Hangzhou Maytime Bio-Tech Co.,Ltd.
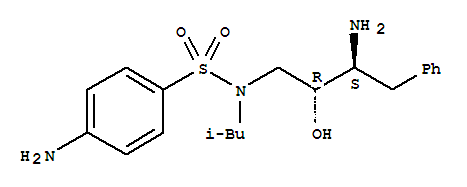
we are supplying below two darunavir intermediates. 4-Amino-N-[(2R,3S)-3-amino-2-hydroxy-4-phenylbutyl]-N-(2-methylpropyl)benzenesulfonamide CAS:169280-56-2 (3aS,4S,6aR)-Tetrahydro-4-methoxy-furo[3,4-b]furan-2(3H)-one CAS:866594-60-7 Appearance
Cas:169280-56-2
Min.Order:1 Kilogram
Negotiable
Type:Other
inquiryDayang Chem (Hangzhou) Co.,Ltd.
Dayangchem's R&D center can offer custom synthesis according to the contract research and development services for the fine chemicals, pharmaceutical, biotechnique and some of the other chemicals. DayangChem can provide different quantities
Cas:169280-56-2
Min.Order:1 Kilogram
FOB Price: $3.0
Type:Lab/Research institutions
inquirySimagchem Corporation
Welcome to Simagchem, your partner in China as a premier supply of bulk specialty chemicals for industry and life science. We introduce experienced quality product and exceptional JIT service with instant market intelligence in China to benefit our
Cas:169280-56-2
Min.Order:1 Metric Ton
Negotiable
Type:Manufacturers
inquiryKono Chem Co.,Ltd
Specifications CAS No.: 169280-56-2 Other Names: Ethyl propiolate MF: C20H29N3O3S EINECS No.: 169280-56-2 Place of Origin: Shandong,
Cas:169280-56-2
Min.Order:1 Kilogram
FOB Price: $300.0 / 400.0
Type:Other
inquiryHangzhou JINLAN Pharm-Drugs Technology Co., Ltd
We can provide GMP validation service that complies with SFDA, FDA, WHO and EU EMPA.Excellent registration team could help us easlily to register our products in different countries.If you and your customer are interested in some products or need CMO
Cas:169280-56-2
Min.Order:1 Gram
Negotiable
Type:Manufacturers
inquiryAlity Chemical Corporation
The above product is Ality Chemical's strong item with best price, good quality and fast supply. Ality Chemical has been focusing on the research and production of this field for over 14 years. At the same time, we are always committed to providi
Cas:169280-56-2
Min.Order:1
Negotiable
Type:Other
inquiryChemwill Asia Co., Ltd.
Our main production base is located in Xuzhou industry park. We are certified both to the ISO 9001 and ISO 14001 Standards, have a safety management system in place.Our R&D team masters core technology for process-design of target building block
Cas:169280-56-2
Min.Order:5 Kiloliter
FOB Price: $1.2 / 5.0
Type:Manufacturers
inquiryHebei Nengqian Chemical Import and Export Co., LTD
With our good experience, we offer detailed technical support and advice to assist customers. We communicate closely with customers to establish their quality requirements. Consistent Quality Our plant has strict quality control in each manufactu
Cas:169280-56-2
Min.Order:1 Kilogram
FOB Price: $1.0 / 10.0
Type:Trading Company
inquiryHenan Tianfu Chemical Co., Ltd.
Our company was built in 2009 with an ISO certificate.In the past 5 years, we have grown up as a famous fine chemicals supplier in China and we had established stable business relationships with Samsung,LG,Merck,Thermo Fisher Scientific and so on.O
Cas:169280-56-2
Min.Order:1 Metric Ton
Negotiable
Type:Lab/Research institutions
inquiryZhuozhou Wenxi import and Export Co., Ltd
WITH US,YOUR MONEY IN SAFE,YOUR BUSINESS IN SAFE 1)Quick Response Within 12 hours; 2)Quality Guarantee: All products are strictly tested by our QC, confirmed by QA and approved by third party lab in China, USA, Canada, Germany, UK, Italy, France et
Cas:169280-56-2
Min.Order:1 Kilogram
FOB Price: $139.0 / 210.0
Type:Trading Company
inquiryHenan Wentao Chemical Product Co., Ltd.
We are leading fine chemicals supplier in China and we had established stable business relationships with Samsung,LG,Merck,Thermo Fisher Scientific and so on.Our main business covers the fields below: 1.Noble Metal Catalysts (Pt.Pd...) 2.Orga
Cas:169280-56-2
Min.Order:1 Kilogram
FOB Price: $5.0
Type:Lab/Research institutions
inquiryShanghai Upbio Tech Co.,Ltd
1.No Less 8 years exporting experience. Clients can 100% received goods 2.Lower Price with higher quality 3,Free sample 4,We are sincerely responsible for the "product quality" and "After Service" Upbio is Specialized
Cas:169280-56-2
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryQingdao Beluga Import and Export Co., LTD
4-AMINO-N-[(2R,3S)-3-AMINO-2-HYDROXY-4-PHENYLBUTYL]-N-ISOBUTYLBENZENE-1-SULFONAMIDE CAS:169280-56-2 Qingdao Belugas Import and Export Co., Ltd. is a scientific and technological company integrating research and development, production and trade of c
Cas:169280-56-2
Min.Order:1 Gram
Negotiable
Type:Lab/Research institutions
inquiryShandong Hanjiang Chemical Co., Ltd.
Hello, dear friend! I'm Hansen and Allen from China. Welcome to my lookchem mall! The following is a brief introduction of our company's products and services. If you are interested in our products, please contact us by emai
Cas:169280-56-2
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryXiamen Hisunny Chemical Co.,Ltd
Best quality & Attractive price & Professional service; Trial & Pilot & Commercial Hisunny Chemical is a leading manufacturer and supplier of chemicals in China. We develop produce and distribute high quality intermediates, specia
Cas:169280-56-2
Min.Order:0
Negotiable
Type:Manufacturers
inquiryTriumph International Development Limilted
