Simagchem Corporation
Welcome to Simagchem, your partner in China as a premier supply of bulk specialty chemicals for industry and life science. We introduce experienced quality product and exceptional JIT service with instant market intelligence in China to benefit our
Cas:12093-10-6
Min.Order:0 Metric Ton
Negotiable
Type:Manufacturers
inquiryAlity Chemical Corporation
The above product is Ality Chemical's strong item with best price, good quality and fast supply. Ality Chemical has been focusing on the research and production of this field for over 14 years. At the same time, we are always committed to providi
Dayang Chem (Hangzhou) Co.,Ltd.
DayangChem exported this product to many countries and regions at best price in China. If you are looking for the product’s supplier in China, DayangChem is your best choice. As a leading manufacturer and supplier of chemicals in China, Day
Cas:12093-10-6
Min.Order:1 Kilogram
FOB Price: $4.0
Type:Lab/Research institutions
inquiryChemwill Asia Co., Ltd.
Our main production base is located in Xuzhou industry park. We are certified both to the ISO 9001 and ISO 14001 Standards, have a safety management system in place.Our R&D team masters core technology for process-design of target building block
Cas:12093-10-6
Min.Order:5 Kiloliter
FOB Price: $1.2 / 5.0
Type:Manufacturers
inquiryZhuozhou Wenxi import and Export Co., Ltd
WITH US,YOUR MONEY IN SAFE,YOUR BUSINESS IN SAFE 1)Quick Response Within 12 hours; 2)Quality Guarantee: All products are strictly tested by our QC, confirmed by QA and approved by third party lab in China, USA, Canada, Germany, UK, Italy, France et
Cas:12093-10-6
Min.Order:1 Kilogram
FOB Price: $139.0 / 210.0
Type:Trading Company
inquiryHangzhou Sartort Biopharma Co., Ltd
Appearance:orange to red to brown powder Storage:room temperature Package:25kg/drum Application:chemicals Transportation:Express/Sea/Air Port:any port in china
Cas:12093-10-6
Min.Order:1 Kilogram
FOB Price: $1.0
Type:Lab/Research institutions
inquiryShanghai Upbio Tech Co.,Ltd
1.No Less 8 years exporting experience. Clients can 100% received goods 2.Lower Price with higher quality 3,Free sample 4,We are sincerely responsible for the "product quality" and "After Service" Upbio is Specialized
Cas:12093-10-6
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryShanghai Minstar Chemical Co., Ltd
Product Name: Ferrocenecarboxaldehyde CAS: 12093-10-6 MF: C11H10FeO10* MW: 214.04 EINECS: 235-158-1 Mol File: 12093-10-6.mol Ferrocenecarboxaldehyde Structure Ferrocenecarboxaldehyde Chemical Properties Melting point 118-120 &
Cas:12093-10-6
Min.Order:1 Gram
Negotiable
Type:Lab/Research institutions
inquiryQingdao Beluga Import and Export Co., LTD
Ferrocenecarboxaldehyde CAS:12093-10-6 Qingdao Belugas Import and Export Co., Ltd. is a scientific and technological company integrating research and development, production and trade of chemical intermediates, specializing in high quality organic
Cas:12093-10-6
Min.Order:1 Gram
Negotiable
Type:Lab/Research institutions
inquiryShandong Hanjiang Chemical Co., Ltd.
Hello, dear friend! I'm Hansen and Allen from China. Welcome to my lookchem mall! The following is a brief introduction of our company's products and services. If you are interested in our products, please contact us by emai
Cas:12093-10-6
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryHenan Wentao Chemical Product Co., Ltd.
Henan Wentao Chemical Product Co.,Ltd is Located in Zhengzhou High-tech Development Zone with import and export license, We passed ISO 9001:2008 as well, Henan Wentao has developed more than 1000 compounds, which are widely used in the fields of prod
Cas:12093-10-6
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryBaoji Guokang Healthchem co.,ltd
Our company has been in existence for 10 years since its establishment. We have our own unique team. The company integrates independent research and development, production and sales. We have established famous brands at home and abroad. At present
Cas:12093-10-6
Min.Order:1 Gram
FOB Price: $93.0
Type:Trading Company
inquiryHubei Jiutian Bio-medical Technology Co., Ltd
1,we produce and sell good chemicals around the world. 2,our success rate is about 95%. this means, if customer order is accepted, the probability that the customer will obtain the ordered substances, is 95%. 3,our staff consists of highly qualifie
Cas:12093-10-6
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryTianjin Kind Pharma Co., Ltd.
Factory direct sales, accept customization. Ferrocenecarboxaldehyde is used to prepare chiral ferrocene aziridinylmethanols for selective azomethine ylide cycloaddtion. It is an important raw material and intermediate used in Organic Synthesis, Phar
Cas:12093-10-6
Min.Order:1 Gram
Negotiable
Type:Lab/Research institutions
inquiryHangzhou J&H Chemical Co., Ltd.
J&H CHEM R&D center can offer custom synthesis according to the contract research and development services for the fine chemicals, pharmaceutical, biotechnique and some of the other chemicals. J&H CHEM has some Manufacturing base in Jia
Cas:12093-10-6
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryHefei Zhaobo Technology Co., Ltd.
Our Advantages Production: Advanced chemical equipment with years of experience Staffs for producing various extract products. Quality Control:A complete set of Testing Professional and Analysis Equipment ensures the Quality Requirements and Specif
Cas:12093-10-6
Min.Order:1 Kilogram
FOB Price: $75.0 / 150.0
Type:Trading Company
inquiryZibo Hangyu Biotechnology Development Co., Ltd
Zibo Hangyu Biotechnology Development Co., Ltd is a leading manufacturer and supplier of chemicals in China. We develop produce and distribute high quality pharmaceuticals, intermediates, special chemicals and OLED intermediates and other fine chemi
Shandong Mopai Biotechnology Co., LTD
Shandong Mopai Biotechnology Co., LTD is a leading manufacturer and supplier of chemicals in China. We develop produce and distribute high quality pharmaceuticals, intermediates, special chemicals and OLED intermediates and other fine chemicals. W
Cas:12093-10-6
Min.Order:1 Gram
Negotiable
Type:Lab/Research institutions
inquiryKAISA GROUP INC
1.Applied in food field.it can improve the immune system and prolong life. 2.Appliedin cosmetic field.it can improve the skin care. 3.Applied in pharmaceutical field.it can treat various dieases. 4.Our product quality assurance will make our customer
Cas:12093-10-6
Min.Order:1 Metric Ton
FOB Price: $7.0 / 8.0
Type:Trading Company
inquiryHunan chemfish Pharmaceutical co.,Ltd
Appearance:95%+ Package:R&D,Pilot run Transportation:per client require Port:Express ,Air, Sea
HANGZHOU YUNUO CHEMICAL CO.,LTD
Superior quality, moderate price & quick delivery. Appearance:colorless transparent liquid Storage:stored in a cool, dry and ventilated place to provent sun and rain Package:25kg/drum, or as per your request. Application:Used in organic syn
Win-Win chemical Co.Ltd
Stock products, own laboratory Package:Grams, Kilograms Application:For R&D Transportation:According to customer request Port:Shanghai
Cas:12093-10-6
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryHenan Tianfu Chemical Co., Ltd.
Melting point 118-120 °C(lit.) Water Solubility INSOLUBLE Stability: Stable. Incompatible with strong oxidizing agents. EPA Substance Registry System Ferrocene, formyl-(12093-10-6) Safety Information Safety Statements 22-24/25 WG
Cas:12093-10-6
Min.Order:1 Metric Ton
FOB Price: $5000.0
Type:Lab/Research institutions
inquiryKono Chem Co.,Ltd
high purity lowest priceAppearance:solid or liquid Storage:in sealed air resistant place Package:drum and bag Application:for pharma use Transportation:by sea or air Port:Beijing or Guangzhou
GIHI CHEMICALS CO.,LIMITED
high purity,in stock Package:25kg/drum,or as per customers'demand Application:API,or Intermediates,fine chemicals Transportation:air,sea,courier
Cas:12093-10-6
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryJINHUA HUAYI CHEMICAL CO., LTD.
Jinhua huayi chemical co., ltd. is dedicated to the development, production and marketing of chemicals. On the basis of equality and mutual benefit, and under the principle of customer first, credit first, quality first, we are ready to join hands
Cas:12093-10-6
Min.Order:100 Gram
Negotiable
Type:Lab/Research institutions
inquiryHangzhou Dingyan Chem Co., Ltd
Ferrocenecarboxaldehyde
Cas:12093-10-6
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryHenan Allgreen Chemical Co.,Ltd
high quality Storage:Sealed, dry, microtherm , avoid light and smell. Package:According to the demand of customer Application:Organic synthesis Transportation:by air or by sea
Aecochem Corp.
Our clients, like BASF,CHEMO,Brenntag,ASR,Evonik,Merck and etc.Appearance:COA Storage:in stock Application:MSDS/TDS
Hubei Taiho Chemical Co.,LTD
TAIHO Unique Advantages:1.We're factory2.Free samples available3.Commodity inspection can be done4.ISO9001,Kosher certifications5.10 years experiences Storage:Store in cool &dry place Package:aluminium foil bag/fiber can/plastic drum Application:comp
Synthetic route

