 This product is a nationally controlled contraband, and the Lookchem platform doesn't provide relevant sales information.
This product is a nationally controlled contraband, and the Lookchem platform doesn't provide relevant sales information.
Synthetic route
-
-
671-50-1
methyl aziridine-1-carboxylate

-
-
62-53-3
aniline

-
A
-
151-56-4
ethyleneimine

-
B
-
79143-47-8
(2-Phenylamino-ethyl)-carbamic acid methyl ester

| Conditions | Yield |
|---|---|
| In ethanol at 18 - 25℃; for 480h; | A n/a B 98% |

-
-
13279-22-6
1-(phenylaminocarbonyl)aziridine

-
-
124-40-3
dimethyl amine

-
A
-
151-56-4
ethyleneimine

-
B
-
101-42-8
fenuron

| Conditions | Yield |
|---|---|
| In ethanol at 18 - 25℃; for 168h; | A n/a B 94% |

-
-
110-89-4
piperidine

-
-
671-50-1
methyl aziridine-1-carboxylate

-
A
-
151-56-4
ethyleneimine

-
B
-
79143-49-0
(2-Piperidin-1-yl-ethyl)-carbamic acid methyl ester

| Conditions | Yield |
|---|---|
| In ethanol at 18 - 25℃; for 24h; | A n/a B 92% |

-
-
13279-22-6
1-(phenylaminocarbonyl)aziridine

-
-
74-89-5
methylamine

-
A
-
151-56-4
ethyleneimine

-
B
-
1007-36-9
1-methyl-3-phenylurea

| Conditions | Yield |
|---|---|
| In ethanol at 18 - 25℃; for 168h; | A n/a B 92% |


| Conditions | Yield |
|---|---|
| With ammonia In ethanol at 18 - 25℃; for 24h; | A n/a B 90% |

| Conditions | Yield |
|---|---|
| With 1 M DTPP at 60℃; for 48h; | 90% |
| With BNTP In dichloromethane at 40℃; for 24h; | 98 % Chromat. |
| With potassium hydroxide; sulfuric acid 1.) 80 deg C, overnight; Multistep reaction; | |
| With cesium nitrate; iron nitrate; magnesium nitrate on fluoridated P2O5/SiO2/TiO2 at 370℃; for 8h; Temperature; Reagent/catalyst; Inert atmosphere; chemoselective reaction; |
-
-
110-91-8
morpholine

-
-
671-50-1
methyl aziridine-1-carboxylate

-
A
-
151-56-4
ethyleneimine

-
B
-
79143-50-3
(2-Morpholin-4-yl-ethyl)-carbamic acid methyl ester

| Conditions | Yield |
|---|---|
| In ethanol at 18 - 25℃; for 24h; | A n/a B 88% |


| Conditions | Yield |
|---|---|
| With ammonia In ethanol at 18 - 25℃; for 168h; | A n/a B 88% |
-
-
671-50-1
methyl aziridine-1-carboxylate

-
-
124-40-3
dimethyl amine

-
A
-
151-56-4
ethyleneimine

-
B
-
79143-42-3
1-methoxycarbonylamino-2-dimethylaminoethane

| Conditions | Yield |
|---|---|
| In ethanol at 18 - 25℃; for 24h; | A n/a B 82% |


| Conditions | Yield |
|---|---|
| With carbon dioxide; DBN; lithium iodide In toluene at 90℃; for 5h; Inert atmosphere; | 82% |
-
-
671-50-1
methyl aziridine-1-carboxylate

-
A
-
151-56-4
ethyleneimine

-
B
-
79143-51-4
(2-Aziridin-1-yl-ethyl)-carbamic acid methyl ester

| Conditions | Yield |
|---|---|
| In ethanol at 18 - 25℃; for 24h; | A n/a B 75% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water at 50℃; for 2h; Inert atmosphere; | 75% |
| With sodium hydroxide In water at 50℃; for 2.5h; | 74% |
| With sodium hydroxide at 50℃; under 25 Torr; for 2h; | 70% |
-
-
671-50-1
methyl aziridine-1-carboxylate

-
-
75-31-0
isopropylamine

-
A
-
151-56-4
ethyleneimine

-
B
-
79143-44-5
(2-Isopropylamino-ethyl)-carbamic acid methyl ester

| Conditions | Yield |
|---|---|
| In ethanol at 18 - 25℃; for 24h; | A n/a B 72% |