Appearance:white or light yellow crystalline powder Storage:Store in a cool,dry place and keep away from direct strong light Package:As customer request Application:Used for research and industrial manufacture. Transportation:By
Cas:169280-56-2
Min.Order:100 Gram
Negotiable
Type:Lab/Research institutions
inquiryAfine Chemicals Limited
Our Services 1. New Molecules R&D 2. Own test center HPLC NMR GC LC-MS 3. API and Intermediates from China reputed manufacturers 4. Documents support COA MOA MSDS DMF open part Our advantages 1. Government awarded company. Top 100 enter
Cas:169280-56-2
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryWuhan Han Sheng New Material Technology Co.,Ltd
Our Advantage: high quality with competitive price High quality standard: BP/USP/EP Enterprise standard All purity customized Fast and safe delivery We have reliable forwarder who can help us deliver our goods more fast and safe. We
Cas:169280-56-2
Min.Order:10 Kilogram
Negotiable
Type:Trading Company
inquiryHANWAYS CHEMPHARM CO.,LIMITED
We are concentrating on the R&D and technical service of APIs and pharmaceutical intermediates Application:Pharmaceutical intermediate Port:Chinese main port
Cas:169280-56-2
Min.Order:1 Kilogram
Negotiable
Type:Trading Company
inquirySHANGHAI T&W PHARMACEUTICAL CO., LTD.
A substitute for perfluorooctanoic acid, mainly used as a surfactant, dispersant, additive, etc Appearance:White solid or Colorless liquid Purity:99.3 % We will ship the goods in a timely manner as required We can provide relevant documents acc
Cas:169280-56-2
Min.Order:4 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryHangzhou J&H Chemical Co., Ltd.
J&H CHEM R&D center can offer custom synthesis according to the contract research and development services for the fine chemicals, pharmaceutical, biotechnique and some of the other chemicals. J&H CHEM has some Manufacturing base in Jia
Cas:169280-56-2
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryShanghai Massive Chemical Technology Co., Ltd.
Massive Chemical is certified with ISO9001 and ISO14001 manufacturer for this product. We will offer all documents as requirement for the materials which includes, Certificate of Analysis, Material Safety Data Sheet, and Method of Analysis and
Cas:169280-56-2
Min.Order:1 Gram
FOB Price: $1.0
Type:Lab/Research institutions
inquiryZibo Hangyu Biotechnology Development Co., Ltd
Zibo Hangyu Biotechnology Development Co., Ltd is a leading manufacturer and supplier of chemicals in China. We develop produce and distribute high quality pharmaceuticals, intermediates, special chemicals and OLED intermediates and other fine chemi
Cas:169280-56-2
Min.Order:10 Gram
FOB Price: $100.0
Type:Lab/Research institutions
inquirySiwei Development Group Ltd.
Product name: 4-Amino-N-(2R,3S)-3-Amino-2-Hydroxy-4-Phenyl-Butyl)-N-Isobutylbenzenesulfonamide CAS No.:169280-56-2 Molecule Formula:C20H29N3O3S Molecule Weight:391.52 Purity: 99% Package: 25kg/drum Description:White crystalline powder Manufact
Cas:169280-56-2
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryXiamen Jenny Chemical Technology Co., Ltd.
GMP standard, high purity, competitive price, in stock 1. Quick Response: within 6 hours after receiving your email. 2. Quality Guarantee: All products are strictly tested by our QC, confirmed by QA, and approved by a third-party lab in China, USA,
Cas:169280-56-2
Min.Order:1 Milligram
Negotiable
Type:Trading Company
inquiryHangzhou Think Chemical Co. Ltd
HANGZHOU THINK CHEMICAL CO., LTD. (THINKCHEM) is an integrative corporation of trade, research and contract manufacture. With about ten years of business experiences on the marketing & distribution, thinkchem specializes in exp
Cas:169280-56-2
Min.Order:1 Kilogram
Negotiable
Type:Other
inquiryEAST CHEMSOURCES LIMITED
factory?direct?saleAppearance:White Powder Storage:Store In Dry, Cool And Ventilated Place Package:25kg/drum, also according to the clients requirement Application:It is widely used as a thickener, emulsifier and stabilizer Transportation:By Sea Or B
Cas:169280-56-2
Min.Order:1 Kilogram
FOB Price: $18.0 / 20.0
Type:Trading Company
inquiryShandong Mopai Biotechnology Co., LTD
Shandong Mopai Biotechnology Co., LTD is a leading manufacturer and supplier of chemicals in China. We develop produce and distribute high quality pharmaceuticals, intermediates, special chemicals and OLED intermediates and other fine chemicals. W
Cas:169280-56-2
Min.Order:1 Gram
Negotiable
Type:Lab/Research institutions
inquiryKAISA GROUP INC
1.Applied in food field.it can improve the immune system and prolong life. 2.Appliedin cosmetic field.it can improve the skin care. 3.Applied in pharmaceutical field.it can treat various dieases. 4.Our product quality assurance will make our customer
Cas:169280-56-2
Min.Order:1 Metric Ton
FOB Price: $1.5
Type:Trading Company
inquiryHangzhou Zhongqi chem Co.,Ltd.
Located in Hangzhou National Hi-Tech Industrial Development Zone, zhongqichem is a technical company mainly focus on the Custom synthesis, manufacturing, sales of chemicals to various industries. Benefiting from the outstanding customer service and h
Cas:169280-56-2
Min.Order:0
Negotiable
Type:Other
inquirySynthetic route
-
-
191226-98-9
[(1S,2R)-3-[(4-nitrophenylsulfonyl)(2-methylpropyl)amino]-2-hydroxy-1-(phenylmethyl)propyl]carbamic acid tert-butyl ester