| Conditions | Yield |
|---|---|
| Stage #1: N-methyl-N-phenylformamide With trichlorophosphate at 0℃; for 0.25h; Stage #2: ferrocene at 20℃; Inert atmosphere; | 98% |
| With phosphorus oxychloride In neat (no solvent) anilide and P-compd. stirring at room temp. for 30 min, Fe-compd. addn.,mixt. stirring at room temp. for 3 d, quenching by pouring onto ice, aq . layer extn. after 2 h with Et2O, org. layer drying (MgSO4), vac. concn.; residue flash column chromy. (SiO2, petrol/E2O 7:3 to 5:5), recrystn. (hot petroleum); | 87% |
| With trichlorophosphate In neat (no solvent) Vilsmeyer formylation; double mol amt. of formylation mixt., few days at ambient temp.;; | 81% |

-
-
12093-10-6
ferrocenecarboxaldehyde

| Conditions | Yield |
|---|---|
| With sodium hydride; sodium iodide In tetrahydrofuran at 40℃; chemoselective reaction; | 94% |
-
-
1273-86-5
1-ferrocenylmethanol

-
-
12093-10-6
ferrocenecarboxaldehyde

| Conditions | Yield |
|---|---|
| With ferrocene-labeled Merrifield resin-supported ionic liquid ([FemDMMerA]RuO4) In tetrahydrofuran for 4h; Reflux; Green chemistry; | 92% |
| With C34H37N4O6Ru2(1+)*Cl(1-); potassium hydroxide In toluene at 70℃; for 6h; Schlenk technique; Inert atmosphere; | 90% |
| With 1-methyl-1H-imidazole; [2,2]bipyridinyl; 2,2,6,6-tetramethyl-piperidine-N-oxyl; TPGS-750-M; copper(I) bromide In water at 20℃; for 24h; | 88% |
-
-
102-54-5
ferrocene