-
-
671-50-1
methyl aziridine-1-carboxylate

-
-
108-91-8
cyclohexylamine

-
A
-
151-56-4
ethyleneimine

-
B
-
79143-46-7
(2-Cyclohexylamino-ethyl)-carbamic acid methyl ester

| Conditions | Yield |
|---|---|
| In ethanol at 18 - 25℃; for 24h; | A n/a B 63% |


| Conditions | Yield |
|---|---|
| With sodium hydroxide In water Heating; | 60% |
| With sodium hydroxide | |
| With potassium hydroxide |
-
-
67-56-1
methanol

-
-
141-43-5
ethanolamine

-
A
-
151-56-4
ethyleneimine

-
B
-
110-85-0
piperazine

-
C
-
280-57-9
1,4-diaza-bicyclo[2.2.2]octane

-
D
-
109-83-1
(2-hydroxyethyl)(methyl)amine

| Conditions | Yield |
|---|---|
| With Cs-P-Si mixed-oxide at 300℃; under 750.06 Torr; Title compound not separated from byproducts; | A 37 % Chromat. B n/a C n/a D 6% |


| Conditions | Yield |
|---|---|
| With water; silver(l) oxide | |
| With potassium hydroxide | |
| With alkaline solution |

| Conditions | Yield |
|---|---|
| With potassium perchlorate In acetic acid at 25℃; Rate constant; | |
| With tetramethyl ammoniumhydroxide In water; dimethyl sulfoxide at 17.9℃; Rate constant; Kinetics; Thermodynamic data; var. temp., var. solvents mixtures, var. bases, ΔGa, ΔHa, ΔSa; | |
| With ammonium bromide In water; dimethyl sulfoxide at 60.3℃; Thermodynamic data; other halide, ΔH(excit.), ΔG(excit.), ΔS(excit.); |

| Conditions | Yield |
|---|---|
| With water In 1,4-dioxane at 39 - 59℃; Kinetics; | |
| With tetramethyl ammoniumhydroxide In water; dimethyl sulfoxide at 31.4℃; Rate constant; Kinetics; Thermodynamic data; var. temp., var. solvents mixtures, var. bases, ΔGa, ΔHa, ΔSa; | |
| With ammonium chloride In water; dimethyl sulfoxide at 60.3℃; Thermodynamic data; other halide, ΔH(excit.), ΔG(excit.), ΔS(excit.); |
-
-
372-47-4
3-Fluoropyridine

-
-
24151-28-8
Aziridin-Kation

-
A
-
151-56-4
ethyleneimine

-
B
-
59278-67-0
2-fluoropyridine-H(1+)

| Conditions | Yield |
|---|---|
| Thermodynamic data; |

-
-
626-60-8
3-Chloropyridine

-
-
24151-28-8
Aziridin-Kation

-
A
-
151-56-4
ethyleneimine

-
B
-
53760-42-2
3-chloropyridinium

| Conditions | Yield |
|---|---|
| Thermodynamic data; |


| Conditions | Yield |
|---|---|
| With sodium hydroxide In acetonitrile at 25℃; Rate constant; var. pH (from 13 to neutral); |
-
-
1195-69-3
amide P,P-bis (1-aziridinyl) N,N-dimethyl phosphinique

-
A
-
151-56-4
ethyleneimine

-
B
-
124-40-3
dimethyl amine

| Conditions | Yield |
|---|---|
| In water-d2 Mechanism; other aziridinyl phosphoramides, other products; |


| Conditions | Yield |
|---|---|
| With sodium hydroxide In acetonitrile at 25℃; Rate constant; var. pH (from 13 to neutral); |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In acetonitrile at 25℃; Rate constant; var. pH (from 13 to neutral); |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In acetonitrile at 25℃; Rate constant; var. pH (from 13 to neutral); |

| Conditions | Yield |
|---|---|
| With tris-(2-chloro-ethyl)-amine Mechanism; Irradiation; also with propene; rel. rate constants; |
-
-
74734-25-1
N,N'-Bis(2-oxo-3-oxazolidin-3-ylcarbonyl)-1,6-hexanediamine

-
A
-
151-56-4
ethyleneimine

-
B
-
497-25-6
dimethylenecyclourethane

-
C
-
925-91-7
Acetonitrile oxide

-
D
-
54680-52-3
trans-nitrosoethylene

| Conditions | Yield |
|---|---|
| at 200 - 600℃; Product distribution; pyrolysis; |

