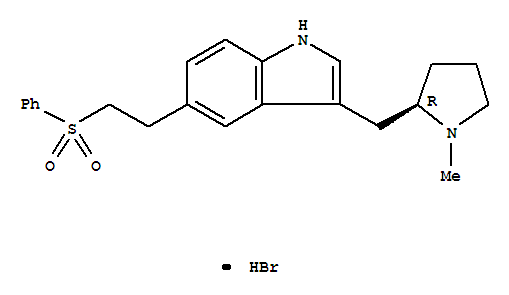
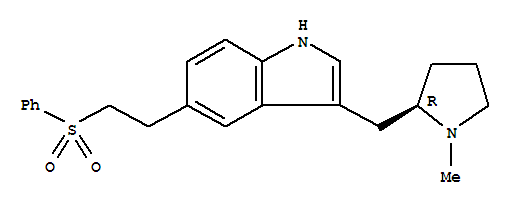
Chemvon Biotechnology Co. Ltd.
molecular formula:C22H26N2O2S.HBr molecular weight:463.43
Cas:177834-92-3
Min.Order:0 Metric Ton
Negotiable
Type:Lab/Research institutions
inquiryHangzhou JINLAN Pharm-Drugs Technology Co., Ltd
Jinlan Pharm-Drugs Technology Co.,Limited (with its export company Hangzhou Royall Import & Export Co.,Ltd.)is located in Hangzhou, Zhejiang Province. Neighboring Ningbo port, Shanghai port, Hangzhou Xiaoshan Int’l Airport and Shanghai Pu
Cas:177834-92-3
Min.Order:1 Gram
Negotiable
Type:Manufacturers
inquiryAlity Chemical Corporation
The above product is Ality Chemical's strong item with best price, good quality and fast supply. Ality Chemical has been focusing on the research and production of this field for over 14 years. At the same time, we are always committed to providi
Xi'an Xszo Chem Co., Ltd.
1. Factory price and high quality must be guaranteed, base on 8 years of production and R&D experience2. Free samples will be provided,ensure specifications and quality are right for customer3. Customers will receive the most professional technical s
Cas:177834-92-3
Min.Order:1 Gram
FOB Price: $0.1
Type:Manufacturers
inquiryEnke Pharma-tech Co.,Ltd. (Cangzhou, China )
Cangzhou Enke Pharma Tech Co.,ltd. is located in Cangzhou City, Hebei province ,where is a famous petroleum chemical industry city in China. Enke Pharma a high-tech enterprise ,and we are dedicated to developing and manufacturing new ap
Cas:177834-92-3
Min.Order:1 Kilogram
FOB Price: $1.0
Type:Manufacturers
inquiryDayang Chem (Hangzhou) Co.,Ltd.
Dayangchem's R&D center can offer custom synthesis according to the contract research and development services for the fine chemicals, pharmaceutical, biotechnique and some of the other chemicals. DayangChem can provide different quantities
Cas:177834-92-3
Min.Order:1 Kilogram
FOB Price: $3.0
Type:Lab/Research institutions
inquiryChemwill Asia Co., Ltd.
Our main production base is located in Xuzhou industry park. We are certified both to the ISO 9001 and ISO 14001 Standards, have a safety management system in place.Our R&D team masters core technology for process-design of target building block
Cas:177834-92-3
Min.Order:5 Kiloliter
FOB Price: $1.2 / 5.0
Type:Manufacturers
inquiryLIDE PHARMACEUTICALS LIMITED
LIDE PHARMACEUTICALS LIMITED is a professional chemicals and APIs leading manufacturer in China. Our core business line covers APIs, Intermediates, Herb extract, etc.
Cas:177834-92-3
Min.Order:1 Kilogram
FOB Price: $0.9 / 1.0
Type:Lab/Research institutions
inquiryHenan Tianfu Chemical Co., Ltd.
Our company was built in 2009 with an ISO certificate.In the past 5 years, we have grown up as a famous fine chemicals supplier in China and we had established stable business relationships with Samsung,LG,Merck,Thermo Fisher Scientific and so on.
Cas:177834-92-3
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryZhuozhou Wenxi import and Export Co., Ltd
WITH US,YOUR MONEY IN SAFE,YOUR BUSINESS IN SAFE 1)Quick Response Within 12 hours; 2)Quality Guarantee: All products are strictly tested by our QC, confirmed by QA and approved by third party lab in China, USA, Canada, Germany, UK, Italy, France et
Cas:177834-92-3
Min.Order:1 Kilogram
FOB Price: $139.0 / 210.0
Type:Trading Company
inquiryHubei Langyou International Trading Co., Ltd
Advantages: Hubei XinRunde Chemical Co., Ltd is a renowned pharmaceutical manufacturer. We can offer high quality products at competitive price in quick delivery with 100% custom pass guaranteed. Never stop striving to offer our best serv
Cas:177834-92-3
Min.Order:10 Gram
Negotiable
Type:Other
inquiryHebei Nengqian Chemical Import and Export Co., LTD
Our Advantage Rich Experience Our products are sold all over Europe,North&South America, Sino-East, Asia and pacific area as well as Africa,we establish long term. Quality service Company cooperates with research institutes. We strictly con
Cas:177834-92-3
Min.Order:1 Kilogram
FOB Price: $1.0 / 10.0
Type:Trading Company
inquiryShanghai Upbio Tech Co.,Ltd
1.No Less 8 years exporting experience. Clients can 100% received goods 2.Lower Price with higher quality 3,Free sample 4,We are sincerely responsible for the "product quality" and "After Service" Upbio is Specialized
Cas:177834-92-3
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryBaoji Guokang Healthchem co.,ltd
Our company has been in existence for 10 years since its establishment. We have our own unique team. The company integrates independent research and development, production and sales. We have established famous brands at home and abroad. At present
Cas:177834-92-3
Min.Order:10 Gram
FOB Price: $20.0 / 25.0
Type:Trading Company
inquiryShandong Hanjiang Chemical Co., Ltd.
Hello, dear friend! I'm Hansen and Allen from China. Welcome to my lookchem mall! The following is a brief introduction of our company's products and services. If you are interested in our products, please contact us by emai