-
-
68-12-2, 33513-42-7
N,N-dimethyl-formamide

-
A
-
12093-10-6
ferrocenecarboxaldehyde

-
B
-
1271-48-3
1,1'-ferrocenyldicarboxaldehyde

| Conditions | Yield |
|---|---|
| With tert.-butyl lithium In tetrahydrofuran; n-heptane under N2; tBuLi in hexane is added to ferrocene in THF at -20°C, stirred for 30 min, DMF is added at -10°C, HCl is added; extd. with CH2Cl2, chromd. (SiO2, CH2Cl2); | A 91% B 1% |
| With potassium tert-butylate; tert.-butyl lithium In tetrahydrofuran N2-atmosphere; dropwise addn. of t-BuLi to mixt. of ferrocene and t-BuOKat -74 to -70°C, stirring at -74°C for 1 h, addn. of exce ss DMF, warming to -40°C over 20 min; water addn., solvent removal (reduced pressure), extn. into CH2Cl2, drying (MgSO4), chromy. (SiO2, hexane, then CH2Cl2, then CH2Cl2/Et2O=1:1); | A 90.7% B 4.9% |
| Stage #1: ferrocene With n-butyllithium; N,N,N,N,-tetramethylethylenediamine In hexane at -80 - 0℃; for 0.25h; Stage #2: N,N-dimethyl-formamide In n-heptane at -80℃; for 2h; | A 20% B 71% |


| Conditions | Yield |
|---|---|
| Stage #1: N,N-dimethyl-formamide With trichlorophosphate at 0℃; for 0.25h; Inert atmosphere; Stage #2: ferrocene at 40℃; for 3h; Inert atmosphere; | 90% |
| With trichlorophosphate In chloroform at -10℃; for 13.5h; Vilsmeier Reaction; Reflux; | 79% |
| Stage #1: N,N-dimethyl-formamide With trichlorophosphate at -10 - -5℃; Inert atmosphere; Stage #2: ferrocene In chloroform at 20℃; Inert atmosphere; | 79% |

| Conditions | Yield |
|---|---|
| In diethyl ether Ar; pptn. on mixing; filtration, evapn. of soln.; | A 90% B n/a |


| Conditions | Yield |
|---|---|
| In diethyl ether Ar; pptn. on mixing; filtration, evapn. of soln.; | A 90% B n/a |


-
-
12093-10-6
ferrocenecarboxaldehyde

| Conditions | Yield |
|---|---|
| With [RhCl2(p-cymene)]2; copper diacetate In tert-Amyl alcohol at 80℃; for 12h; Catalytic behavior; Reagent/catalyst; Solvent; Temperature; Inert atmosphere; Schlenk technique; | 75% |
| With ferrocene; copper diacetate In 1,2-dichloro-ethane at 80℃; Reagent/catalyst; | 50% |

| Conditions | Yield |
|---|---|
| With trichlorophosphate for 72h; | 73% |

| Conditions | Yield |
|---|---|
| In dichloromethane presence of AlCl3, Cl2CHOC2H5 excess, 0°C;; | 72% |

-
-
594-19-4
tert.-butyl lithium

-
A
-
12093-10-6
ferrocenecarboxaldehyde

| Conditions | Yield |
|---|---|
| Stage #1: ferrocenecarboxaldoxime; tert.-butyl lithium In diethyl ether at -80℃; for 0.5h; Inert atmosphere; Schlenk technique; Stage #2: With C20H34ClO3P In diethyl ether at -30 - 20℃; for 18h; Inert atmosphere; Schlenk technique; | A 8% B 72% C 3% |


| Conditions | Yield |
|---|---|
| With AlCl3 In dichloromethane triethyl orthoformate added dropwise to mixt. of ferrocene and CH2Cl2 under stirring, after complete dissolution of ferrocene anhydrous AlCl3 slowly added, mixt. stirred at room temp. for 3 h; quenched with satd. soln. of sodium hydrosulfite, extraction with Et2O, extract concd. under vac., chromy. (silica gel, petroleum ether/ethyl acetate (5/1)); | 70% |
| With aluminum (III) chloride In dichloromethane at 20℃; for 4h; | 70% |
| With aluminum (III) chloride In benzene at 0 - 25℃; |
-
-
116693-42-6, 54841-23-5
ferrocenyl-pentacarbonylmanganese

-
A
-
12093-10-6
ferrocenecarboxaldehyde

| Conditions | Yield |
|---|---|
| With methanol In methanol under N2, soln. of Mn(CO)5(CpFeC5H4) in MeOH stirred at room temp. for 67 h; evapd., extd. with pentane, evapd., taken up in CH2Cl2, sepd. by columnchromy. (Kieselgel, CH2Cl2); | A <1 B 69% |

-
A
-
12093-10-6
ferrocenecarboxaldehyde

-
B
-
216527-69-4, 254977-75-8
(Sp,Sp)-[1,1'-biferrocenyl]-2,2'-dicarboxaldehyde

| Conditions | Yield |
|---|---|
| With copper at 105℃; Inert atmosphere; Darkness; | A n/a B 66.8% C n/a |


-
A
-
12093-10-6
ferrocenecarboxaldehyde

-
B
-
1273-86-5
1-ferrocenylmethanol

| Conditions | Yield |
|---|---|
| With sodium azide In water boiling; | A 2% B 8.6% C 62% |
| With NaN3 In water boiling; | A 2% B 8.6% C 62% |