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| Stage #1: [(1S,2R)-3-[(4-nitrophenylsulfonyl)(2-methylpropyl)amino]-2-hydroxy-1-(phenylmethyl)propyl]carbamic acid tert-butyl ester With palladium 10% on activated carbon; ammonium formate; acetic acid In tetrahydrofuran at 15 - 20℃; Large scale; Stage #2: With hydrogenchloride In tetrahydrofuran; water at 35 - 45℃; Large scale; | 99.4% |
| Stage #1: [(1S,2R)-3-[(4-nitrophenylsulfonyl)(2-methylpropyl)amino]-2-hydroxy-1-(phenylmethyl)propyl]carbamic acid tert-butyl ester With triethanolamine; hydrogen; palladium 10% on activated carbon In methanol at 40 - 45℃; for 2h; Stage #2: With hydrogenchloride; water for 2h; Reflux; Stage #3: With sodium hydroxide In water; isopropyl alcohol at 20℃; for 10h; pH=9 - 10; | 95% |
| Multi-step reaction with 2 steps 1: 95 percent / H2 / Pd/C / ethyl acetate / 11 h / 23 °C 2: CF3COOH / CH2Cl2 / 0.67 h / 23 °C View Scheme |
-
-
183004-94-6
3-S-(N-tert-butyloxyformamido)-[2R-hydroxy-1-[(4-aminophenylsulfonyl)(2-methylpropyl)]amino]-4-phenylbutane

