °C View Scheme | |
| Multi-step reaction with 2 steps 1: hydrogenchloride; water / methanol / 2.5 h / Inert atmosphere; Reflux 2: palladium 10% on activated carbon; cyclohexa-1,4-diene / methanol / 2 h / 20 °C View Scheme |

-
-
118525-40-9
icaritin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: N-ethyl-N,N-diisopropylamine / dichloromethane / 6 h / 20 °C 2: 18-crown-6 ether; potassium carbonate / acetone / 19.33 h / 20 °C 3: tris(6,6,7,7,8,8-heptafluoro-2,2-dimethyl-3,5-octadionato)europium(III) / chlorobenzene / 24 h / 85 °C / Inert atmosphere 4: hydrogenchloride / methanol; water / 2.5 h / Reflux; Inert atmosphere 5: palladium 10% on activated carbon; cyclohexa-1,4-diene / methanol / 2 h / 20 °C View Scheme | |
| Multi-step reaction with 5 steps 1: N-ethyl-N,N-diisopropylamine / dichloromethane / 6 h / 0 - 20 °C 2: potassium carbonate; 18-crown-6 ether / acetone / 19 h / 20 °C 3: tris(6,6,7,7,8,8-heptafluoro-2,2-dimethyl-3,5-octadionato)europium(III); sodium hydrogencarbonate / chlorobenzene / 24 h / 85 °C / Inert atmosphere 4: hydrogenchloride; water / methanol / 2.5 h / Inert atmosphere; Reflux 5: palladium 10% on activated carbon; cyclohexa-1,4-diene / methanol / 2 h / 20 °C View Scheme |
-
-
16274-11-6
3,5,7-triacetoxy-2-(4-acetoxy-phenyl)-chromen-4-one

-
-
118525-40-9
icaritin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: potassium iodide; potassium carbonate / acetone 2: dimethyl sulfate; potassium carbonate / acetone 3: N-ethyl-N,N-diisopropylamine / dichloromethane / 6 h / 20 °C 4: 18-crown-6 ether; potassium carbonate / acetone / 19.33 h / 20 °C 5: tris(6,6,7,7,8,8-heptafluoro-2,2-dimethyl-3,5-octadionato)europium(III) / chlorobenzene / 24 h / 85 °C / Inert atmosphere 6: hydrogenchloride / methanol; water / 2.5 h / Reflux; Inert atmosphere 7: palladium 10% on activated carbon; cyclohexa-1,4-diene / methanol / 2 h / 20 °C View Scheme | |
| Multi-step reaction with 7 steps 1.1: potassium iodide; potassium carbonate / N,N-dimethyl-formamide / 24 h / Reflux 2.1: potassium carbonate / tetrahydrofuran / 2 h / Reflux 2.2: 24 h / Reflux 3.1: N-ethyl-N,N-diisopropylamine / dichloromethane / 6 h / 0 - 20 °C 4.1: potassium carbonate; 18-crown-6 ether / acetone / 19 h / 20 °C 5.1: tris(6,6,7,7,8,8-heptafluoro-2,2-dimethyl-3,5-octadionato)europium(III); sodium hydrogencarbonate / chlorobenzene / 24 h / 85 °C / Inert atmosphere 6.1: hydrogenchloride; water / methanol / 2.5 h / Inert atmosphere; Reflux 7.1: palladium 10% on activated carbon; cyclohexa-1,4-diene / methanol / 2 h / 20 °C View Scheme |

-
-
118525-40-9
icaritin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 18-crown-6 ether; potassium carbonate / acetone / 19.33 h / 20 °C 2: tris(6,6,7,7,8,8-heptafluoro-2,2-dimethyl-3,5-octadionato)europium(III) / chlorobenzene / 24 h / 85 °C / Inert atmosphere 3: hydrogenchloride / methanol; water / 2.5 h / Reflux; Inert atmosphere 4: palladium 10% on activated carbon; cyclohexa-1,4-diene / methanol / 2 h / 20 °C View Scheme | |
| Multi-step reaction with 4 steps 1: potassium carbonate; 18-crown-6 ether / acetone / 19 h / 20 °C 2: tris(6,6,7,7,8,8-heptafluoro-2,2-dimethyl-3,5-octadionato)europium(III); sodium hydrogencarbonate / chlorobenzene / 24 h / 85 °C / Inert atmosphere 3: hydrogenchloride; water / methanol / 2.5 h / Inert atmosphere; Reflux 4: palladium 10% on activated carbon; cyclohexa-1,4-diene / methanol / 2 h / 20 °C View Scheme |
-
-
520-18-3
kaempferol