-
-
14873-63-3
cis-bis(benzonitrile)dichloroplatinum(II)

-
A
-
12093-10-6
ferrocenecarboxaldehyde

-
-
657414-35-2
[Pt([(η5-C5H3)-CH=N-(C6H4-2-SMe)]Fe(η5-C5H5))Cl]

| Conditions | Yield |
|---|---|
| In toluene suspn. of Pt complex in toluene heated under reflux until solid dissolved, filtered, filtrate treated with Fe complex, mixt. heated under refluxfor 5.5 h; evapd. under vac., residue washed with hexane, dissolved in CH2Cl2, hexane added, slowly evapd. at room temp., crystals collected, air-dried; elem. anal.; | A n/a B 59% |

-
-
38959-35-2
η-fluorene-η-cyclopentadienyliron(II) hexafluorophosphate

-
-
12093-10-6
ferrocenecarboxaldehyde

| Conditions | Yield |
|---|---|
| With t-BuOK; n-BuLi In tetrahydrofuran; hexane dropwise addn. of BuLi (2.5 M, hexane, 1 h) to soln. (THF) of Cp-deriv. (-78°C), mixing with soln. (THF) of Fe-intermediate (made from t-BuOK and Fe-compd., 0°C, 30 min), heating (60°C, 6 h); cooling to room temp. extn. (hexane-water (+ HCl drop)), org. phase drying (MgSO4), concg., chromy. (SiO2, hexane); | 56% |

-
-
696-68-4
6-(dimethylamino)fulvene

-
-
12093-10-6
ferrocenecarboxaldehyde

| Conditions | Yield |
|---|---|
| With sodium hydroxide In dichloromethane Irradiation (UV/VIS); a stirred soln. of Fe-complex and pentafulvene in CH2Cl2 was irradiated for 14 h with a 300-W sunlamp (Osram) in an atm. of N2, cooled to room temp., filtered, EtOH and 2 N NaOH were added, stirring was continued for another 90 min; layers were sepd., aq. layer dild., extd. with CH2Cl2, combined org. layers were washed twice with water, dried (Na2SO4), concd., residue was chromd. over alumina column (B II), recrystd. from ether-hexane or sublimed at 0.001 Torr; | 53% |
-
-
16853-85-3
lithium aluminium tetrahydride

-
-
403819-33-0
1-ferrocenoyl-1H-imidazole

-
-
12093-10-6
ferrocenecarboxaldehyde

| Conditions | Yield |
|---|---|
| In diethyl ether (Ar or N2); addn. of iron complex to Et2O with LiAlH4, stirring for 15 min at room temp.; addn. to a mixt. of cold water and ethyl acetate, extn. with Et2O, drying (Na2SO4), evapn., column chromy. (Al2O3, petroleum ether/Et2O 10:1), evapn.; | 52% |

-
-
124-38-9
carbon dioxide

-
A
-
12093-10-6
ferrocenecarboxaldehyde

-
B
-
433301-35-0
(Sp)-2-formylferrocene-1-carboxylic acid

| Conditions | Yield |
|---|---|
| With t-butyl lithium; hydrochloric acid; SnCl2 In diethyl ether; water; pentane Schlenk techniques used under Ar, Fc(C4H6O2)CH2OMe (20 mmol) in Et2O treated with 1.7M pentane soln. of tBuLi (22 mmol) at -78°C for 10 min, stirred for 1 h at room temp., cooled to -20°C, CO2 gas introduced for 20 min, aq. HCl/SnCl2 added; stirred overnight at room temp., organic phase sepd., aq. phase extd. with EtOAc, combined organic phase washed with H2O, dried over Na2SO4, evapd., chromd. (SiO2, CH2Cl2/MeOH (80:1, 30:1)), evapd., petroleum ether added, filtered, dried; elem. anal.; | A 18% B 52% |

-
-
15321-51-4
diiron nonacarbonyl

-
-
1273-86-5
1-ferrocenylmethanol

-
A
-
12093-10-6
ferrocenecarboxaldehyde

-
B
-
69661-83-2
bis(ferrocenylmethyl)ether

| Conditions | Yield |
|---|---|
| In benzene soln. of ferrocenylcarbinol is degassed (N2) and refluxed for 30 h in presence of Fe2(CO)9; react. mixt. is filtered over Celite, concd. and chromatographed (silica gel 60, CH2Cl2); | A 20% B 51% |


-
-
7732-18-5
water

-
A
-
12093-10-6
ferrocenecarboxaldehyde

| Conditions | Yield |
|---|---|
| With K3Fe(CN)6/K2CO3; hydroquinidine 2,5-Ph2-4,6-pyrimidinediyl diether; K2OsO2(OH)4 In water; tert-butyl alcohol (N2); solid Os complex (0.2 equiv.) was added to soln. of K3Fe(CN)6 (6 equiv.), K2CO3 (6 equiv.) and hydroquinidine compd. (0.2 equiv.) in t-BuOH/H2O; mixt. was stirred for 2 h; ferrocenyl complex was added over 1.5 h; stirred at room temp. for 66 h; Na2SO3 added; extd. (EtOAc); org. extracts washed (brine) and dried (Na2SO4); evapd. (vac.); chromd. (SiO2, C6H14/EtOAc); elem. anal.; | A 20% B 51% |
| With K3Fe(CN)6/K2CO3; hydroquinidine 2,5-Ph2-4,6-pyrimidinediyl diether; K2OsO2(OH)4 In water; acetonitrile (N2); solid Os complex (0.1 equiv.) was added to soln. of K3Fe(CN)6 (3 equiv.), K2CO3 (3 equiv.) and hydroquinidine compd. (0.1 equiv.) in CH3CN/H2O; mixt. was stirred for 2 h; ferrocenyl complex was added over 1.5 h; stirred at room temp. for 110 h; Na2SO3 added; extd. (EtOAc); org. extracts washed (brine) and dried (Na2SO4); evapd. (vac.); chromd. (SiO2, C6H14/EtOAc); | A 4% B 30% |