Cas:177834-92-3
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryHenan Wentao Chemical Product Co., Ltd.
Henan Wentao Chemical Product Co.,Ltd is Located in Zhengzhou High-tech Development Zone with import and export license, We passed ISO 9001:2008 as well, Henan Wentao has developed more than 1000 compounds, which are widely used in the fields of prod
Cas:177834-92-3
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryShangHai Soyoung Biotechnology Inc
Trade Assurance Get full protection for your orders 100% Product quality protection 100% On-time shipment protection 100% Payment protection Appearance:Yellow brown solid powder Storage:Store at a low temperature of 2℃~8℃. preserve in tight containe
Cas:177834-92-3
Min.Order:1 Kilogram
FOB Price: $80.0
Type:Lab/Research institutions
inquiryTaizhou Crene Biotechnology co.ltd
Our company provides one-stop services of research - development - production for a variety of special prouducts. Not only do we make effective use of our strong technological strength, but also establish of cooperative relations with several well-
Cas:177834-92-3
Min.Order:1 Gram
Negotiable
Type:Lab/Research institutions
inquiryHangzhou Maytime Bio-Tech Co.,Ltd.
production in commercial lot . Storage:keep from moisture,store in tight container Application:Relieve migraine
Afine Chemicals Limited
Our Services 1. New Molecules R&D 2. Own test center HPLC NMR GC LC-MS 3. API and Intermediates from China reputed manufacturers 4. Documents support COA MOA MSDS DMF open part Our advantages 1. Government awarded company. Top 100 enter
Cas:177834-92-3
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryHangzhou J&H Chemical Co., Ltd.
J&H CHEM R&D center can offer custom synthesis according to the contract research and development services for the fine chemicals, pharmaceutical, biotechnique and some of the other chemicals. J&H CHEM has some Manufacturing base in Jia
Cas:177834-92-3
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryZibo Hangyu Biotechnology Development Co., Ltd
Zibo Hangyu Biotechnology Development Co., Ltd is a leading manufacturer and supplier of chemicals in China. We develop produce and distribute high quality pharmaceuticals, intermediates, special chemicals and OLED intermediates and other fine chemi
Cas:177834-92-3
Min.Order:10 Gram
FOB Price: $100.0
Type:Lab/Research institutions
inquiryHangzhou Lingrui Chemical Co.,Ltd.
Eletriptan hydrobromideAppearance:powder Storage:store in cool, dry place Package:according to customers' requirements Application:177834-92-3 Transportation:By air(EMS or EUB or FedEx or TNT ect...) or by sea(FOB or CIF or CNF ect...)or according to
Xiamen Jenny Chemical Technology Co., Ltd.
GMP standard, high purity, competitive price, in stock 1. Quick Response: within 6 hours after receiving your email. 2. Quality Guarantee: All products are strictly tested by our QC, confirmed by QA, and approved by a third-party lab in China, USA,
Cas:177834-92-3
Min.Order:1 Milligram
Negotiable
Type:Trading Company
inquiryShanghai Minstar Chemical Co., Ltd
Eletriptan hydrobromide Basic information Product Name: Eletriptan hydrobromide Synonyms: Unii-m41W832ta3;Relpax Also see: E505000;Eletriptan HCl;1H-Indole,3-[[(2R)-1-methyl-2-pyrrolidinyl]methyl]-5-[2-(phenylsulfonyl)ethyl]-,hydrobromide;(R)-
Cas:177834-92-3
Min.Order:1 Gram
Negotiable
Type:Lab/Research institutions
inquiryHangzhou Zhongqi chem Co.,Ltd.
Located in Hangzhou National Hi-Tech Industrial Development Zone, zhongqichem is a technical company mainly focus on the Custom synthesis, manufacturing, sales of chemicals to various industries. Benefiting from the outstanding customer service and h
Hunan chemfish Pharmaceutical co.,Ltd
Appearance:95%+ Package:R&D,Pilot run Transportation:per client require Port:Express ,Air, Sea
Suzhou Health Chemicals Co., Ltd.
High quality,stable supply chain.Appearance:white/off-white or light yellow Storage:Store in cool and dry place, keep away from strong light and heat. Package:aluminum bottle,glass bottle,PTFE bottle,cardboard drum Application:Active Pharmaceutical I
Cas:177834-92-3
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryHANGZHOU YUNUO CHEMICAL CO.,LTD
supplier in China Appearance:Tan solid Storage:Stored in cool, dry and ventilation place; Away from fire and heat Package:100g/bottle,1kg/bottle,25kg/drum or as per your request Application:A second generation triptan drug used in the treatment
Cas:177834-92-3
Min.Order:1 Kilogram
Negotiable
Type:Trading Company
inquiryBluecrystal chem-union
We are a Union of chemistry in China, consists of chemists,engineers, laboratories,factories in China. We organize surplus capacity of R&D and production as well as custom synthesis for chemical products and chemical business project. We are supp
Synthetic route
-
-
143322-58-1
eletriptan

