-
Name
4-AMINO-N-[(2R,3S)-3-AMINO-2-HYDROXY-4-PHENYLBUTYL]-N-ISOBUTYLBENZENE-1-SULFONAMIDE
- EINECS
- CAS No. 169280-56-2
- Article Data41
- CAS DataBase
- Density 1.226 g/cm3
- Solubility
- Melting Point
- Formula C20H29N3O3S
- Boiling Point 609.105 °C at 760 mmHg
- Molecular Weight 391.535
- Flash Point 322.175 °C
- Transport Information
- Appearance
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms Benzenesulfonamide,4-amino-N-(3-amino-2-hydroxy-4-phenylbutyl)-N-(2-methylpropyl)-, [R-(R*,S*)]-;
- PSA 118.03000
- LogP 4.20870
Synthetic route
-
-
191226-98-9
[(1S,2R)-3-[(4-nitrophenylsulfonyl)(2-methylpropyl)amino]-2-hydroxy-1-(phenylmethyl)propyl]carbamic acid tert-butyl ester

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| Stage #1: [(1S,2R)-3-[(4-nitrophenylsulfonyl)(2-methylpropyl)amino]-2-hydroxy-1-(phenylmethyl)propyl]carbamic acid tert-butyl ester With palladium 10% on activated carbon; ammonium formate; acetic acid In tetrahydrofuran at 15 - 20℃; Large scale; Stage #2: With hydrogenchloride In tetrahydrofuran; water at 35 - 45℃; Large scale; | 99.4% |
| Stage #1: [(1S,2R)-3-[(4-nitrophenylsulfonyl)(2-methylpropyl)amino]-2-hydroxy-1-(phenylmethyl)propyl]carbamic acid tert-butyl ester With triethanolamine; hydrogen; palladium 10% on activated carbon In methanol at 40 - 45℃; for 2h; Stage #2: With hydrogenchloride; water for 2h; Reflux; Stage #3: With sodium hydroxide In water; isopropyl alcohol at 20℃; for 10h; pH=9 - 10; | 95% |
| Multi-step reaction with 2 steps 1: 95 percent / H2 / Pd/C / ethyl acetate / 11 h / 23 °C 2: CF3COOH / CH2Cl2 / 0.67 h / 23 °C View Scheme |
-
-
183004-94-6
3-S-(N-tert-butyloxyformamido)-[2R-hydroxy-1-[(4-aminophenylsulfonyl)(2-methylpropyl)]amino]-4-phenylbutane

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid In dichloromethane for 4h; | 96% |
| With trifluoroacetic acid In dichloromethane at 23℃; for 0.666667h; | |
| With trifluoroacetic acid In dichloromethane at 20℃; | 0.75 g |
-
-
244641-42-7
N-(3-dibenzylamino-2-hydroxy-4-phenylbutyl)-N-isobutyl-4-nitrobenzenesulfonamide

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal In methanol at 20℃; | 95% |
| With hydrogen; palladium dihydroxide In methanol at 23℃; for 4h; Catalytic hydrogenation; hydrogenolysis; |
-
-
251105-80-3
N-(3S-amino-2R-hydroxy-4-phenylbutyl)-N-isobutyl-4-nitro-benzenesulfonamide hydrochloride

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| With palladium 10% on activated carbon; hydrogen In methanol at 20℃; under 2585.81 Torr; for 2h; Inert atmosphere; | 94% |
| With palladium 10% on activated carbon; hydrogen In methanol at 20℃; under 2585.81 Torr; for 2h; | 94% |
| With palladium 10% on activated carbon; hydrogen In methanol at 25℃; under 2585.81 Torr; for 2h; | 94% |
-
-
159005-59-1
4-nitro-N-((2R(syn),3S)-3-(N-benzyloxycarbonylamino)-2-hydroxy-4-phenylbutyl)-N-isobutyl-benzenesulfonamide