-
-
116693-42-6, 54841-23-5
ferrocenyl-pentacarbonylmanganese

-
A
-
102-54-5
ferrocene

-
B
-
12093-10-6
ferrocenecarboxaldehyde

-
C
-
1273-86-5
1-ferrocenylmethanol

| Conditions | Yield |
|---|---|
| With propene; carbon monoxide; hydrogen In N,N-dimethyl-formamide High Pressure; soln. of Mn(CO)5(CpFeC5H4) in DMF pressurised in autoclave successivelywith 8 bar of propene, 15 bar of CO, and 15 bar of H2, stirred at 100°C for 20 h; evapd. in vac., extd. with CH2Cl2, sepd. by preparative thin layer chromy. (CH2Cl2); | A n/a B 17% C 47% |


-
-
7732-18-5
water

-
A
-
12093-10-6
ferrocenecarboxaldehyde

| Conditions | Yield |
|---|---|
| With K3Fe(CN)6/K2CO3; hydroquinine 2,5-Ph2-4,6-pyrimidinediyl diether; K2OsO2(OH)4 In water; tert-butyl alcohol (N2); solid Os complex (0.2 equiv.) was added to soln. of K3Fe(CN)6 (6 equiv.), K2CO3 (6 equiv.) and hydroquinine compd. (0.2 equiv.) in t-BuOH/H2O (1/1); mixt. was stirred for 2 h; ferrocenyl complex was added over 1.5 h; stirred at room temp. for 47 h; Na2SO3 added; extd. (EtOAc); org. extracts washed (brine) and dried (Na2SO4); evapd. (vac.); chromd. (SiO2, C6H14/EtOAc); | A 26% B 47% |
| With K3Fe(CN)6/K2CO3; hydroquinine 1,4-phthalazinediyl diether; K2OsO2(OH)4 In water; acetonitrile (N2); solid Os complex (0.1 equiv.) was added to soln. of K3Fe(CN)6 (3 equiv.), K2CO3 (3 equiv.) and hydroquinine compd. (0.1 equiv.) in CH3CN/H2O (1/1); mixt. was stirred for 2 h; ferrocenyl complex was added over 1.5 h; stirred at room temp. for 44 h; Na2SO3 added; extd. (EtOAc); org. extracts washed (brine) and dried (Na2SO4); evapd. (vac.); chromd. (SiO2, C6H14/EtOAc); | A n/a B 10% |

-
-
16940-66-2
sodium tetrahydroborate

-
-
12093-10-6
ferrocenecarboxaldehyde

| Conditions | Yield |
|---|---|
| In tetrahydrofuran N2 atmosphere; addn. of NaBH4 (0°C), stirring (room temp., 3 h); H2O addn., extn. (hexane), drying (Na2SO4), filtration, evapn., extn. (hexane), chromy. (SiO2, CH2Cl2); | 43% |

-
-
64-17-5
ethanol

-
-
7732-18-5
water

-
-
311-77-3
1,1-dimethylguanidine dihydrogensulfate

-
A
-
12093-10-6
ferrocenecarboxaldehyde

-
B
-
1416854-27-7
6-amino-2-ethoxy-4-ferrocenyl-5-ferrocenylmethyl-4,5-dihydropyridine-3,5-dicarbonitrile

-
C
-
1416854-25-5
2-amino-6-ethoxy-4-ferrocenylpyridine-3,5-dicarbonitrile

-
D
-
1416854-24-4
4-amino-2-dimethylamino-6-ferrocenylpyrimidine-5-carbonitrile

| Conditions | Yield |
|---|---|
| With sodium carbonate at 80℃; for 8h; | A 10% B 15% C 23% D 42% |

-
-
116693-42-6, 54841-23-5
ferrocenyl-pentacarbonylmanganese

-
-
75-05-8
acetonitrile

-
A
-
102-54-5
ferrocene

-
B
-
12093-10-6
ferrocenecarboxaldehyde

| Conditions | Yield |
|---|---|
| In acetonitrile under N2, soln. of Mn(CO)5(CpFeC5H4) in MeCN stirred for 63 h at room temp.; filtered through Na2SO4, evapd., extd. with pentane, concd., chromd. (Kieselgel; pentane, CH2Cl2, Et2O); | A n/a B 37% |


-
-
12093-10-6
ferrocenecarboxaldehyde

| Conditions | Yield |
|---|---|
| With hexamethylenetetramine In acetic acid boiling; | 37% |
| With hexamethylenetetramine In acetic acid boiling; | 37% |

-
-
64-17-5
ethanol

-
-
7732-18-5
water

-
-
1670-14-0
benzamidine monohydrochloride

-
A
-
12093-10-6
ferrocenecarboxaldehyde

-
B
-
1416854-27-7
6-amino-2-ethoxy-4-ferrocenyl-5-ferrocenylmethyl-4,5-dihydropyridine-3,5-dicarbonitrile