-
-
671-50-1
methyl aziridine-1-carboxylate

-
-
75-04-7
ethylamine

-
A
-
151-56-4
ethyleneimine

-
B
-
6135-31-5
methyl N-ethylcarbamate

-
C
-
79143-43-4
(2-Ethylamino-ethyl)-carbamic acid methyl ester

| Conditions | Yield |
|---|---|
| In ethanol at 18 - 25℃; for 24h; | A n/a B 35 % Chromat. C 65 % Chromat. |

-
-
151-56-4
ethyleneimine

-
-
78993-89-2
2-chloro-3,6-diphenyl-3,4-dihydro-1,3,2-oxazophosporin-2-oxide

-
-
95886-05-8
2-Aziridin-1-yl-3,6-diphenyl-3,4-dihydro-[1,3,2]oxazaphosphinine 2-oxide

| Conditions | Yield |
|---|---|
| In benzene for 0.5h; Ambient temperature; | 100% |
-
-
151-56-4
ethyleneimine

-
-
78993-90-5
2-Chloro-6-phenyl-3-p-tolyl-3,4-dihydro-[1,3,2]oxazaphosphinine 2-oxide

-
-
95886-04-7
2-(1-Aziridinyl)-6-phenyl-3-(p-tolyl)-3,4-dihydro-1,3,2-oxazaphosphorin-2-oxide

| Conditions | Yield |
|---|---|
| In benzene for 0.5h; Ambient temperature; | 100% |
-
-
151-56-4
ethyleneimine

-
-
78993-91-6
2-Chloro-3-(4-methoxy-phenyl)-6-phenyl-3,4-dihydro-[1,3,2]oxazaphosphinine 2-oxide

-
-
95886-03-6
2-Aziridin-1-yl-3-(4-methoxy-phenyl)-6-phenyl-3,4-dihydro-[1,3,2]oxazaphosphinine 2-oxide

| Conditions | Yield |
|---|---|
| In benzene for 0.5h; Ambient temperature; | 100% |
-
-
151-56-4
ethyleneimine

-
-
10165-13-6
1-chloroaziridine

| Conditions | Yield |
|---|---|
| With N-chloro-succinimide at 20℃; under 0.1 Torr; | 99% |
| With sodium hypochlorite; water at -10℃; | |
| With N-chloro-succinimide under 0.001 Torr; Ambient temperature; |
-
-
151-56-4
ethyleneimine

-
-
32837-87-9
3-hydroxy-3-methyl-1-cyano-1-butyne

| Conditions | Yield |
|---|---|
| In chloroform at 10℃; for 2h; | 99% |
-
-
151-56-4
ethyleneimine

-
-
40920-46-5
3-chloro-2-phenyl-1-indenone

-
-
94796-57-3
3-aziridino-2-phenyl-1-indenone

| Conditions | Yield |
|---|---|
| With triethylamine for 0.5h; | 99% |
-
-
151-56-4
ethyleneimine

-
-
94796-76-6
3-chloro-2-(4-chlorophenyl)-1-indenone

-
-
94796-59-5
3-aziridino-2-(4-chlorophenyl)-1-indenone

| Conditions | Yield |
|---|---|
| With triethylamine for 0.5h; | 99% |
-
-
151-56-4
ethyleneimine

-
-
94796-75-5
3-chloro-2-(4-methoxyphenyl)-1-indenone

-
-
94796-58-4
3-aziridino-2-(4-methoxyphenyl)-1-indenone

| Conditions | Yield |
|---|---|
| With triethylamine for 0.5h; | 99% |
-
-
151-56-4
ethyleneimine

-
-
94796-77-7
3-chloro-2-(1-naphthyl)-1-indenone

-
-
94796-60-8
3-aziridino-2-(1-naphthyl)-1-indenone

| Conditions | Yield |
|---|---|
| With triethylamine for 0.5h; | 99% |
-
-
151-56-4
ethyleneimine

-
-
84336-27-6
diethoxyphosphoryldichloroacetaldehyde

| Conditions | Yield |
|---|---|
| In ethanol at 5 - 10℃; for 1h; Addition; | 99% |
-
-
151-56-4
ethyleneimine

-
-
864467-21-0
1-{2-[1,3-(oxytetraethylenoxy)-3,5,5-trichloro-cyclotriphosphazatrien-1-yl]-aminoethylamino}anthraquinone

| Conditions | Yield |
|---|---|
| With sodium hydroxide In benzene at 20℃; for 21.5h; | 99% |
-
-
17084-13-8
potassium hexafluorophosphate