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| With hydrogen; palladium(II) hydroxide/carbon In methanol at 40℃; under 760.051 Torr; for 3.5h; | 91% |
| With hydrogen; palladium(II) hydroxide/carbon In methanol at 40℃; under 760.051 Torr; for 3 - 5.5h; | 90% |
| Stage #1: 4-nitro-N-((2R(syn),3S)-3-(N-benzyloxycarbonylamino)-2-hydroxy-4-phenylbutyl)-N-isobutyl-benzenesulfonamide With hydrogenchloride; hydrogen; palladium(II) hydroxide/carbon In methanol; water at 40℃; under 760.051 Torr; for 21h; Stage #2: With sodium hydroxide In water at 4℃; for 5.7h; | 71% |

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| With hydrogenchloride In methanol; water at 25 - 35℃; Solvent; Reagent/catalyst; | 89.6% |
-
-
143224-62-8
(2R,3S)-3-benzyloxycarbonylamino-2-hydroxy-1-(N-isobutylamino)-4-phenylbutane

-
-
98-74-8
4-Nitrobenzenesulfonyl chloride

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| Stage #1: (2R,3S)-3-benzyloxycarbonylamino-2-hydroxy-1-(N-isobutylamino)-4-phenylbutane; 4-Nitrobenzenesulfonyl chloride With triethylamine In ethyl acetate at 40℃; for 3h; Stage #2: With hydrogen; 5% Pd(OH)2/C In methanol at 40℃; under 760.051 Torr; for 5h; | 87% |

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| With potassium carbonate In water at 50 - 60℃; for 3h; | 76.39% |

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal In ethyl acetate |
-
-
98760-08-8, 98818-34-9, 98818-35-0, 103127-56-6, 98737-29-2
(1-oxiranyl-2-phenylethyl)carbamic acid tert-butyl ester

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: propan-2-ol / 2 h / 80 °C 2: 0.88 g / triethylamine / CH2Cl2 / 4 h / 20 °C 3: H2 / Pd(OH)2 on carbon / ethyl acetate / 1 h 4: 0.75 g / trifluoroacetic acid / CH2Cl2 / 20 °C View Scheme | |
| Multi-step reaction with 4 steps 1: 97 percent / propan-2-ol / 3 h / 80 °C 2: 90 percent / Et3N / CH2Cl2 / 16 h 3: 95 percent / H2 / Pd(OH)2 / ethyl acetate / 4 h 4: 96 percent / TFA / CH2Cl2 / 4 h View Scheme | |
| Multi-step reaction with 4 steps 1: 99 percent / propan-2-ol / 6 h / Heating 2: 96 percent / NaHCO3 / H2O; CH2Cl2 / 12 h / 23 °C 3: 95 percent / H2 / Pd/C / ethyl acetate / 11 h / 23 °C 4: CF3CO2H / CH2Cl2 / 0.67 h / 23 °C View Scheme |
-
-
160232-08-6
(2R,3S)-3-tert-butoxycarbonylamino-1-isobutylamino-4-phenyl-2-butanol

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 0.88 g / triethylamine / CH2Cl2 / 4 h / 20 °C 2: H2 / Pd(OH)2 on carbon / ethyl acetate / 1 h 3: 0.75 g / trifluoroacetic acid / CH2Cl2 / 20 °C View Scheme | |
| Multi-step reaction with 3 steps 1: 90 percent / Et3N / CH2Cl2 / 16 h 2: 95 percent / H2 / Pd(OH)2 / ethyl acetate / 4 h 3: 96 percent / TFA / CH2Cl2 / 4 h View Scheme | |
| Multi-step reaction with 3 steps 1: 96 percent / NaHCO3 / H2O; CH2Cl2 / 12 h / 23 °C 2: 95 percent / H2 / Pd/C / ethyl acetate / 11 h / 23 °C 3: CF3CO2H / CH2Cl2 / 0.67 h / 23 °C View Scheme |
-
-
169331-42-4
βS--αR-<<(2-methylpropyl)amino>methyl>-benzenepropanol

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 79 percent / Et3N / CH2Cl2 / 4 h / 20 °C 2: 95 percent / H2 / Pd/C / methanol / 20 °C View Scheme | |
| Multi-step reaction with 2 steps 1: 94 percent / Et3N / CH2Cl2 / 2 h / 23 °C 2: H2 / Pd(OH)2/C / methanol / 4 h / 23 °C View Scheme |
-
-
98-74-8
4-Nitrobenzenesulfonyl chloride