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid In dichloromethane for 4h; | 96% |
| With trifluoroacetic acid In dichloromethane at 23℃; for 0.666667h; | |
| With trifluoroacetic acid In dichloromethane at 20℃; | 0.75 g |
-
-
244641-42-7
N-(3-dibenzylamino-2-hydroxy-4-phenylbutyl)-N-isobutyl-4-nitrobenzenesulfonamide

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal In methanol at 20℃; | 95% |
| With hydrogen; palladium dihydroxide In methanol at 23℃; for 4h; Catalytic hydrogenation; hydrogenolysis; |
-
-
251105-80-3
N-(3S-amino-2R-hydroxy-4-phenylbutyl)-N-isobutyl-4-nitro-benzenesulfonamide hydrochloride

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| With palladium 10% on activated carbon; hydrogen In methanol at 20℃; under 2585.81 Torr; for 2h; Inert atmosphere; | 94% |
| With palladium 10% on activated carbon; hydrogen In methanol at 20℃; under 2585.81 Torr; for 2h; | 94% |
| With palladium 10% on activated carbon; hydrogen In methanol at 25℃; under 2585.81 Torr; for 2h; | 94% |
-
-
159005-59-1
4-nitro-N-((2R(syn),3S)-3-(N-benzyloxycarbonylamino)-2-hydroxy-4-phenylbutyl)-N-isobutyl-benzenesulfonamide

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| With hydrogen; palladium(II) hydroxide/carbon In methanol at 40℃; under 760.051 Torr; for 3.5h; | 91% |
| With hydrogen; palladium(II) hydroxide/carbon In methanol at 40℃; under 760.051 Torr; for 3 - 5.5h; | 90% |
| Stage #1: 4-nitro-N-((2R(syn),3S)-3-(N-benzyloxycarbonylamino)-2-hydroxy-4-phenylbutyl)-N-isobutyl-benzenesulfonamide With hydrogenchloride; hydrogen; palladium(II) hydroxide/carbon In methanol; water at 40℃; under 760.051 Torr; for 21h; Stage #2: With sodium hydroxide In water at 4℃; for 5.7h; | 71% |

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| With hydrogenchloride In methanol; water at 25 - 35℃; Solvent; Reagent/catalyst; | 89.6% |
-
-
143224-62-8
(2R,3S)-3-benzyloxycarbonylamino-2-hydroxy-1-(N-isobutylamino)-4-phenylbutane

-
-
98-74-8
4-Nitrobenzenesulfonyl chloride

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| Stage #1: (2R,3S)-3-benzyloxycarbonylamino-2-hydroxy-1-(N-isobutylamino)-4-phenylbutane; 4-Nitrobenzenesulfonyl chloride With triethylamine In ethyl acetate at 40℃; for 3h; Stage #2: With hydrogen; 5% Pd(OH)2/C In methanol at 40℃; under 760.051 Torr; for 5h; | 87% |

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| With potassium carbonate In water at 50 - 60℃; for 3h; | 76.39% |

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal In ethyl acetate |
-
-
98760-08-8, 98818-34-9, 98818-35-0, 103127-56-6, 98737-29-2
(1-oxiranyl-2-phenylethyl)carbamic acid tert-butyl ester

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: propan-2-ol / 2 h / 80 °C 2: 0.88 g / triethylamine / CH2Cl2 / 4 h / 20 °C 3: H2 / Pd(OH)2 on carbon / ethyl acetate / 1 h 4: 0.75 g / trifluoroacetic acid / CH2Cl2 / 20 °C View Scheme | |
| Multi-step reaction with 4 steps 1: 97 percent / propan-2-ol / 3 h / 80 °C 2: 90 percent / Et3N / CH2Cl2 / 16 h 3: 95 percent / H2 / Pd(OH)2 / ethyl acetate / 4 h 4: 96 percent / TFA / CH2Cl2 / 4 h View Scheme | |
| Multi-step reaction with 4 steps 1: 99 percent / propan-2-ol / 6 h / Heating 2: 96 percent / NaHCO3 / H2O; CH2Cl2 / 12 h / 23 °C 3: 95 percent / H2 / Pd/C / ethyl acetate / 11 h / 23 °C 4: CF3CO2H / CH2Cl2 / 0.67 h / 23 °C View Scheme |
-
-
160232-08-6
(2R,3S)-3-tert-butoxycarbonylamino-1-isobutylamino-4-phenyl-2-butanol