-
-
151-56-4
ethyleneimine

-
-
69532-52-1
[C5H5(CO)2Fe(COCH2CH2NH)](1+)*PF6(1-)=[C5H5(CO)2Fe(COCH2CH2NH)]PF6

| Conditions | Yield |
|---|---|
| With catalyst: (BrCH2CH2NH3)Br In acetonitrile addn. of aziridine to soln. of complex and bromide via syringe; stirring for 10 min at 25°C under N2; adding an excess of KPF6; stirringfor 5 min; evapn.; washing (Et2O); extg. (CH2Cl29; filtration through MgSO4; reducing volume until crystn. occured; adding Et2O; keeping at -20°C overnight; crystn.; drying in vac.; elem. anal.; | 99% |
| With catalyst: (BrCH2CH2CH2NH3)Br In acetonitrile addn. of aziridine to soln. of complex and bromide via syringe; stirring for 10 min at 25°C under N2; adding an excess of KPF6; stirringfor 5 min; evapn.; washing (Et2O); extg. (CH2Cl29; filtration through MgSO4; reducing volume until crystn. occured; adding Et2O; keeping at -20°C overnight; crystn.; drying in vac.; elem. anal.; | 89% |

-
-
151-56-4
ethyleneimine

-
-
12354-84-6, 12354-85-7
bis[dichloro(pentamethylcyclopentadienyl)iridium(III)]

-
-
1018682-30-8
[IrCl(Cp(*))(aziridine)2]Cl

| Conditions | Yield |
|---|---|
| In dichloromethane (Ar); 5 equiv. of aziridine added to metal-complex in CH2Cl2; stirred atroom temp. for 2 h; solvent removed in vacuo; purified by stirring in dry n-hexane overnight; decanted; dried in vacuo; elem. anal.; | 99% |
-
-
151-56-4
ethyleneimine

-
-
73954-60-6
bis(N,N-diethylamido)-N1-ethyl(bromo)imidophosphate

| Conditions | Yield |
|---|---|
| In benzene at 10℃; | 98% |
-
-
151-56-4
ethyleneimine

-
-
90429-62-2
8-Methoxy-5-quinolinesulfonyl chloride

-
-
98267-09-5
N-(8-methoxy-5-quinolylsulfonyl)aziridine

| Conditions | Yield |
|---|---|
| With triethylamine In benzene for 3h; O deg C - 20 deg C; | 98% |
-
-
151-56-4
ethyleneimine

-
-
107036-23-7
Acetic acid 4-[(1S,2R)-2-(4-acetoxy-phenyl)-1-chlorocarbonyl-butyl]-phenyl ester

-
-
107036-26-0
(2R*,3S*)-1-<2,3-bis(4-acetoxyphenyl)-1-pentanoyl>aziridine

| Conditions | Yield |
|---|---|
| With triethylamine In diethyl ether 1.) -4 deg C, 20 min, 2.) RT, 1 h; | 98% |
-
-
151-56-4
ethyleneimine

-
-
73954-61-7
pentapropylphosphorodiamidimidic bromide

| Conditions | Yield |
|---|---|
| In benzene at 10℃; | 98% |

| Conditions | Yield |
|---|---|
| With caesium carbonate; 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene; palladium diacetate In toluene at 100℃; for 24h; | 98% |

| Conditions | Yield |
|---|---|
| In chloroform for 2h; darkness; | 98% |
-
-
151-56-4
ethyleneimine