-
-
118525-40-9
icaritin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1: pyridine 2: potassium iodide; potassium carbonate / acetone 3: dimethyl sulfate; potassium carbonate / acetone 4: N-ethyl-N,N-diisopropylamine / dichloromethane / 6 h / 20 °C 5: 18-crown-6 ether; potassium carbonate / acetone / 19.33 h / 20 °C 6: tris(6,6,7,7,8,8-heptafluoro-2,2-dimethyl-3,5-octadionato)europium(III) / chlorobenzene / 24 h / 85 °C / Inert atmosphere 7: hydrogenchloride / methanol; water / 2.5 h / Reflux; Inert atmosphere 8: palladium 10% on activated carbon; cyclohexa-1,4-diene / methanol / 2 h / 20 °C View Scheme | |
| Multi-step reaction with 8 steps 1.1: pyridine / 20 °C 2.1: potassium iodide; potassium carbonate / N,N-dimethyl-formamide / 24 h / Reflux 3.1: potassium carbonate / tetrahydrofuran / 2 h / Reflux 3.2: 24 h / Reflux 4.1: N-ethyl-N,N-diisopropylamine / dichloromethane / 6 h / 0 - 20 °C 5.1: potassium carbonate; 18-crown-6 ether / acetone / 19 h / 20 °C 6.1: tris(6,6,7,7,8,8-heptafluoro-2,2-dimethyl-3,5-octadionato)europium(III); sodium hydrogencarbonate / chlorobenzene / 24 h / 85 °C / Inert atmosphere 7.1: hydrogenchloride; water / methanol / 2.5 h / Inert atmosphere; Reflux 8.1: palladium 10% on activated carbon; cyclohexa-1,4-diene / methanol / 2 h / 20 °C View Scheme |

-
-
118525-40-9
icaritin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: dimethyl sulfate; potassium carbonate / acetone 2: N-ethyl-N,N-diisopropylamine / dichloromethane / 6 h / 20 °C 3: 18-crown-6 ether; potassium carbonate / acetone / 19.33 h / 20 °C 4: tris(6,6,7,7,8,8-heptafluoro-2,2-dimethyl-3,5-octadionato)europium(III) / chlorobenzene / 24 h / 85 °C / Inert atmosphere 5: hydrogenchloride / methanol; water / 2.5 h / Reflux; Inert atmosphere 6: palladium 10% on activated carbon; cyclohexa-1,4-diene / methanol / 2 h / 20 °C View Scheme | |
| Multi-step reaction with 6 steps 1.1: potassium carbonate / tetrahydrofuran / 2 h / Reflux 1.2: 24 h / Reflux 2.1: N-ethyl-N,N-diisopropylamine / dichloromethane / 6 h / 0 - 20 °C 3.1: potassium carbonate; 18-crown-6 ether / acetone / 19 h / 20 °C 4.1: tris(6,6,7,7,8,8-heptafluoro-2,2-dimethyl-3,5-octadionato)europium(III); sodium hydrogencarbonate / chlorobenzene / 24 h / 85 °C / Inert atmosphere 5.1: hydrogenchloride; water / methanol / 2.5 h / Inert atmosphere; Reflux 6.1: palladium 10% on activated carbon; cyclohexa-1,4-diene / methanol / 2 h / 20 °C View Scheme |

-
-
118525-40-9
icaritin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: tris(6,6,7,7,8,8-heptafluoro-2,2-dimethyl-3,5-octadionato)europium(III) / chlorobenzene / 24 h / 85 °C / Inert atmosphere 2: hydrogenchloride / methanol; water / 2.5 h / Reflux; Inert atmosphere 3: palladium 10% on activated carbon; cyclohexa-1,4-diene / methanol / 2 h / 20 °C View Scheme | |
| Multi-step reaction with 3 steps 1: tris(6,6,7,7,8,8-heptafluoro-2,2-dimethyl-3,5-octadionato)europium(III); sodium hydrogencarbonate / chlorobenzene / 24 h / 85 °C / Inert atmosphere 2: hydrogenchloride; water / methanol / 2.5 h / Inert atmosphere; Reflux 3: palladium 10% on activated carbon; cyclohexa-1,4-diene / methanol / 2 h / 20 °C View Scheme |

-
-
118525-40-9
icaritin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: sodium acetate; hydrogenchloride / ethanol; water / 5 h / 50 °C / pH 5 / Enzymatic reaction 2: sulfuric acid / ethanol; water / 5 h / 50 °C View Scheme |
-
-
64-18-6
formic acid