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 79 percent / Et3N / CH2Cl2 / 4 h / 20 °C 2: 95 percent / H2 / Pd/C / methanol / 20 °C View Scheme |
-
-
143224-62-8
(2R,3S)-3-benzyloxycarbonylamino-2-hydroxy-1-(N-isobutylamino)-4-phenylbutane

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 75 percent / Et3N / CH2Cl2 / 0 - 20 °C 2: 70 percent / H2 / Pd/C / ethyl acetate View Scheme |
-
-
98-74-8
4-Nitrobenzenesulfonyl chloride

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 75 percent / Et3N / CH2Cl2 / 0 - 20 °C 2: 70 percent / H2 / Pd/C / ethyl acetate View Scheme | |
| Multi-step reaction with 2 steps 1.1: 3 h / Heating 1.2: Reflux 2.1: hydrogen; triethanolamine / palladium 10% on activated carbon / methanol / 2 h / 40 - 45 °C 2.2: 2 h / Reflux 2.3: 10 h / 20 °C / pH 9 - 10 View Scheme | |
| Multi-step reaction with 4 steps 1.1: 1,4-dioxane / 1 h / 10 - 25 °C 2.1: potassium carbonate; N-benzyl-N,N,N-triethylammonium chloride / 1,4-dioxane / 6 h / 80 - 85 °C 3.1: trifluoroacetic acid / dichloromethane / 2 h / 20 - 25 °C 3.2: pH 8.5 - 9.2 4.1: hydrogen; palladium 10% on activated carbon / ethanol / 0.58 h / 20 - 25 °C / 2068.65 Torr View Scheme |
-
-
123054-12-6, 123808-74-2, 136370-73-5, 111060-64-1
(2S)-N,N-dibenzylphenylalaninal

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: 86 percent / KF; O(9)-benzyl-N-(9-anthracenylmethyl)cinchonidium fluoride / tetrahydrofuran / 6 h / -10 °C 2: 85 percent / NaBH4; NiCl2 / methanol / 0.17 h / 0 °C 3: MgSO4 4: NaBH4 / ethanol / 4 h / 0 - 23 °C 5: 94 percent / Et3N / CH2Cl2 / 2 h / 23 °C 6: H2 / Pd(OH)2/C / methanol / 4 h / 23 °C View Scheme |
-
-
244641-41-6
(2R,3S)-3-(dibenzylamino)-1-nitro-4-phenylbutan-2-ol

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 85 percent / NaBH4; NiCl2 / methanol / 0.17 h / 0 °C 2: MgSO4 3: NaBH4 / ethanol / 4 h / 0 - 23 °C 4: 94 percent / Et3N / CH2Cl2 / 2 h / 23 °C 5: H2 / Pd(OH)2/C / methanol / 4 h / 23 °C View Scheme |
-
-
170359-24-7
(2R,3S)-1-amino-3-(dibenzylamino)-4-phenylbutan-2-ol

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: MgSO4 2: NaBH4 / ethanol / 4 h / 0 - 23 °C 3: 94 percent / Et3N / CH2Cl2 / 2 h / 23 °C 4: H2 / Pd(OH)2/C / methanol / 4 h / 23 °C View Scheme |

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: NaBH4 / ethanol / 4 h / 0 - 23 °C 2: 94 percent / Et3N / CH2Cl2 / 2 h / 23 °C 3: H2 / Pd(OH)2/C / methanol / 4 h / 23 °C View Scheme |
-
-
136465-89-9
(2S)-2-[1'(S)-1-azido-2-phenylethyl]oxirane

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: propan-2-ol / 12 h / 80 °C 2: aq. NaHCO3 3: H2 / 10percent Pd/C / ethyl acetate View Scheme |
-
-
206361-96-8
(2R,3R)-3-azido-4-phenylbutane-1,2-diol

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: aq. NaHCO3 2: H2 / 10percent Pd/C / ethyl acetate View Scheme |
-
-
1415750-61-6
[(1S,2R)-3-[(4-aminophenylsulfonyl)(2-methylpropyl)amino]-2-hydroxy-1-(phenylmethyl)propyl]amine hydrochloride