-
-
38834-26-3
(η5-Cp)Fe(CO)3PF6

-
-
69532-52-1
[C5H5(CO)2Fe(COCH2CH2NH)](1+)*PF6(1-)=[C5H5(CO)2Fe(COCH2CH2NH)]PF6

| Conditions | Yield |
|---|---|
| sodium bromide In acetonitrile ratio of educts and catalyst: 1:1:1; evapn. in vac. to dryness, extrd. with CH2Cl2, pptd. with ether at -20°C; | 98% |
| With catalyst: (nBu)4N(1+)*Br(1-) or (nBu4)N(1+)*I(1-) In acetonitrile ratio of educts and catalyst: 1:1:1; evapn. in vac. to dryness, extrd. with CH2Cl2, pptd. with ether at -20°C; | 98% |
| With catalyst: Br(CH2)2NH3(1+)*Br(1-) In acetonitrile ratio of educts and catalyst: 1:1:1; evapn. in vac. to dryness, extrd. with CH2Cl2, pptd. with ether at -20°C; | 98% |
| With catalyst: Br(CH2)3NH3(1+)*Br(1-) In acetonitrile ratio of educts and catalyst: 1:1:1; evapn. in vac. to dryness, extrd. with CH2Cl2, pptd. with ether at -20°C; | 98% |
| With catalyst: Et3NH(1+)*Br(1-) In acetonitrile ratio of educts and catalyst: 1:1:1; evapn. in vac. to dryness, extrd. with CH2Cl2, pptd. with ether at -20°C; | 98% |

| Conditions | Yield |
|---|---|
| In toluene (Schlenk flask); soln. of Re-complex is cooled to -45°C (CH3CN/CO2), CF3SO3H is added dropwise, after 5 min cyclic amine is added and the cold bath is removed; after 60 min hexane is added with stirring, filtrate is washed with hexane and dried under vacuum, elem. anal.; | 98% |


| Conditions | Yield |
|---|---|
| In dichloromethane anhydrous metal chloride suspd. in dry CH2Cl2; 5 equiv. of aziridine added; stirred overnight at 21°C; filtered off; solvent removed in vacuo; purified by stirring in dry n-hexane overnight at ambient temp.; n-hexane phase removed by decantation; dried in vacuo; elem. anal.; | 98% |
| In not given (Ar); mole ratio ZnCl2:ethylenimine = 1:2; soln. of ethylenimine added dropwise to a soln. of ZnCl2 with stirring and cooling to -20°C; stirred for 3-4 h; ppt. sepd.; washed (cold soln.); dried (vac.); elem. anal.; |
-
-
151-56-4
ethyleneimine

-
-
92344-36-0, 21654-58-0
trans-[bis(aziridine)dichloridopalladium(II)]

| Conditions | Yield |
|---|---|
| In dichloromethane anhydrous metal chloride suspd. in dry CH2Cl2; 2 equiv. of aziridine added; stirred overnight at 21°C; filtered off; solvent removed in vacuo; purified by stirring in dry n-hexane overnight at ambient temp.; n-hexane phase removed by decantation; dried in vacuo; elem. anal.; | 98% |

| Conditions | Yield |
|---|---|
| With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In 4-(dicyanomethylene)-2-methyl-6-(p-dimethylaminostyryl)-4H-pyran at 0 - 20℃; for 2h; Temperature; | 97.5% |
-
-
151-56-4
ethyleneimine

-
-
81675-82-3
pentabutylphosphorodiamidimic bromide

| Conditions | Yield |
|---|---|
| In benzene at 10℃; | 97% |
-
-
151-56-4
ethyleneimine

| Conditions | Yield |
|---|---|
| at 20℃; for 1h; | 97% |
-
-
151-56-4
ethyleneimine

-
-
59848-50-9
4-(bis-methylsulfanyl-methylene)-2-phenyl-5-p-tolyl-2,4-dihydro-pyrazol-3-one

-
-
61254-28-2
4-<(N-aziridinomethylthio)methylene>-3-(p-methylphenyl)-5-oxo-1-phenyl-Δ2-pyrazoline

| Conditions | Yield |
|---|---|
| In diethyl ether at 0 - 20℃; for 3h; | 97% |
-
-
151-56-4
ethyleneimine

| Conditions | Yield |
|---|---|
| With C2H5OH In neat (no solvent) careful addn. of aziridine to solid cis-(Co(NH3)(en)2Br)Br2*H2O under stirring and cooling in a dry ice/acetone bath in N2 atm.; stirring at room temp. for 6 h; pptn. by slow addn. of ethanol and diethyl ether;; filtration; washing with ether; drying in vac. over P2O5; elem. anal.;; | 97% |
-
-
151-56-4
ethyleneimine

| Conditions | Yield |
|---|---|
| In tetrahydrofuran reaction under N2: a suspn. of Pt-complex in THF is treated with aziridine at 0°C, removing the ice-bath, warming to room temp., stirring for 5 h; reaction is controlled by IR-spectroscopy, filtn., vac. drying, elem. anal.; | 97% |
Related products
Downstream Products
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View













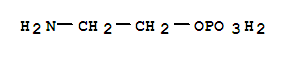


 F,
F, T
T