-
-
177834-92-3
eletriptan hydrobromide

| Conditions | Yield |
|---|---|
| With hydrogen bromide In acetone at 20℃; for 0.25h; Product distribution / selectivity; | 98.34% |
| With hydrogen bromide In water; butanone Industry scale; | 96.3% |
| With hydrogen bromide In water; acetone at -45 - 15℃; Product distribution / selectivity; | 90% |
-
-
143577-60-0
(R)-5-(2-phenylsulphonylethenyl)-3-(N-methylpyrrolidine-2-yl-methyl)-1H-indole hydrobromide

-
-
177834-92-3
eletriptan hydrobromide

| Conditions | Yield |
|---|---|
| Stage #1: (R)-5-(2-phenylsulphonylethenyl)-3-(N-methylpyrrolidine-2-yl-methyl)-1H-indole hydrobromide With hydrogen; palladium 10% on activated carbon In methanol at 40℃; under 3677.86 Torr; Stage #2: With hydrogen bromide In isopropyl alcohol at 25 - 70℃; for 5h; Product distribution / selectivity; | 88.33% |
-
-
273211-28-2
3-(N-methyl-2(R)-pyrrolidinylmethyl)-5-(2-phenylsulphonylethyl)-1H-indole hydrobromide monohydrate

-
-
177834-92-3
eletriptan hydrobromide

| Conditions | Yield |
|---|---|
| In water; acetone at 20℃; for 120h; Product distribution / selectivity; | |
| In ethanol; ethyl acetate at 20 - 80℃; for 17.5h; Product distribution / selectivity; | |
| In water; ethyl acetate at 10 - 80℃; for 3.5h; Product distribution / selectivity; |
-
-
1162655-06-2
3-[[(R)-1-methyl-2-pyrrolidinyl]methyl]-5-[2-(phenyl-sulfonyl)ethyl]indole para-toluenesulfonate

-
-
177834-92-3
eletriptan hydrobromide

| Conditions | Yield |
|---|---|
| Stage #1: 3-[[(R)-1-methyl-2-pyrrolidinyl]methyl]-5-[2-(phenyl-sulfonyl)ethyl]indole para-toluenesulfonate With ammonia In tert-butyl methyl ether; water pH=10.5 - 11.0; Stage #2: With sodium carbonate In tert-butyl methyl ether; water Stage #3: With hydrogen bromide In ipa; acetone pH=6.6 - 7.5; Product distribution / selectivity; | |
| Stage #1: 3-[[(R)-1-methyl-2-pyrrolidinyl]methyl]-5-[2-(phenyl-sulfonyl)ethyl]indole para-toluenesulfonate With ammonia In tert-butyl methyl ether; water pH=10.5 - 11.0; Stage #2: With sodium carbonate In tert-butyl methyl ether; water Stage #3: With hydrogen bromide In ipa pH=6.6 - 7.5; Product distribution / selectivity; |

-
-
177834-92-3
eletriptan hydrobromide

| Conditions | Yield |
|---|---|
| With hydrogen bromide In water; isopropyl alcohol at 25 - 30℃; for 2.5 - 3h; pH=6 - 7; Product distribution / selectivity; | |
| With hydrogen bromide In water; ethyl acetate at 25 - 30℃; for 2.5 - 3h; Product distribution / selectivity; | |
| With hydrogen bromide In water; acetone at 25 - 30℃; for 2.5h; pH=6 - 7; Product distribution / selectivity; |

-
-
177834-92-3
eletriptan hydrobromide

| Conditions | Yield |
|---|---|
| With hydrogen; 5% Pd(II)/C(eggshell) In methanol; water at 20 - 25℃; under 3800.26 Torr; Product distribution / selectivity; | |
| With hydrogen; 5% Pd(II)/C(eggshell) In water at 20 - 50℃; Product distribution / selectivity; Autoclave; |

-
-
177834-92-3
eletriptan hydrobromide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: triethylamine / acetonitrile / 25 - 83 °C 2.1: triethylamine / palladium diacetate; tris-(o-tolyl)phosphine / acetonitrile / 25 - 35 °C 2.2: 25 - 83 °C 3.1: potassium carbonate; methanol / 25 - 35 °C 3.2: 25 - 30 °C 4.1: hydrogen / 5% Pd(II)/C(eggshell) / water; acetone / 25 - 35 °C / Inert atmosphere 5.1: sodium hydroxide / ethyl acetate; water / 0.33 h / 25 - 30 °C / pH 7 - 10 5.2: 10.5 h / 20 - 25 °C View Scheme | |
| Multi-step reaction with 4 steps 1.1: triethylamine / acetonitrile / 25 - 83 °C 2.1: triethylamine / palladium diacetate; tris-(o-tolyl)phosphine / acetonitrile / 25 - 35 °C 2.2: 25 - 83 °C 3.1: potassium carbonate; methanol / 25 - 35 °C 3.2: 25 - 35 °C / Inert atmosphere 4.1: sodium hydroxide / ethyl acetate; water / 0.33 h / 25 - 30 °C / pH 7 - 10 4.2: 10.5 h / 20 - 25 °C View Scheme |
-
-
180637-88-1
(R)-1-acetyl-5-[2-(phenylsulfonyl)ethyenyl]-3-(N-methylpyrrolidin-2-ylmethyl)-1H-indole

-
-
177834-92-3
eletriptan hydrobromide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: potassium carbonate; methanol / 25 - 35 °C 1.2: 25 - 30 °C 2.1: hydrogen / 5% Pd(II)/C(eggshell) / water; acetone / 25 - 35 °C / Inert atmosphere 3.1: sodium hydroxide / ethyl acetate; water / 0.33 h / 25 - 30 °C / pH 7 - 10 3.2: 10.5 h / 20 - 25 °C View Scheme | |
| Multi-step reaction with 2 steps 1.1: potassium carbonate; methanol / 25 - 35 °C 1.2: 25 - 35 °C / Inert atmosphere 2.1: sodium hydroxide / ethyl acetate; water / 0.33 h / 25 - 30 °C / pH 7 - 10 2.2: 10.5 h / 20 - 25 °C View Scheme | |
| Multi-step reaction with 3 steps 1.1: water; sodium carbonate; methanol / 2 h / 25 - 30 °C 2.1: hydrogen; methanesulfonic acid / 5%-palladium/activated carbon / acetone / 20 °C / 2585.81 Torr 2.2: 0.5 h / 25 - 30 °C 3.1: hydrogen bromide / dichloromethane; water; ethylene glycol / 0.5 h / 5 - 10 °C / Inert atmosphere View Scheme |