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 0.88 g / triethylamine / CH2Cl2 / 4 h / 20 °C 2: H2 / Pd(OH)2 on carbon / ethyl acetate / 1 h 3: 0.75 g / trifluoroacetic acid / CH2Cl2 / 20 °C View Scheme | |
| Multi-step reaction with 3 steps 1: 90 percent / Et3N / CH2Cl2 / 16 h 2: 95 percent / H2 / Pd(OH)2 / ethyl acetate / 4 h 3: 96 percent / TFA / CH2Cl2 / 4 h View Scheme | |
| Multi-step reaction with 3 steps 1: 96 percent / NaHCO3 / H2O; CH2Cl2 / 12 h / 23 °C 2: 95 percent / H2 / Pd/C / ethyl acetate / 11 h / 23 °C 3: CF3CO2H / CH2Cl2 / 0.67 h / 23 °C View Scheme |
-
-
169331-42-4
βS--αR-<<(2-methylpropyl)amino>methyl>-benzenepropanol

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 79 percent / Et3N / CH2Cl2 / 4 h / 20 °C 2: 95 percent / H2 / Pd/C / methanol / 20 °C View Scheme | |
| Multi-step reaction with 2 steps 1: 94 percent / Et3N / CH2Cl2 / 2 h / 23 °C 2: H2 / Pd(OH)2/C / methanol / 4 h / 23 °C View Scheme |
-
-
98-74-8
4-Nitrobenzenesulfonyl chloride

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 79 percent / Et3N / CH2Cl2 / 4 h / 20 °C 2: 95 percent / H2 / Pd/C / methanol / 20 °C View Scheme |
-
-
143224-62-8
(2R,3S)-3-benzyloxycarbonylamino-2-hydroxy-1-(N-isobutylamino)-4-phenylbutane

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 75 percent / Et3N / CH2Cl2 / 0 - 20 °C 2: 70 percent / H2 / Pd/C / ethyl acetate View Scheme |
-
-
98-74-8
4-Nitrobenzenesulfonyl chloride

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 75 percent / Et3N / CH2Cl2 / 0 - 20 °C 2: 70 percent / H2 / Pd/C / ethyl acetate View Scheme | |
| Multi-step reaction with 2 steps 1.1: 3 h / Heating 1.2: Reflux 2.1: hydrogen; triethanolamine / palladium 10% on activated carbon / methanol / 2 h / 40 - 45 °C 2.2: 2 h / Reflux 2.3: 10 h / 20 °C / pH 9 - 10 View Scheme | |
| Multi-step reaction with 4 steps 1.1: 1,4-dioxane / 1 h / 10 - 25 °C 2.1: potassium carbonate; N-benzyl-N,N,N-triethylammonium chloride / 1,4-dioxane / 6 h / 80 - 85 °C 3.1: trifluoroacetic acid / dichloromethane / 2 h / 20 - 25 °C 3.2: pH 8.5 - 9.2 4.1: hydrogen; palladium 10% on activated carbon / ethanol / 0.58 h / 20 - 25 °C / 2068.65 Torr View Scheme |
-
-
123054-12-6, 123808-74-2, 136370-73-5, 111060-64-1
(2S)-N,N-dibenzylphenylalaninal

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: 86 percent / KF; O(9)-benzyl-N-(9-anthracenylmethyl)cinchonidium fluoride / tetrahydrofuran / 6 h / -10 °C 2: 85 percent / NaBH4; NiCl2 / methanol / 0.17 h / 0 °C 3: MgSO4 4: NaBH4 / ethanol / 4 h / 0 - 23 °C 5: 94 percent / Et3N / CH2Cl2 / 2 h / 23 °C 6: H2 / Pd(OH)2/C / methanol / 4 h / 23 °C View Scheme |
-
-
244641-41-6
(2R,3S)-3-(dibenzylamino)-1-nitro-4-phenylbutan-2-ol