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol; water at 0 - 7℃; for 4 - 48h; pH=> 12.5; | |
| With sodium carbonate In dichloromethane; water at 20 - 25℃; for 0.333333h; | 11.2 g |
-
-
1229623-13-5
tert-butyl ((2S)-4-chloro-3-hydroxy-1-phenylbutan-2-yl)carbamate

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: sodium hydrogencarbonate / dichloromethane / 5 h / Reflux 2.1: triethylamine / dichloromethane / Reflux 2.2: Reflux 3.1: hydrogen; triethanolamine / palladium 10% on activated carbon / methanol / 2 h / 40 - 45 °C 3.2: 2 h / Reflux 3.3: 10 h / 20 °C / pH 9 - 10 View Scheme |

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: sodium hydrogencarbonate / dichloromethane / 5 h / Reflux 2: triethylamine / dichloromethane / Reflux 3: hydrogen; triethanolamine / palladium 10% on activated carbon / methanol / 2 h / 40 - 45 °C 4: hydrogenchloride; water / 2 h / Reflux View Scheme |
-
-
89840-80-2
N-isobutyl-4-nitrobenzenesulfonamide

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: potassium carbonate; N-benzyl-N,N,N-triethylammonium chloride / 1,4-dioxane / 6 h / 80 - 85 °C 2.1: trifluoroacetic acid / dichloromethane / 2 h / 20 - 25 °C 2.2: pH 8.5 - 9.2 3.1: hydrogen; palladium 10% on activated carbon / ethanol / 0.58 h / 20 - 25 °C / 2068.65 Torr View Scheme |
-
-
24939-24-0
4-aminobenzenesulfonyl chloride

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: 1,4-dioxane / 0.83 h / 5 - 25 °C 2.1: potassium carbonate; N-benzyl-N,N,N-triethylammonium chloride / 1,4-dioxane / 6 h / 80 - 85 °C 3.1: trifluoroacetic acid / dichloromethane / 2 h / 20 - 25 °C 3.2: pH 8.5 - 9 View Scheme |
-
-
53668-36-3
4-amino-N-isobutylbenzenesulfonamide

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: potassium carbonate; N-benzyl-N,N,N-triethylammonium chloride / 1,4-dioxane / 6 h / 80 - 85 °C 2.1: trifluoroacetic acid / dichloromethane / 2 h / 20 - 25 °C 2.2: pH 8.5 - 9 View Scheme |
-
-
72225-62-8
N-(4-Isobutylsulfamoyl-phenyl)-acetamide

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: potassium carbonate; N-benzyl-N,N,N-triethylammonium chloride / 1,4-dioxane / 14 h / 85 - 90 °C 2.1: hydrogenchloride; water / ethanol / 10 h / 55 - 65 °C 2.2: 0.5 h / 25 - 30 °C / pH 11.5 - 12.5 View Scheme | |
| Multi-step reaction with 4 steps 1.1: potassium carbonate; N-benzyl-N,N,N-triethylammonium chloride / 1,4-dioxane / 14 h / 85 - 90 °C 2.1: trifluoroacetic acid / dichloromethane / 5 h / 20 - 25 °C 2.2: pH 9.11 3.1: hydrogenchloride / water; ethanol / 6.5 h / 60 - 65 °C 4.1: sodium carbonate / dichloromethane; water / 0.33 h / 20 - 25 °C View Scheme |
-
-
121-60-8
p-acetylaminobenzenesulfonyl chloride

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: 1,4-dioxane / 3.58 h / 5 - 25 °C 2.1: potassium carbonate; N-benzyl-N,N,N-triethylammonium chloride / 1,4-dioxane / 14 h / 85 - 90 °C 3.1: hydrogenchloride; water / ethanol / 10 h / 55 - 65 °C 3.2: 0.5 h / 25 - 30 °C / pH 11.5 - 12.5 View Scheme | |
| Multi-step reaction with 5 steps 1.1: 1,4-dioxane / 3.58 h / 5 - 25 °C 2.1: potassium carbonate; N-benzyl-N,N,N-triethylammonium chloride / 1,4-dioxane / 14 h / 85 - 90 °C 3.1: trifluoroacetic acid / dichloromethane / 5 h / 20 - 25 °C 3.2: pH 9.11 4.1: hydrogenchloride / water; ethanol / 6.5 h / 60 - 65 °C 5.1: sodium carbonate / dichloromethane; water / 0.33 h / 20 - 25 °C View Scheme |