-
-
177834-92-3
eletriptan hydrobromide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: triethylamine / palladium diacetate; tris-(o-tolyl)phosphine / acetonitrile / 25 - 35 °C 1.2: 25 - 83 °C 2.1: potassium carbonate; methanol / 25 - 35 °C 2.2: 25 - 30 °C 3.1: hydrogen / 5% Pd(II)/C(eggshell) / water; acetone / 25 - 35 °C / Inert atmosphere 4.1: sodium hydroxide / ethyl acetate; water / 0.33 h / 25 - 30 °C / pH 7 - 10 4.2: 10.5 h / 20 - 25 °C View Scheme | |
| Multi-step reaction with 3 steps 1.1: triethylamine / palladium diacetate; tris-(o-tolyl)phosphine / acetonitrile / 25 - 35 °C 1.2: 25 - 83 °C 2.1: potassium carbonate; methanol / 25 - 35 °C 2.2: 25 - 35 °C / Inert atmosphere 3.1: sodium hydroxide / ethyl acetate; water / 0.33 h / 25 - 30 °C / pH 7 - 10 3.2: 10.5 h / 20 - 25 °C View Scheme |
-
-
1261286-61-6
(R)-5-(2-phenylsulphonylethyl)-3-(N-methylpyrrolidine-2-yl-methyl)-1H-indole methanesulphonate

-
-
177834-92-3
eletriptan hydrobromide

| Conditions | Yield |
|---|---|
| Stage #1: (R)-5-(2-phenylsulphonylethyl)-3-(N-methylpyrrolidine-2-yl-methyl)-1H-indole methanesulphonate With sodium hydroxide In water; ethyl acetate at 25 - 30℃; for 0.333333h; pH=7 - 10; Stage #2: With hydrogen bromide In water; acetone at 20 - 25℃; for 10.5h; Product distribution / selectivity; |
-
-
143322-57-0
(R)-5-bromo-3-(N-methylpyrrolidine-2-ylmethyl)-1H-indole

-
-
177834-92-3
eletriptan hydrobromide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1.1: isopropyl alcohol; water / 4 h / 20 - 35 °C 2.1: potassium hydroxide / toluene; water / 0.25 h / 21 - 25 °C 2.2: 4 h / 100 °C 3.1: triethylamine / palladium diacetate; tris-(o-tolyl)phosphine / N,N-dimethyl-formamide / 5 h / 115 °C 4.1: potassium carbonate; water / methanol 5.1: hydrogen bromide / water / pH 1.5 6.1: hydrogen / 5% Pd(II)/C(eggshell) / water / 20 - 50 °C / Autoclave View Scheme | |
| Multi-step reaction with 5 steps 1.1: N,N-dimethyl-formamide / 95 - 100 °C 1.2: 0 - 5 °C / pH 8 - 9 2.1: N-ethyl-N,N-diisopropylamine / palladium diacetate; tris-(o-tolyl)phosphine / N,N-dimethyl-formamide / 90 - 95 °C / Inert atmosphere 3.1: water; sodium carbonate; methanol / 2 h / 25 - 30 °C 4.1: hydrogen; methanesulfonic acid / 5%-palladium/activated carbon / acetone / 20 °C / 2585.81 Torr 4.2: 0.5 h / 25 - 30 °C 5.1: hydrogen bromide / dichloromethane; water; ethylene glycol / 0.5 h / 5 - 10 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 3 steps 1.1: triethylamine / N,N-dimethyl-formamide / 2 h / 90 - 100 °C 1.2: 3 h / Reflux 2.1: water; sodium carbonate; methanol / 2 h / 25 - 30 °C 3.1: hydrogen; methanesulfonic acid / 5%-palladium/activated carbon / acetone 3.3: 0.5 h / 20 °C View Scheme |
-
-
143322-56-9
(R)-3-[(N-benzyloxycarbonylpyrrolidin-2-yl)carbonyl]-5-bromo-1H-indole

-
-
177834-92-3
eletriptan hydrobromide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1.1: lithium aluminium tetrahydride / tetrahydrofuran / 3.5 h / 60 °C / Reflux 2.1: isopropyl alcohol; water / 4 h / 20 - 35 °C 3.1: potassium hydroxide / toluene; water / 0.25 h / 21 - 25 °C 3.2: 4 h / 100 °C 4.1: triethylamine / palladium diacetate; tris-(o-tolyl)phosphine / N,N-dimethyl-formamide / 5 h / 115 °C 5.1: potassium carbonate; water / methanol 6.1: hydrogen bromide / water / pH 1.5 7.1: hydrogen / 5% Pd(II)/C(eggshell) / water / 20 - 50 °C / Autoclave View Scheme | |
| Multi-step reaction with 3 steps 1.1: lithium aluminium tetrahydride / tetrahydrofuran / 39 h / 25 °C / 760.05 Torr / Inert atmosphere; Reflux 2.1: triethylamine / palladium diacetate; tris-(o-tolyl)phosphine / acetonitrile / 75 - 80 °C 3.1: hydrogen / palladium 10% on activated carbon / methanol / 40 °C / 3677.86 Torr 3.2: 5 h / 25 - 70 °C View Scheme |

-
-
177834-92-3
eletriptan hydrobromide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: potassium carbonate; water / methanol 2: hydrogen bromide / water / pH 1.5 3: hydrogen / 5% Pd(II)/C(eggshell) / water / 20 - 50 °C / Autoclave View Scheme |
-
-
6404-31-5
Z-D-proline