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 85 percent / NaBH4; NiCl2 / methanol / 0.17 h / 0 °C 2: MgSO4 3: NaBH4 / ethanol / 4 h / 0 - 23 °C 4: 94 percent / Et3N / CH2Cl2 / 2 h / 23 °C 5: H2 / Pd(OH)2/C / methanol / 4 h / 23 °C View Scheme |
-
-
170359-24-7
(2R,3S)-1-amino-3-(dibenzylamino)-4-phenylbutan-2-ol

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: MgSO4 2: NaBH4 / ethanol / 4 h / 0 - 23 °C 3: 94 percent / Et3N / CH2Cl2 / 2 h / 23 °C 4: H2 / Pd(OH)2/C / methanol / 4 h / 23 °C View Scheme |

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: NaBH4 / ethanol / 4 h / 0 - 23 °C 2: 94 percent / Et3N / CH2Cl2 / 2 h / 23 °C 3: H2 / Pd(OH)2/C / methanol / 4 h / 23 °C View Scheme |
-
-
136465-89-9
(2S)-2-[1'(S)-1-azido-2-phenylethyl]oxirane

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: propan-2-ol / 12 h / 80 °C 2: aq. NaHCO3 3: H2 / 10percent Pd/C / ethyl acetate View Scheme |
-
-
206361-96-8
(2R,3R)-3-azido-4-phenylbutane-1,2-diol

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: aq. NaHCO3 2: H2 / 10percent Pd/C / ethyl acetate View Scheme |
-
-
1415750-61-6
[(1S,2R)-3-[(4-aminophenylsulfonyl)(2-methylpropyl)amino]-2-hydroxy-1-(phenylmethyl)propyl]amine hydrochloride

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol; water at 0 - 7℃; for 4 - 48h; pH=> 12.5; | |
| With sodium carbonate In dichloromethane; water at 20 - 25℃; for 0.333333h; | 11.2 g |
-
-
1229623-13-5
tert-butyl ((2S)-4-chloro-3-hydroxy-1-phenylbutan-2-yl)carbamate

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: sodium hydrogencarbonate / dichloromethane / 5 h / Reflux 2.1: triethylamine / dichloromethane / Reflux 2.2: Reflux 3.1: hydrogen; triethanolamine / palladium 10% on activated carbon / methanol / 2 h / 40 - 45 °C 3.2: 2 h / Reflux 3.3: 10 h / 20 °C / pH 9 - 10 View Scheme |

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: sodium hydrogencarbonate / dichloromethane / 5 h / Reflux 2: triethylamine / dichloromethane / Reflux 3: hydrogen; triethanolamine / palladium 10% on activated carbon / methanol / 2 h / 40 - 45 °C 4: hydrogenchloride; water / 2 h / Reflux View Scheme |
-
-
89840-80-2
N-isobutyl-4-nitrobenzenesulfonamide

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: potassium carbonate; N-benzyl-N,N,N-triethylammonium chloride / 1,4-dioxane / 6 h / 80 - 85 °C 2.1: trifluoroacetic acid / dichloromethane / 2 h / 20 - 25 °C 2.2: pH 8.5 - 9.2 3.1: hydrogen; palladium 10% on activated carbon / ethanol / 0.58 h / 20 - 25 °C / 2068.65 Torr View Scheme |
-
-
24939-24-0
4-aminobenzenesulfonyl chloride

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: 1,4-dioxane / 0.83 h / 5 - 25 °C 2.1: potassium carbonate; N-benzyl-N,N,N-triethylammonium chloride / 1,4-dioxane / 6 h / 80 - 85 °C 3.1: trifluoroacetic acid / dichloromethane / 2 h / 20 - 25 °C 3.2: pH 8.5 - 9 View Scheme |
-
-
53668-36-3
4-amino-N-isobutylbenzenesulfonamide

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: potassium carbonate; N-benzyl-N,N,N-triethylammonium chloride / 1,4-dioxane / 6 h / 80 - 85 °C 2.1: trifluoroacetic acid / dichloromethane / 2 h / 20 - 25 °C 2.2: pH 8.5 - 9 View Scheme |
-
-
72225-62-8
N-(4-Isobutylsulfamoyl-phenyl)-acetamide