-
-
118525-40-9
icaritin

-
-
38226-86-7
3,5-dihydroxy-2-(4-methoxyphenyl)-8,8-dimethyl-9,10-dihydro-8H-pyrano[2,3-f]chromen-4-one

| Conditions | Yield |
|---|---|
| for 20h; Reflux; | 93.3% |
-
-
118525-40-9
icaritin

-
-
38226-86-7
3,5-dihydroxy-2-(4-methoxyphenyl)-8,8-dimethyl-9,10-dihydro-8H-pyrano[2,3-f]chromen-4-one

| Conditions | Yield |
|---|---|
| With sulfuric acid; acetic acid at 90℃; for 6h; | 92% |
| With sulfuric acid; acetic acid at 90℃; for 6h; | 92% |
| With polyphosphoric acid; air In N,N-dimethyl-formamide at 130℃; for 2h; | 81% |
-
-
118525-40-9
icaritin

-
-
105-36-2
ethyl bromoacetate

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone for 6h; Reflux; | 90% |
-
-
110-52-1
1,4-dibromo-butane

-
-
118525-40-9
icaritin

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone for 12h; Reflux; | 87% |

| Conditions | Yield |
|---|---|
| With recombinant rhamnosyl transferase from Epimedium pseudowushanense; UDP-rhamnose synthase from Epimedium pseudowushanense; nicotinamide adenine dinucleotide; NADPH In dimethyl sulfoxide at 30℃; for 12h; pH=7.4; Enzymatic reaction; regioselective reaction; | 87% |

| Conditions | Yield |
|---|---|
| Stage #1: N-Boc-(S/R)-valine; icaritin With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 20℃; for 1h; Stage #2: With hydrogenchloride In dichloromethane; water at 20℃; for 0.333333h; | 81% |
-
-
2969-81-5
4-bromoethylbutanoate

-
-
118525-40-9
icaritin

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone for 6h; Reflux; | 80% |
-
-
3744-87-4, 7764-95-6, 15761-38-3
N-t-butyloxycarbonyl-DL-alanine

-
-
118525-40-9
icaritin

| Conditions | Yield |
|---|---|
| Stage #1: N-t-butyloxycarbonyl-DL-alanine; icaritin With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 20℃; for 1h; Stage #2: With hydrogenchloride In dichloromethane; water at 20℃; for 0.333333h; | 80% |
-
-
118525-40-9
icaritin

-
-
623-48-3
ethyl iodoacetae

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone for 12h; Reflux; | 79% |
-
-
629-03-8
1 ,6-dibromohexane

-
-
118525-40-9
icaritin

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone for 12h; Reflux; | 78% |
-
-
4549-32-0
1,8-dibromooctane

-
-
118525-40-9
icaritin

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone for 12h; Reflux; | 75% |
-
-
118525-40-9
icaritin

-
-
540-51-2
2-bromoethanol

-
-
1067198-74-6
5-hydroxy-3,7-bis(2-hydroxyethoxy)-2-(4-methoxyphenyl)-8-(3-methylbut-2-en-1-yl)-4H-chromen-4-one

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone for 6h; Reflux; | 73% |
| With potassium carbonate In acetone Reflux; | 61% |
| With potassium carbonate In acetone Reflux; | 61% |

| Conditions | Yield |
|---|---|
| Stage #1: bis(trichloromethyl) carbonate; icaritin With N-ethyl-N,N-diisopropylamine In dichloromethane at 0 - 20℃; for 3.5h; Inert atmosphere; Stage #2: 1-methyl-piperazine With N-ethyl-N,N-diisopropylamine In dichloromethane at 0 - 20℃; | 69% |

-
-
118525-40-9
icaritin

-
-
623-48-3
ethyl iodoacetae

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone for 12h; Reflux; | 65% |

| Conditions | Yield |
|---|---|
| With dmap; N-ethyl-N,N-diisopropylamine In tetrahydrofuran; tetrachloromethane at -10 - 20℃; | 64% |
-
-
37746-78-4
4-bromo-trans-crotonic acid ethyl ester

-
-
118525-40-9
icaritin

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone for 6h; Reflux; | 60% |

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; | 59.8% |
-
-
118525-40-9
icaritin

-
-
627-18-9
1-bromo-3-propanol

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone for 6h; Reflux; | 55% |
| With potassium carbonate In acetone at 56℃; for 8h; | 850 mg |
-
-
118525-40-9
icaritin

-
-
6482-24-2
2-Bromoethyl methyl ether

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone Reflux; Inert atmosphere; | 55% |
-
-
118525-40-9
icaritin

-
-
106-93-4
ethylene dibromide

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone for 12h; Reflux; | 54% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone for 6h; Reflux; | A 41% B 12% |


| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone for 6h; Reflux; | A 40% B 10% |

Related products
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View