-
-
177834-92-3
eletriptan hydrobromide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 9 steps 1.1: oxalyl dichloride / N,N-dimethyl-formamide; toluene / 2.5 h / 20 - 25 °C 2.1: methylmagnesium chloride / tert-butyl methyl ether; tetrahydrofuran / 0.75 h / 20 °C 2.2: 0.5 h / 3 - 20 °C 3.1: lithium aluminium tetrahydride / tetrahydrofuran / 3.5 h / 60 °C / Reflux 4.1: isopropyl alcohol; water / 4 h / 20 - 35 °C 5.1: potassium hydroxide / toluene; water / 0.25 h / 21 - 25 °C 5.2: 4 h / 100 °C 6.1: triethylamine / palladium diacetate; tris-(o-tolyl)phosphine / N,N-dimethyl-formamide / 5 h / 115 °C 7.1: potassium carbonate; water / methanol 8.1: hydrogen bromide / water / pH 1.5 9.1: hydrogen / 5% Pd(II)/C(eggshell) / water / 20 - 50 °C / Autoclave View Scheme | |
| Multi-step reaction with 5 steps 1.1: oxalyl dichloride / N,N-dimethyl-formamide / dichloromethane / 25 - 30 °C 2.1: ethylmagnesium bromide / diethyl ether / 2.25 h / 25 °C / 760.05 Torr / Reflux 2.2: 1 h / -30 °C 2.3: 0.17 h / -30 - 30 °C 3.1: lithium aluminium tetrahydride / tetrahydrofuran / 39 h / 25 °C / 760.05 Torr / Inert atmosphere; Reflux 4.1: triethylamine / palladium diacetate; tris-(o-tolyl)phosphine / acetonitrile / 75 - 80 °C 5.1: hydrogen / palladium 10% on activated carbon / methanol / 40 °C / 3677.86 Torr 5.2: 5 h / 25 - 70 °C View Scheme |
-
-
61350-62-7
N-benzyloxycarbonyl-D-proline acid chloride

-
-
177834-92-3
eletriptan hydrobromide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1.1: methylmagnesium chloride / tert-butyl methyl ether; tetrahydrofuran / 0.75 h / 20 °C 1.2: 0.5 h / 3 - 20 °C 2.1: lithium aluminium tetrahydride / tetrahydrofuran / 3.5 h / 60 °C / Reflux 3.1: isopropyl alcohol; water / 4 h / 20 - 35 °C 4.1: potassium hydroxide / toluene; water / 0.25 h / 21 - 25 °C 4.2: 4 h / 100 °C 5.1: triethylamine / palladium diacetate; tris-(o-tolyl)phosphine / N,N-dimethyl-formamide / 5 h / 115 °C 6.1: potassium carbonate; water / methanol 7.1: hydrogen bromide / water / pH 1.5 8.1: hydrogen / 5% Pd(II)/C(eggshell) / water / 20 - 50 °C / Autoclave View Scheme | |
| Multi-step reaction with 4 steps 1.1: ethylmagnesium bromide / diethyl ether / 2.25 h / 25 °C / 760.05 Torr / Reflux 1.2: 1 h / -30 °C 1.3: 0.17 h / -30 - 30 °C 2.1: lithium aluminium tetrahydride / tetrahydrofuran / 39 h / 25 °C / 760.05 Torr / Inert atmosphere; Reflux 3.1: triethylamine / palladium diacetate; tris-(o-tolyl)phosphine / acetonitrile / 75 - 80 °C 4.1: hydrogen / palladium 10% on activated carbon / methanol / 40 °C / 3677.86 Torr 4.2: 5 h / 25 - 70 °C View Scheme |
-
-
205369-12-6
(R)-1-acetyl-5-bromo-3-(N-methylpyrrolidin-2-ylmethyl)-1H-indole

-
-
177834-92-3
eletriptan hydrobromide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: triethylamine / palladium diacetate; tris-(o-tolyl)phosphine / N,N-dimethyl-formamide / 5 h / 115 °C 2: potassium carbonate; water / methanol 3: hydrogen bromide / water / pH 1.5 4: hydrogen / 5% Pd(II)/C(eggshell) / water / 20 - 50 °C / Autoclave View Scheme | |
| Multi-step reaction with 4 steps 1.1: N-ethyl-N,N-diisopropylamine / palladium diacetate; tris-(o-tolyl)phosphine / N,N-dimethyl-formamide / 90 - 95 °C / Inert atmosphere 2.1: water; sodium carbonate; methanol / 2 h / 25 - 30 °C 3.1: hydrogen; methanesulfonic acid / 5%-palladium/activated carbon / acetone / 20 °C / 2585.81 Torr 3.2: 0.5 h / 25 - 30 °C 4.1: hydrogen bromide / dichloromethane; water; ethylene glycol / 0.5 h / 5 - 10 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 3 steps 1.1: N-ethyl-N,N-diisopropylamine / palladium diacetate; tris-(o-tolyl)phosphine / N,N-dimethyl-formamide / 90 - 95 °C / Inert atmosphere 2.1: water; sodium carbonate; methanol / 2 h / 25 - 30 °C 3.1: hydrogen; methanesulfonic acid / 5%-palladium/activated carbon / acetone 3.3: 0.5 h / 20 °C View Scheme |
-
-
1196663-29-2
5-bromo-3-{[(2R)-1-methylpyrrolidin-2-yl]methyl}-1H-indole ethanedioate

-
-
177834-92-3
eletriptan hydrobromide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: potassium hydroxide / toluene; water / 0.25 h / 21 - 25 °C 1.2: 4 h / 100 °C 2.1: triethylamine / palladium diacetate; tris-(o-tolyl)phosphine / N,N-dimethyl-formamide / 5 h / 115 °C 3.1: potassium carbonate; water / methanol 4.1: hydrogen bromide / water / pH 1.5 5.1: hydrogen / 5% Pd(II)/C(eggshell) / water / 20 - 50 °C / Autoclave View Scheme |