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: potassium carbonate; N-benzyl-N,N,N-triethylammonium chloride / 1,4-dioxane / 14 h / 85 - 90 °C 2.1: hydrogenchloride; water / ethanol / 10 h / 55 - 65 °C 2.2: 0.5 h / 25 - 30 °C / pH 11.5 - 12.5 View Scheme | |
| Multi-step reaction with 4 steps 1.1: potassium carbonate; N-benzyl-N,N,N-triethylammonium chloride / 1,4-dioxane / 14 h / 85 - 90 °C 2.1: trifluoroacetic acid / dichloromethane / 5 h / 20 - 25 °C 2.2: pH 9.11 3.1: hydrogenchloride / water; ethanol / 6.5 h / 60 - 65 °C 4.1: sodium carbonate / dichloromethane; water / 0.33 h / 20 - 25 °C View Scheme |
-
-
121-60-8
p-acetylaminobenzenesulfonyl chloride

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: 1,4-dioxane / 3.58 h / 5 - 25 °C 2.1: potassium carbonate; N-benzyl-N,N,N-triethylammonium chloride / 1,4-dioxane / 14 h / 85 - 90 °C 3.1: hydrogenchloride; water / ethanol / 10 h / 55 - 65 °C 3.2: 0.5 h / 25 - 30 °C / pH 11.5 - 12.5 View Scheme | |
| Multi-step reaction with 5 steps 1.1: 1,4-dioxane / 3.58 h / 5 - 25 °C 2.1: potassium carbonate; N-benzyl-N,N,N-triethylammonium chloride / 1,4-dioxane / 14 h / 85 - 90 °C 3.1: trifluoroacetic acid / dichloromethane / 5 h / 20 - 25 °C 3.2: pH 9.11 4.1: hydrogenchloride / water; ethanol / 6.5 h / 60 - 65 °C 5.1: sodium carbonate / dichloromethane; water / 0.33 h / 20 - 25 °C View Scheme |

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 0 - 25℃; for 5h; Inert atmosphere; | 98.2% |

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

-
-
1262482-52-9
(3aS,4S,7aR)-hexahydro-2H-furo[2,3-b]pyran-4-yl-(2S,3R)-4-(4-amino-N-isobutylphenylsulfonamido)-3-hydroxy-1-phenylbutan-2-yl carbamate

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In dichloromethane at 23℃; for 1h; Inert atmosphere; | 96% |
-
-
84358-13-4
N-[(tert-butoxy)carbonyl]piperidine-4-carboxylic acid

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| With dmap; benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 0 - 25℃; for 8h; Inert atmosphere; | 95.4% |
-
-
253265-97-3
1-[[[(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yloxy]carbonyl]oxy]pyrrolidine-2,5-dione

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| In dimethyl sulfoxide at 15 - 30℃; Solvent; | 94% |
| With triethylamine In dichloromethane at 20℃; | 90% |
| With potassium carbonate In Isopropyl acetate; water at 15 - 35℃; for 4h; | 83.33% |
-
-
58632-95-4
2-(tert-Butoxycarbonyloxyimino)-2-phenylacetonitrile

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

-
-
183004-94-6
3-S-(N-tert-butyloxyformamido)-[2R-hydroxy-1-[(4-aminophenylsulfonyl)(2-methylpropyl)]amino]-4-phenylbutane

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran for 16h; | 94% |
| With triethylamine In tetrahydrofuran for 16h; | 94% |
-
-
88495-54-9
(3S)-1-(tert-butoxycarbonyl)piperidine-3-carboxylic acid

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| With dmap; benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 0 - 25℃; for 8h; Inert atmosphere; | 93.4% |

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; for 25h; | 90% |
-
-
779-27-1
7-hydroxy-2-oxo-2H-chromene-3-carboxylic acid

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| Stage #1: 7-hydroxy-2-oxo-2H-chromene-3-carboxylic acid; 4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 0 - 20℃; for 1.16667h; Inert atmosphere; Stage #2: With dmap In N,N-dimethyl-formamide at 20℃; for 2h; Inert atmosphere; | 88% |
-
-
1609973-50-3
C14H15NO8

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

-
-
1609973-55-8
C28H39N3O8S

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; for 30h; | 85% |

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 0 - 25℃; for 5h; Inert atmosphere; | 84.9% |
-
-
7734-80-7
2-oxo-2H-benzopyran-6-carboxylic acid