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 0 - 25℃; for 5h; Inert atmosphere; | 98.2% |

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

-
-
1262482-52-9
(3aS,4S,7aR)-hexahydro-2H-furo[2,3-b]pyran-4-yl-(2S,3R)-4-(4-amino-N-isobutylphenylsulfonamido)-3-hydroxy-1-phenylbutan-2-yl carbamate

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In dichloromethane at 23℃; for 1h; Inert atmosphere; | 96% |
-
-
84358-13-4
N-[(tert-butoxy)carbonyl]piperidine-4-carboxylic acid

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| With dmap; benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 0 - 25℃; for 8h; Inert atmosphere; | 95.4% |
-
-
253265-97-3
1-[[[(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yloxy]carbonyl]oxy]pyrrolidine-2,5-dione

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| In dimethyl sulfoxide at 15 - 30℃; Solvent; | 94% |
| With triethylamine In dichloromethane at 20℃; | 90% |
| With potassium carbonate In Isopropyl acetate; water at 15 - 35℃; for 4h; | 83.33% |
-
-
58632-95-4
2-(tert-Butoxycarbonyloxyimino)-2-phenylacetonitrile

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

-
-
183004-94-6
3-S-(N-tert-butyloxyformamido)-[2R-hydroxy-1-[(4-aminophenylsulfonyl)(2-methylpropyl)]amino]-4-phenylbutane

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran for 16h; | 94% |
| With triethylamine In tetrahydrofuran for 16h; | 94% |
-
-
88495-54-9
(3S)-1-(tert-butoxycarbonyl)piperidine-3-carboxylic acid

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| With dmap; benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 0 - 25℃; for 8h; Inert atmosphere; | 93.4% |

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; for 25h; | 90% |
-
-
779-27-1
7-hydroxy-2-oxo-2H-chromene-3-carboxylic acid

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| Stage #1: 7-hydroxy-2-oxo-2H-chromene-3-carboxylic acid; 4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 0 - 20℃; for 1.16667h; Inert atmosphere; Stage #2: With dmap In N,N-dimethyl-formamide at 20℃; for 2h; Inert atmosphere; | 88% |
-
-
1609973-50-3
C14H15NO8

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

-
-
1609973-55-8
C28H39N3O8S

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; for 30h; | 85% |

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 0 - 25℃; for 5h; Inert atmosphere; | 84.9% |
-
-
7734-80-7
2-oxo-2H-benzopyran-6-carboxylic acid

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| Stage #1: 2-oxo-2H-benzopyran-6-carboxylic acid; 4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 0 - 20℃; for 1.16667h; Inert atmosphere; Stage #2: With dmap In N,N-dimethyl-formamide at 20℃; for 2h; Inert atmosphere; | 84% |
-
-
20300-59-8
7-methoxycoumarin-3-carboxylic acid

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| Stage #1: 7-methoxycoumarin-3-carboxylic acid; 4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 0 - 20℃; for 1.16667h; Inert atmosphere; Stage #2: With dmap In N,N-dimethyl-formamide at 20℃; for 2h; Inert atmosphere; | 83% |
-
-
192725-55-6
(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl-(4-nitrophenyl)carbonic acid

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| In 1-methyl-pyrrolidin-2-one at -4 - 30℃; for 10h; | 82.5% |
-
-
64-17-5
ethanol

-
-
192725-55-6
(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl-(4-nitrophenyl)carbonic acid

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

-
-
635728-49-3
[14C]-Darunavir ethanolate

| Conditions | Yield |
|---|---|
| Stage #1: (3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl-(4-nitrophenyl)carbonic acid; 4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide In 1-methyl-pyrrolidin-2-one at -4 - 30℃; for 10h; Stage #2: ethanol at 45 - 50℃; for 1h; | 82.5% |