-
D
-
1416854-25-5
2-amino-6-ethoxy-4-ferrocenylpyridine-3,5-dicarbonitrile

| Conditions | Yield |
|---|---|
| With sodium carbonate at 80℃; for 8h; | A 11% B 12% C 33% D 22% E 9% |


-
-
64-17-5
ethanol

-
-
7732-18-5
water

-
-
124-42-5
acetamidine hydrochloride

-
A
-
12093-10-6
ferrocenecarboxaldehyde

-
B
-
1416854-27-7
6-amino-2-ethoxy-4-ferrocenyl-5-ferrocenylmethyl-4,5-dihydropyridine-3,5-dicarbonitrile

-
C
-
1416854-28-8
6-amino-4-ferrocenyl-2-methyl-3,4-dihydropyrimidine-5-carbonitrile

-
D
-
1416854-25-5
2-amino-6-ethoxy-4-ferrocenylpyridine-3,5-dicarbonitrile

-
E
-
1416854-26-6
4-amino-6-ferrocenyl-2-methylpyrimidine-5-carbonitrile

| Conditions | Yield |
|---|---|
| With sodium carbonate at 80℃; for 8h; | A 9% B 14% C n/a D 27.5% E 10% |


| Conditions | Yield |
|---|---|
| lithium perchlorate In dichloromethane (Ar); mixt. of Fe complex and LiClO4 (2 equiv.) in CH2Cl2 was stirred for 3 min; amine was added via syringe; after 5 min Me3SiCN was added; mixt. was stirred at room temp. for 20-30 min; CH2Cl2 added; filtered; org. layer washed (aq. NaHCO3, H2O); dried (Na2SO4); solvent removed; chromd. (silica gel, petroleum ether/EtOAc); | 100% |

-
-
123-75-1
pyrrolidine

-
-
12093-10-6
ferrocenecarboxaldehyde

-
-
7677-24-9
trimethylsilyl cyanide

-
-
843618-94-0
[(C5H5)Fe(C5H4CH(CN)NC4H8)]

| Conditions | Yield |
|---|---|
| lithium perchlorate In dichloromethane (Ar); mixt. of Fe complex and LiClO4 (2 equiv.) in CH2Cl2 was stirred for 3 min; amine was added via syringe; after 5 min Me3SiCN was added; mixt. was stirred at room temp. for 20-30 min; CH2Cl2 added; filtered; org. layer washed (aq. NaHCO3, H2O); dried (Na2SO4); solvent removed; chromd. (silica gel, petroleum ether/EtOAc); | 100% |


| Conditions | Yield |
|---|---|
| lithium perchlorate In dichloromethane (Ar); mixt. of Fe complex and LiClO4 (2 equiv.) in CH2Cl2 was stirred for 3 min; amine was added via syringe; after 5 min Me3SiCN was added; mixt. was stirred at room temp. for 20-30 min; CH2Cl2 added; filtered; org. layer washed (aq. NaHCO3, H2O); dried (Na2SO4); solvent removed; chromd. (silica gel, petroleum ether/EtOAc); | 100% |


| Conditions | Yield |
|---|---|
| lithium perchlorate In dichloromethane (Ar); mixt. of Fe complex and LiClO4 (2 equiv.) in CH2Cl2 was stirred for 3 min; amine was added via syringe; after 5 min Me3SiCN was added; mixt. was stirred at room temp. for 20-30 min; CH2Cl2 added; filtered; org. layer washed (aq. NaHCO3, H2O); dried (Na2SO4); solvent removed; chromd. (silica gel, petroleum ether/EtOAc); | 100% |


| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol for 16h; Inert atmosphere; Schlenk technique; Reflux; | 100% |
| With sodium acetate In ethanol; water under N2; H2NOH*HCl (2 equiv.) in water added to Fe complex in EtOH; AcONa (3 equiv.) added; refluxed for 3 h; cooled; concd. in vac.; CHCl3 added dropwise; stirred for 0.5 h; according to K. Schoegl, et al., Monatsh. Chem. 97 (1966) 150; filtered; filtrate concd. under vac.; | 95.8% |
| With sodium hydroxide In ethanol for 3h; Reflux; | 92% |

| Conditions | Yield |
|---|---|
| In methanol in CH3OH/piperidine; | 100% |
| In methanol in CH3OH/piperidine; | 100% |
| With dichloro[2,6-bis(4-isopropyloxazolin-2-yl)pyridine]zinc In water at 20℃; for 0.05h; Catalytic behavior; Reagent/catalyst; Solvent; Knoevenagel Condensation; Inert atmosphere; Schlenk technique; | 99% |
-
-
12093-10-6
ferrocenecarboxaldehyde

-
-
3886-69-9
(R)-1-phenyl-ethyl-amine

| Conditions | Yield |
|---|---|
| With MgSO4 In tetrahydrofuran (Ar) soln. ferrocenecarboxaldehyde and (R)-1-phenylethylamine in THF wasstirred at room temp. over MgSO4 for 24 h; soln. was filtered through Celite and evapd. in vacuo; | 100% |
| In methanol the mixt. in methanol was stirred for 20 h at room temp.; evapd., the solid was dissolved in benzene and pptd. with hexane; elem. anal.; | 90% |
| In methanol in presence of molecular sieves under Ar at room temp.; GC; solvent emoved in vacuo; recrystd. from Et2O; | 78% |
| In methanol Fe compd. reacted with org. compd. in MeOH according to Wang, H. X. et al., Inorg. Chem. Commun. 9 (2006) 658; Wang, H. X. et al., Polyhedron 25(2006) 2530; Wang, H. X. et al., Inorg. Chim. Acta 359 (2006) 4114; elem. anal.; | 75% |
| In chloroform for 3h; Reflux; |

| Conditions | Yield |
|---|---|
| With pyrrolidine In neat (no solvent) at 20℃; for 0.116667h; Aldol Condensation; | 100% |
-
-
12093-10-6
ferrocenecarboxaldehyde