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| Stage #1: 2-oxo-2H-benzopyran-6-carboxylic acid; 4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 0 - 20℃; for 1.16667h; Inert atmosphere; Stage #2: With dmap In N,N-dimethyl-formamide at 20℃; for 2h; Inert atmosphere; | 84% |
-
-
20300-59-8
7-methoxycoumarin-3-carboxylic acid

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| Stage #1: 7-methoxycoumarin-3-carboxylic acid; 4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 0 - 20℃; for 1.16667h; Inert atmosphere; Stage #2: With dmap In N,N-dimethyl-formamide at 20℃; for 2h; Inert atmosphere; | 83% |
-
-
192725-55-6
(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl-(4-nitrophenyl)carbonic acid

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| In 1-methyl-pyrrolidin-2-one at -4 - 30℃; for 10h; | 82.5% |
-
-
64-17-5
ethanol

-
-
192725-55-6
(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl-(4-nitrophenyl)carbonic acid

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

-
-
635728-49-3
[14C]-Darunavir ethanolate

| Conditions | Yield |
|---|---|
| Stage #1: (3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl-(4-nitrophenyl)carbonic acid; 4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide In 1-methyl-pyrrolidin-2-one at -4 - 30℃; for 10h; Stage #2: ethanol at 45 - 50℃; for 1h; | 82.5% |

-
-
74124-79-1
di(succinimido) carbonate

-
-
156928-09-5
(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-ol

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| With pyridine; triethylamine; methylamine In ethanol; water; acetonitrile Product distribution / selectivity; | 81% |
| With triethylamine; methylamine In ethanol; water; ethyl acetate; acetonitrile | 71% |
| Stage #1: di(succinimido) carbonate; (3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-ol With pyridine In acetonitrile at 25 - 30℃; for 1h; Stage #2: 4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide In acetonitrile at 0 - 5℃; for 0.5h; Stage #3: With triethylamine; methylamine In acetonitrile at 0 - 30℃; |

-
-
5070-13-3
bis-(p-nitrophenyl) carbonate

-
-
156928-09-5
(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-ol

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| With triethylamine; methylamine In ethanol; water; ethyl acetate; acetonitrile Product distribution / selectivity; | 81% |

-
-
14897-78-0
dihydrosinapic acid

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| Stage #1: dihydrosinapic acid; 4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 0 - 25℃; for 1.16667h; Inert atmosphere; Stage #2: With dmap In N,N-dimethyl-formamide at 25℃; for 2h; Inert atmosphere; | 81% |

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In acetonitrile at 23℃; for 144h; | 80% |
| With N-ethyl-N,N-diisopropylamine In acetonitrile at 23℃; for 72h; Inert atmosphere; | 80% |
-
-
149376-70-5
2-amino-6-chloro-9-carboxymethylpurine

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| Stage #1: 2-amino-6-chloro-9-carboxymethylpurine; 4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 0 - 20℃; for 1.16667h; Inert atmosphere; Stage #2: With dmap In N,N-dimethyl-formamide at 20℃; for 2h; Inert atmosphere; | 80% |
-
-
1135-24-6
(E)-3-(4-hydroxy-3-methoxyphenyl)acrylic acid

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| Stage #1: (E)-3-(4-hydroxy-3-methoxyphenyl)acrylic acid; 4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 0 - 25℃; for 1.16667h; Inert atmosphere; Stage #2: With dmap In N,N-dimethyl-formamide at 25℃; for 2h; Inert atmosphere; | 80% |
-
-
163438-09-3
(R)-1-Boc-nipecotic acid

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| With dmap; benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 0 - 25℃; for 8h; Inert atmosphere; | 79.4% |
-
-
1609973-49-0
C13H12N2O10

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

-
-
1609973-54-7
C27H36N4O10S

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; for 30h; | 79% |

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

-
-
1075209-51-6
N-((2S,3R)-4-((4-amino-N-isobutylphenyl)sulfonamido)-3-hydroxy-1-phenylbutan-2-yl)-2-((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl)acetamide

| Conditions | Yield |
|---|---|
| Stage #1: 2-((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl)acetic acid; 4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide With N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 23℃; for 0.0833333h; Stage #2: With N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate In N,N-dimethyl-formamide at 23℃; for 24h; | 79% |
Related products
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View