-
-
177834-92-3
eletriptan hydrobromide

| Conditions | Yield |
|---|---|
| Stage #1: (R)-5-(2-Benzenesulphonylethenyl)-3-(N-methylpyrrolidin-2-ylmethyl)-1H-indole With hydrogen; Raney nickel In methanol under 517.162 - 775.743 Torr; for 5h; Industry scale; Stage #2: With hydrogen bromide In water; ethyl acetate for 0.5h; Industry scale; Stage #3: In toluene Reflux; Industry scale; | |
| Multi-step reaction with 2 steps 1: hydrogen bromide / water / pH 1.5 2: hydrogen / 5% Pd(II)/C(eggshell) / water / 20 - 50 °C / Autoclave View Scheme | |
| Multi-step reaction with 2 steps 1.1: methanesulfonic acid / acetone; water / 0.08 h 1.2: 6 h / 30 °C / 2250.23 Torr 2.1: hydrogen bromide / water; ethanol / 6 h / 20 °C View Scheme |
-
-
180637-89-2
(R)-5-[(2-phenylsulfonyl)ethenyl]-3-(N-methylpyrrolidine-2-ylmethyl)-1H-indole

-
-
177834-92-3
eletriptan hydrobromide

| Conditions | Yield |
|---|---|
| Stage #1: (R)-5-[(2-phenylsulfonyl)ethenyl]-3-(N-methylpyrrolidine-2-ylmethyl)-1H-indole With methanesulfonic acid; hydrogen; 5%-palladium/activated carbon In acetone Stage #2: With ammonia In dichloromethane; water Stage #3: With hydrogen bromide In dichloromethane; water at 20℃; for 0.5h; | |
| Multi-step reaction with 2 steps 1.1: hydrogen; methanesulfonic acid / 5%-palladium/activated carbon / acetone / 20 °C / 2585.81 Torr 1.2: 0.5 h / 25 - 30 °C 2.1: hydrogen bromide / dichloromethane; water; ethylene glycol / 0.5 h / 5 - 10 °C / Inert atmosphere View Scheme | |
| With 10% Pd/C; hydrogen bromide In water; acetone at 25 - 30℃; | |
| Multi-step reaction with 2 steps 1: methanesulfonic acid; 5%-palladium/activated carbon; hydrogen / water; acetone / 0.08 h / 25 - 35 °C / 3800.26 - 4560.31 Torr 2: hydrogen bromide / water; isopropyl alcohol / 4 - 5 h / 25 - 35 °C View Scheme |
-
-
5535-48-8
PVS

-
-
177834-92-3
eletriptan hydrobromide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: triethylamine / N,N-dimethyl-formamide / 2 h / 90 - 100 °C 1.2: 3 h / Reflux 2.1: water; sodium carbonate; methanol / 2 h / 25 - 30 °C 3.1: hydrogen; methanesulfonic acid / 5%-palladium/activated carbon / acetone / 20 °C / 2585.81 Torr 3.2: 0.5 h / 25 - 30 °C 4.1: hydrogen bromide / dichloromethane; water; ethylene glycol / 0.5 h / 5 - 10 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 3 steps 1.1: triethylamine / N,N-dimethyl-formamide / 2 h / 90 - 100 °C 1.2: 3 h / Reflux 2.1: water; sodium carbonate; methanol / 2 h / 25 - 30 °C 3.1: hydrogen; methanesulfonic acid / 5%-palladium/activated carbon / acetone 3.3: 0.5 h / 20 °C View Scheme |
-
-
105370-80-7
(R)-pyrrolidine-1,2-dicarboxylic acid 1-phenyl ester