-
-
88-05-1
2,4,6-trimethylaniline

| Conditions | Yield |
|---|---|
| In chloroform soln. of Fe-complex and ligand in CHCl3 was heated under reflux, cooled to room temp.; solvent was evapd., dried under vac.; elem. anal.; | 99.62% |
| With toluene-4-sulfonic acid In toluene Reflux; Dean-Stark; | 90% |
| In chloroform refluxing (6 h); removal of solvent, trituration with hexane; | 82% |
| In ethanol boiling; | |
| In toluene for 8h; Inert atmosphere; Reflux; Molecular sieve; |
-
-
12093-10-6
ferrocenecarboxaldehyde

-
-
506-59-2
N,N-dimethylammonium chloride

-
-
56553-60-7
sodium tris(acetoxy)borohydride

-
-
1271-86-9
N,N-dimethylaminomethylferrocene

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane Fe complex reacted with HNMe2*HCl and B compd. in presence of Et3N in CH2Cl2; | 99% |

-
-
12093-10-6
ferrocenecarboxaldehyde

-
-
107-15-3
ethylenediamine

-
-
63000-26-0
N,N'-bis(ferrocenylmethylene)ethylenediamine Schiff base

| Conditions | Yield |
|---|---|
| In neat (no solvent, solid phase) Fe complex (2 equiv.) and ligand were ground at room temp.; kept in vac.overnight; recrystd. (cold MeOH); | 99% |
| With potassium carbonate In ethanol reaction in boiling absolute EtOH in the presence of anhydrous K2CO3 under N2 and with protection from light; recrystn. from EtOH; elem. anal.; | 87% |
| In ethanol Inert atmosphere; Schlenk technique; | 87% |
-
-
16853-85-3
lithium aluminium tetrahydride

-
-
12093-10-6
ferrocenecarboxaldehyde

-
-
1273-86-5
1-ferrocenylmethanol

| Conditions | Yield |
|---|---|
| In diethyl ether under Ar; LiAlH4 in anhyd. Et2O added dropwise to Fe complex in anhyd. Et2O; stirred at room temp. for 30 min; aq. soln. of NH4Cl slowly added; ether phase sepd.; aq. phase extd. withEt2O; combined org. phases washed with H2O and brine; dried over Na2SO4 ; filtrate evapd. under vac.; | 99% |
| In not given |
-
-
16940-66-2
sodium tetrahydroborate

-
-
12093-10-6
ferrocenecarboxaldehyde

-
-
1273-86-5
1-ferrocenylmethanol

| Conditions | Yield |
|---|---|
| In methanol; sodium hydroxide aq. NaOH; (N2); a soln. of NaBH4 in aq. NaOH added to a MeOH soln. of Fe complex at 0°C, stirred and allowed to warm to room temp. overnight; evapd., extd. (Et2O), the org. layer washed (H2O), dried (MgSO4), filtered, evapd. (vac.); | 99% |
| With H2O In ethanol | 95% |
| In methanol synthesized by Broadhead, G. D., Osgerby, J. M., Pauson, P. L., J. Chem.Soc. (1958) 650; | 90% |
| In ethanol synthesized by Broadhead, G. D., Osgerby, J. M., Pauson, P. L., J. Chem.Soc. (1958) 650; | 90% |
-
-
12093-10-6
ferrocenecarboxaldehyde

-
-
1273-86-5
1-ferrocenylmethanol

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In methanol at 0 - 20℃; Inert atmosphere; | 99% |
| With C46H49CoN3P4(2+)*2BF4(1-); hydrogen; potassium hydroxide In ethanol; acetonitrile at 60℃; under 22801.5 Torr; for 24h; Autoclave; Glovebox; chemoselective reaction; | 98% |
| With lithium aluminium tetrahydride at 45℃; for 2h; Inert atmosphere; Reflux; | 97% |
-
-
16853-85-3
lithium aluminium tetrahydride

-
-
12093-10-6
ferrocenecarboxaldehyde

-
-
542-92-7
cyclopenta-1,3-diene

| Conditions | Yield |
|---|---|
| With potassium hydroxide In ethanol cyclopentadiene (ca.250 mmol) was added to a soln. of KOH (153 mmol) in ethanol; the mixt. was added to a stirred soln. of Fe-contg. compd (44 mmol) in ethanol; after 2.5 h TLC showed the react. completness; LiAlH4 (44 mmol) in Et2O was added; after 20 h the ppt. was collected on a frit, washed with Et2O and dried for 24 h in a vac.; | A 99% B 80% |


| Conditions | Yield |
|---|---|
| With iodine In chloroform for 1h; | 99% |
| With boron trifluoride diethyl etherate In dichloromethane at 20℃; for 10h; Schlenk technique; Sealed tube; Inert atmosphere; | 95.2% |
| presence of acidic catalysts: HCl, ZnCl2, p-CH3C6H4SO3H or BF3*O(C2H5)2; | 93% |
| presence of acidic catalysts: HCl, ZnCl2, p-CH3C6H4SO3H or BF3*O(C2H5)2; | 93% |
| With toluene-4-sulfonic acid In toluene at 60℃; for 24h; | 76% |

| Conditions | Yield |
|---|---|
| In pyridine standing of a soln. of ferrocene-compd. and hydroxylamine hydrochloridein dry pyridine at room temp. for 24 h under N2; pouring into water, pptn., recrystn. from MeOH; | 99% |

| Conditions | Yield |
|---|---|
| In chloroform byproducts: water; refluxing aldehyde and amine for 3 h, evapn. (reduced pressure), dissoln. in MeOH, addn. of excess NaBH4 (in portions, 0°C), stirring for30 min; addn. of aq. NaOH. extn. into CHCl3, drying, evapn.; elem. anal.; | 99% |