-
-
74124-79-1
di(succinimido) carbonate

-
-
156928-09-5
(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-ol

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| With pyridine; triethylamine; methylamine In ethanol; water; acetonitrile Product distribution / selectivity; | 81% |
| With triethylamine; methylamine In ethanol; water; ethyl acetate; acetonitrile | 71% |
| Stage #1: di(succinimido) carbonate; (3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-ol With pyridine In acetonitrile at 25 - 30℃; for 1h; Stage #2: 4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide In acetonitrile at 0 - 5℃; for 0.5h; Stage #3: With triethylamine; methylamine In acetonitrile at 0 - 30℃; |

-
-
5070-13-3
bis-(p-nitrophenyl) carbonate

-
-
156928-09-5
(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-ol

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| With triethylamine; methylamine In ethanol; water; ethyl acetate; acetonitrile Product distribution / selectivity; | 81% |

-
-
14897-78-0
dihydrosinapic acid

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| Stage #1: dihydrosinapic acid; 4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 0 - 25℃; for 1.16667h; Inert atmosphere; Stage #2: With dmap In N,N-dimethyl-formamide at 25℃; for 2h; Inert atmosphere; | 81% |

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In acetonitrile at 23℃; for 144h; | 80% |
| With N-ethyl-N,N-diisopropylamine In acetonitrile at 23℃; for 72h; Inert atmosphere; | 80% |
-
-
149376-70-5
2-amino-6-chloro-9-carboxymethylpurine

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| Stage #1: 2-amino-6-chloro-9-carboxymethylpurine; 4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 0 - 20℃; for 1.16667h; Inert atmosphere; Stage #2: With dmap In N,N-dimethyl-formamide at 20℃; for 2h; Inert atmosphere; | 80% |
-
-
1135-24-6
(E)-3-(4-hydroxy-3-methoxyphenyl)acrylic acid

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| Stage #1: (E)-3-(4-hydroxy-3-methoxyphenyl)acrylic acid; 4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 0 - 25℃; for 1.16667h; Inert atmosphere; Stage #2: With dmap In N,N-dimethyl-formamide at 25℃; for 2h; Inert atmosphere; | 80% |
-
-
163438-09-3
(R)-1-Boc-nipecotic acid

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| With dmap; benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 0 - 25℃; for 8h; Inert atmosphere; | 79.4% |
-
-
1609973-49-0
C13H12N2O10

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

-
-
1609973-54-7
C27H36N4O10S

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; for 30h; | 79% |

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

-
-
1075209-51-6
N-((2S,3R)-4-((4-amino-N-isobutylphenyl)sulfonamido)-3-hydroxy-1-phenylbutan-2-yl)-2-((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl)acetamide

| Conditions | Yield |
|---|---|
| Stage #1: 2-((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl)acetic acid; 4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide With N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 23℃; for 0.0833333h; Stage #2: With N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate In N,N-dimethyl-formamide at 23℃; for 24h; | 79% |
4-Amino-N-[(2R,3S)-3-amino-2-hydroxy-4-phenylbutyl]-N-(2-methylpropyl)benzenesulfonamide Chemical Properties
Molecule structure of 4-Amino-N-((2R,3S)-3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide (CAS NO.169280-56-2):
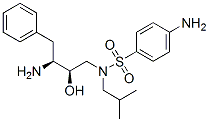
Molecular Weight: 391.5276 g/mol
Molecular Formula: C20H29N3O3S
Density: 1.226 g/cm3
Boiling Point: 609.105 °C at 760 mmHg
Flash Point: 322.175 °C
Index of Refraction: 1.605
Molar Refractivity: 109.917 cm3
Molar Volume: 319.307 cm3
Polarizability: 43.575×10-24 cm3
Surface Tension: 55.775 dyne/cm
Enthalpy of Vaporization: 95.139 kJ/mol
InChI: InChI=1/C20H29N3O3S/c1-15(2)13-23(27(25,26)18-10-8-17(21)9-11-18)14-20(24)19(22)12-16-6-4-3-5-7-16/h3-11,15,19-20,24H,12-14,21-22H2,1-2H3/t19-,20/m0/s1
InChIKey of 4-Amino-N-((2R,3S)-3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide (CAS NO.169280-56-2): NUMJNKDUHFCFJO-XJDOXCRVBY
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View