-
-
177834-92-3
eletriptan hydrobromide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1.1: N,N-dimethyl-formamide; thionyl chloride / dichloromethane / 3 h / Reflux 2.1: ethylmagnesium bromide; zinc(II) chloride / dichloromethane; tetrahydrofuran / 5 - 30 °C 2.2: -10 - 25 °C 3.1: sodium bis(2-methoxyethoxy)aluminium dihydride / toluene / 10 - 50 °C / Inert atmosphere; Darkness 3.2: 0 - 35 °C / pH 12 - 13 4.1: N,N-dimethyl-formamide / 95 - 100 °C 4.2: 0 - 5 °C / pH 8 - 9 5.1: N-ethyl-N,N-diisopropylamine / palladium diacetate; tris-(o-tolyl)phosphine / N,N-dimethyl-formamide / 90 - 95 °C / Inert atmosphere 6.1: water; sodium carbonate; methanol / 2 h / 25 - 30 °C 7.1: hydrogen; methanesulfonic acid / 5%-palladium/activated carbon / acetone / 20 °C / 2585.81 Torr 7.2: 0.5 h / 25 - 30 °C 8.1: hydrogen bromide / dichloromethane; water; ethylene glycol / 0.5 h / 5 - 10 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 6 steps 1.1: N,N-dimethyl-formamide; thionyl chloride / dichloromethane / 3 h / Reflux 2.1: ethylmagnesium bromide; zinc(II) chloride / dichloromethane; tetrahydrofuran / 5 - 30 °C 2.2: -10 - 25 °C 3.1: sodium bis(2-methoxyethoxy)aluminium dihydride / toluene / 10 - 50 °C / Inert atmosphere; Darkness 3.2: 0 - 35 °C / pH 12 - 13 4.1: triethylamine / N,N-dimethyl-formamide / 2 h / 90 - 100 °C 4.2: 3 h / Reflux 5.1: water; sodium carbonate; methanol / 2 h / 25 - 30 °C 6.1: hydrogen; methanesulfonic acid / 5%-palladium/activated carbon / acetone 6.3: 0.5 h / 20 °C View Scheme | |
| Multi-step reaction with 7 steps 1.1: N,N-dimethyl-formamide; thionyl chloride / dichloromethane / 3 h / Reflux 2.1: ethylmagnesium bromide; zinc(II) chloride / dichloromethane; tetrahydrofuran / 5 - 30 °C 2.2: -10 - 25 °C 3.1: sodium bis(2-methoxyethoxy)aluminium dihydride / toluene / 10 - 50 °C / Inert atmosphere; Darkness 3.2: 0 - 35 °C / pH 12 - 13 4.1: triethylamine / N,N-dimethyl-formamide / 2 h / 90 - 100 °C 4.2: 3 h / Reflux 5.1: water; sodium carbonate; methanol / 2 h / 25 - 30 °C 6.1: hydrogen; methanesulfonic acid / 5%-palladium/activated carbon / acetone / 20 °C / 2585.81 Torr 6.2: 0.5 h / 25 - 30 °C 7.1: hydrogen bromide / dichloromethane; water; ethylene glycol / 0.5 h / 5 - 10 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 7 steps 1.1: N,N-dimethyl-formamide; thionyl chloride / dichloromethane / 3 h / Reflux 2.1: ethylmagnesium bromide; zinc(II) chloride / dichloromethane; tetrahydrofuran / 5 - 30 °C 2.2: -10 - 25 °C 3.1: sodium bis(2-methoxyethoxy)aluminium dihydride / toluene / 10 - 50 °C / Inert atmosphere; Darkness 3.2: 0 - 35 °C / pH 12 - 13 4.1: N,N-dimethyl-formamide / 95 - 100 °C 4.2: 0 - 5 °C / pH 8 - 9 5.1: N-ethyl-N,N-diisopropylamine / palladium diacetate; tris-(o-tolyl)phosphine / N,N-dimethyl-formamide / 90 - 95 °C / Inert atmosphere 6.1: water; sodium carbonate; methanol / 2 h / 25 - 30 °C 7.1: hydrogen; methanesulfonic acid / 5%-palladium/activated carbon / acetone 7.3: 0.5 h / 20 °C View Scheme |
-
-
1353991-67-9
(R)-2-chlorocarbonylpyrrolidine-1-carboxylic acid phenyl ester

-
-
177834-92-3
eletriptan hydrobromide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1.1: ethylmagnesium bromide; zinc(II) chloride / dichloromethane; tetrahydrofuran / 5 - 30 °C 1.2: -10 - 25 °C 2.1: sodium bis(2-methoxyethoxy)aluminium dihydride / toluene / 10 - 50 °C / Inert atmosphere; Darkness 2.2: 0 - 35 °C / pH 12 - 13 3.1: N,N-dimethyl-formamide / 95 - 100 °C 3.2: 0 - 5 °C / pH 8 - 9 4.1: N-ethyl-N,N-diisopropylamine / palladium diacetate; tris-(o-tolyl)phosphine / N,N-dimethyl-formamide / 90 - 95 °C / Inert atmosphere 5.1: water; sodium carbonate; methanol / 2 h / 25 - 30 °C 6.1: hydrogen; methanesulfonic acid / 5%-palladium/activated carbon / acetone / 20 °C / 2585.81 Torr 6.2: 0.5 h / 25 - 30 °C 7.1: hydrogen bromide / dichloromethane; water; ethylene glycol / 0.5 h / 5 - 10 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 5 steps 1.1: ethylmagnesium bromide; zinc(II) chloride / dichloromethane; tetrahydrofuran / 5 - 30 °C 1.2: -10 - 25 °C 2.1: sodium bis(2-methoxyethoxy)aluminium dihydride / toluene / 10 - 50 °C / Inert atmosphere; Darkness 2.2: 0 - 35 °C / pH 12 - 13 3.1: triethylamine / N,N-dimethyl-formamide / 2 h / 90 - 100 °C 3.2: 3 h / Reflux 4.1: water; sodium carbonate; methanol / 2 h / 25 - 30 °C 5.1: hydrogen; methanesulfonic acid / 5%-palladium/activated carbon / acetone 5.3: 0.5 h / 20 °C View Scheme | |
| Multi-step reaction with 6 steps 1.1: ethylmagnesium bromide; zinc(II) chloride / dichloromethane; tetrahydrofuran / 5 - 30 °C 1.2: -10 - 25 °C 2.1: sodium bis(2-methoxyethoxy)aluminium dihydride / toluene / 10 - 50 °C / Inert atmosphere; Darkness 2.2: 0 - 35 °C / pH 12 - 13 3.1: triethylamine / N,N-dimethyl-formamide / 2 h / 90 - 100 °C 3.2: 3 h / Reflux 4.1: water; sodium carbonate; methanol / 2 h / 25 - 30 °C 5.1: hydrogen; methanesulfonic acid / 5%-palladium/activated carbon / acetone / 20 °C / 2585.81 Torr 5.2: 0.5 h / 25 - 30 °C 6.1: hydrogen bromide / dichloromethane; water; ethylene glycol / 0.5 h / 5 - 10 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 6 steps 1.1: ethylmagnesium bromide; zinc(II) chloride / dichloromethane; tetrahydrofuran / 5 - 30 °C 1.2: -10 - 25 °C 2.1: sodium bis(2-methoxyethoxy)aluminium dihydride / toluene / 10 - 50 °C / Inert atmosphere; Darkness 2.2: 0 - 35 °C / pH 12 - 13 3.1: N,N-dimethyl-formamide / 95 - 100 °C 3.2: 0 - 5 °C / pH 8 - 9 4.1: N-ethyl-N,N-diisopropylamine / palladium diacetate; tris-(o-tolyl)phosphine / N,N-dimethyl-formamide / 90 - 95 °C / Inert atmosphere 5.1: water; sodium carbonate; methanol / 2 h / 25 - 30 °C 6.1: hydrogen; methanesulfonic acid / 5%-palladium/activated carbon / acetone 6.3: 0.5 h / 20 °C View Scheme |
-
-
1353991-68-0
(R)-2-(5-bromo-1H-indole-3-carbonyl)pyrrolidine-1-carboxylic acid phenyl ester