-
-
12093-10-6
ferrocenecarboxaldehyde

-
-
7677-24-9
trimethylsilyl cyanide

| Conditions | Yield |
|---|---|
| With C29H38AlN4O2(1+)*CF3O3S(1-) In tetrahydrofuran at 20℃; for 0.416667h; Catalytic behavior; Inert atmosphere; Schlenk technique; | 99% |
| With zinc(II) iodide In neat (no solvent) under N2; mixt. stirred overnight; evapd., column chromy. (alumina, hexane/CH2Cl2 75:25), recrystd. (hexane/CH2Cl2); elem. anal.; | 61% |
-
-
16940-66-2
sodium tetrahydroborate

-
-
12093-10-6
ferrocenecarboxaldehyde

-
-
104-84-7
para-methylbenzylamine

| Conditions | Yield |
|---|---|
| In chloroform byproducts: water; refluxing aldehyde and amine for 3 h, evapn. (reduced pressure), dissoln. in MeOH, addn. of excess NaBH4 (in portions, 0°C), stirring for30 min; addn. of aq. NaOH. extn. into CHCl3, drying, evapn.; elem. anal.; | 99% |

-
-
12093-10-6
ferrocenecarboxaldehyde

-
-
107-15-3
ethylenediamine

| Conditions | Yield |
|---|---|
| In benzene byproducts: H2O; Fe complex added to a soln. of ethylenediamine, refluxed overnight with a Dean-Stark apparatus for azeotropic distn.; evapd. (vac.); elem. anal.; | 99% |
| In not given byproducts: H2O; Fe complex reacted with H2NCH2CH2NH2 using Dean-Stark apparatus for removal of H2O by azeotropic distn.; |

| Conditions | Yield |
|---|---|
| With potassium carbonate In neat (no solvent) stirring (darkness); hexane and K2CO3 addn., filtn., concn.; elem. anal.; | 99% |
-
-
12093-10-6
ferrocenecarboxaldehyde

-
-
119364-52-2
(1S,2R)-2-amino-1-phenylpropane-1,3-diol

| Conditions | Yield |
|---|---|
| With potassium carbonate In neat (no solvent) stirring (darkness); hexane and K2CO3 addn., filtn., concn.; elem. anal.; | 99% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In neat (no solvent) stirring (darkness); hexane and K2CO3 addn., filtn., concn.; elem. anal.; | 99% |
-
-
12093-10-6
ferrocenecarboxaldehyde

-
-
555-21-5
4-Nitrophenylacetonitrile

| Conditions | Yield |
|---|---|
| With piperidine In neat (no solvent, solid phase) (under N2, Schlenk); Fe-complex and ligand added to tube, mixed, piperidine added, mixed at room temp., sealed, shaken for 30 min at room temp.; dried in air overnight, column chromy. with hexane/Et2O (4:1); elem. anal.; | 99% |

| Conditions | Yield |
|---|---|
| 99% |
-
-
12093-10-6
ferrocenecarboxaldehyde

-
-
64715-81-7
(S)-(+)-2-amino-1-methoxy-3-phenylpropane hydrochloride

| Conditions | Yield |
|---|---|
| With potassium carbonate In neat (no solvent) standing (darkness), stirring; K2CO3 and hexane addn., filtn., evapn. (vac.), recrystn.; elem. anal.; | 99% |
-
-
12093-10-6
ferrocenecarboxaldehyde

-
-
1552-41-6
diethyl [(4-cyanophenyl)methyl]phosphonate

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In tetrahydrofuran at 20℃; for 2h; Darkness; | 99% |
-
-
69556-70-3
4-methoxy-5H-furan-2-one

-
-
12093-10-6
ferrocenecarboxaldehyde

| Conditions | Yield |
|---|---|
| Stage #1: 4-methoxy-5H-furan-2-one With n-butyllithium Stage #2: ferrocenecarboxaldehyde In tetrahydrofuran at -78 - 20℃; | 99% |
| Stage #1: 4-methoxy-5H-furan-2-one With n-butyllithium In tetrahydrofuran; hexane at -78℃; for 0.25h; Inert atmosphere; Stage #2: ferrocenecarboxaldehyde In tetrahydrofuran; hexane at -78 - 20℃; Inert atmosphere; Further stages; | 95% |
-
-
12093-10-6
ferrocenecarboxaldehyde

-
-
1075-08-7
4-Chloro-5-hydrazino-2-methyl-3(2H)-pyridazinone

| Conditions | Yield |
|---|---|
| In ethanol Reflux; Inert atmosphere; | 99% |
Related products
Raw Materials
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View








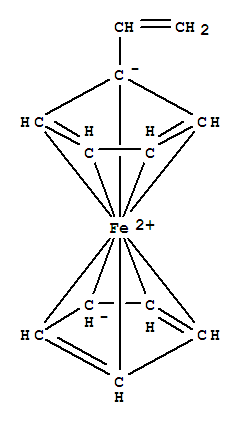






 T
T