-
-
177834-92-3
eletriptan hydrobromide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1.1: sodium bis(2-methoxyethoxy)aluminium dihydride / toluene / 10 - 50 °C / Inert atmosphere; Darkness 1.2: 0 - 35 °C / pH 12 - 13 2.1: N,N-dimethyl-formamide / 95 - 100 °C 2.2: 0 - 5 °C / pH 8 - 9 3.1: N-ethyl-N,N-diisopropylamine / palladium diacetate; tris-(o-tolyl)phosphine / N,N-dimethyl-formamide / 90 - 95 °C / Inert atmosphere 4.1: water; sodium carbonate; methanol / 2 h / 25 - 30 °C 5.1: hydrogen; methanesulfonic acid / 5%-palladium/activated carbon / acetone / 20 °C / 2585.81 Torr 5.2: 0.5 h / 25 - 30 °C 6.1: hydrogen bromide / dichloromethane; water; ethylene glycol / 0.5 h / 5 - 10 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 4 steps 1.1: sodium bis(2-methoxyethoxy)aluminium dihydride / toluene / 10 - 50 °C / Inert atmosphere; Darkness 1.2: 0 - 35 °C / pH 12 - 13 2.1: triethylamine / N,N-dimethyl-formamide / 2 h / 90 - 100 °C 2.2: 3 h / Reflux 3.1: water; sodium carbonate; methanol / 2 h / 25 - 30 °C 4.1: hydrogen; methanesulfonic acid / 5%-palladium/activated carbon / acetone 4.3: 0.5 h / 20 °C View Scheme | |
| Multi-step reaction with 5 steps 1.1: sodium bis(2-methoxyethoxy)aluminium dihydride / toluene / 10 - 50 °C / Inert atmosphere; Darkness 1.2: 0 - 35 °C / pH 12 - 13 2.1: N,N-dimethyl-formamide / 95 - 100 °C 2.2: 0 - 5 °C / pH 8 - 9 3.1: N-ethyl-N,N-diisopropylamine / palladium diacetate; tris-(o-tolyl)phosphine / N,N-dimethyl-formamide / 90 - 95 °C / Inert atmosphere 4.1: water; sodium carbonate; methanol / 2 h / 25 - 30 °C 5.1: hydrogen; methanesulfonic acid / 5%-palladium/activated carbon / acetone 5.3: 0.5 h / 20 °C View Scheme | |
| Multi-step reaction with 5 steps 1.1: sodium bis(2-methoxyethoxy)aluminium dihydride / toluene / 10 - 50 °C / Inert atmosphere; Darkness 1.2: 0 - 35 °C / pH 12 - 13 2.1: triethylamine / N,N-dimethyl-formamide / 2 h / 90 - 100 °C 2.2: 3 h / Reflux 3.1: water; sodium carbonate; methanol / 2 h / 25 - 30 °C 4.1: hydrogen; methanesulfonic acid / 5%-palladium/activated carbon / acetone / 20 °C / 2585.81 Torr 4.2: 0.5 h / 25 - 30 °C 5.1: hydrogen bromide / dichloromethane; water; ethylene glycol / 0.5 h / 5 - 10 °C / Inert atmosphere View Scheme |
-
-
5535-48-8
PVS

-
-
143322-57-0
(R)-5-bromo-3-(N-methylpyrrolidine-2-ylmethyl)-1H-indole

-
-
177834-92-3
eletriptan hydrobromide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: triethylamine / palladium diacetate; tris-(o-tolyl)phosphine / acetonitrile / 75 - 80 °C 2.1: hydrogen / palladium 10% on activated carbon / methanol / 40 °C / 3677.86 Torr 2.2: 5 h / 25 - 70 °C View Scheme |

-
-
177834-92-3
eletriptan hydrobromide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: trifluoroacetic acid / dichloromethane / 4 h / 20 °C 2.1: methanesulfonic acid / acetone; water / 0.08 h 2.2: 6 h / 30 °C / 2250.23 Torr 3.1: hydrogen bromide / water; ethanol / 6 h / 20 °C View Scheme |

-
-
177834-92-3
eletriptan hydrobromide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: tris-(o-tolyl)phosphine; triethylamine; palladium diacetate / acetonitrile / 15 h / Inert atmosphere; Reflux 2.1: trifluoroacetic acid / dichloromethane / 4 h / 20 °C 3.1: methanesulfonic acid / acetone; water / 0.08 h 3.2: 6 h / 30 °C / 2250.23 Torr 4.1: hydrogen bromide / water; ethanol / 6 h / 20 °C View Scheme |
-
-
177834-92-3
eletriptan hydrobromide

-
-
1408337-90-5
4-methyl-8-[2-(phenylsulfonyl)ethyl]-1,2,3,5,10,10a-hexahydropyrrolidino[3,2-b]indol-4-ium

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide In water; acetonitrile at 80 - 85℃; for 0.75h; | 60% |
-
-
177834-92-3
eletriptan hydrobromide

-
-
273211-28-2
3-(N-methyl-2(R)-pyrrolidinylmethyl)-5-(2-phenylsulphonylethyl)-1H-indole hydrobromide monohydrate

| Conditions | Yield |
|---|---|
| With water at 60℃; under 20 Torr; for 16h; Product distribution / selectivity; |
Related products
Raw Materials
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View