-
Name
Eletriptan hydrobromide
- EINECS 639-688-8
- CAS No. 177834-92-3
- Article Data23
- CAS DataBase
- Density
- Solubility
- Melting Point 169-171 °C
- Formula C22H27BrN2O2S
- Boiling Point 633.9 °C at 760 mmHg
- Molecular Weight 463.439
- Flash Point 337.2 °C
- Transport Information
- Appearance Tan solid
- Safety 26
- Risk Codes 36
-
Molecular Structure
- Hazard Symbols Xi
- Synonyms 1H-Indole,3-[(1-methyl-2-pyrrolidinyl)methyl]-5-[2-(phenylsulfonyl)ethyl]-,monohydrobromide, (R)-;1H-Indole,3-[[(2R)-1-methyl-2-pyrrolidinyl]methyl]-5-[2-(phenylsulfonyl)ethyl]-,monohydrobromide (9CI);(R)-5-[2-(Benzenesulfonyl)ethyl]-3-[(N-methylpyrrolidin-2-yl)methyl]-1H-indolehydrobromide;1H-Indole,3-[[(2R)-1-methyl-2-pyrrolidinyl]methyl]-5-[2-(phenylsulfonyl)ethyl]-,hydrobromide (1:1);Relert;Relpax;UK 116044-04;
- PSA 61.55000
- LogP 4.83970
Synthetic route
-
-
143322-58-1
eletriptan

-
-
177834-92-3
eletriptan hydrobromide

| Conditions | Yield |
|---|---|
| With hydrogen bromide In acetone at 20℃; for 0.25h; Product distribution / selectivity; | 98.34% |
| With hydrogen bromide In water; butanone Industry scale; | 96.3% |
| With hydrogen bromide In water; acetone at -45 - 15℃; Product distribution / selectivity; | 90% |
-
-
143577-60-0
(R)-5-(2-phenylsulphonylethenyl)-3-(N-methylpyrrolidine-2-yl-methyl)-1H-indole hydrobromide

-
-
177834-92-3
eletriptan hydrobromide

| Conditions | Yield |
|---|---|
| Stage #1: (R)-5-(2-phenylsulphonylethenyl)-3-(N-methylpyrrolidine-2-yl-methyl)-1H-indole hydrobromide With hydrogen; palladium 10% on activated carbon In methanol at 40℃; under 3677.86 Torr; Stage #2: With hydrogen bromide In isopropyl alcohol at 25 - 70℃; for 5h; Product distribution / selectivity; | 88.33% |
-
-
273211-28-2
3-(N-methyl-2(R)-pyrrolidinylmethyl)-5-(2-phenylsulphonylethyl)-1H-indole hydrobromide monohydrate

-
-
177834-92-3
eletriptan hydrobromide

| Conditions | Yield |
|---|---|
| In water; acetone at 20℃; for 120h; Product distribution / selectivity; | |
| In ethanol; ethyl acetate at 20 - 80℃; for 17.5h; Product distribution / selectivity; | |
| In water; ethyl acetate at 10 - 80℃; for 3.5h; Product distribution / selectivity; |
-
-
1162655-06-2
3-[[(R)-1-methyl-2-pyrrolidinyl]methyl]-5-[2-(phenyl-sulfonyl)ethyl]indole para-toluenesulfonate

-
-
177834-92-3
eletriptan hydrobromide

| Conditions | Yield |
|---|---|
| Stage #1: 3-[[(R)-1-methyl-2-pyrrolidinyl]methyl]-5-[2-(phenyl-sulfonyl)ethyl]indole para-toluenesulfonate With ammonia In tert-butyl methyl ether; water pH=10.5 - 11.0; Stage #2: With sodium carbonate In tert-butyl methyl ether; water Stage #3: With hydrogen bromide In ipa; acetone pH=6.6 - 7.5; Product distribution / selectivity; | |
| Stage #1: 3-[[(R)-1-methyl-2-pyrrolidinyl]methyl]-5-[2-(phenyl-sulfonyl)ethyl]indole para-toluenesulfonate With ammonia In tert-butyl methyl ether; water pH=10.5 - 11.0; Stage #2: With sodium carbonate In tert-butyl methyl ether; water Stage #3: With hydrogen bromide In ipa pH=6.6 - 7.5; Product distribution / selectivity; |

-
-
177834-92-3
eletriptan hydrobromide

| Conditions | Yield |
|---|---|
| With hydrogen bromide In water; isopropyl alcohol at 25 - 30℃; for 2.5 - 3h; pH=6 - 7; Product distribution / selectivity; | |
| With hydrogen bromide In water; ethyl acetate at 25 - 30℃; for 2.5 - 3h; Product distribution / selectivity; | |
| With hydrogen bromide In water; acetone at 25 - 30℃; for 2.5h; pH=6 - 7; Product distribution / selectivity; |

-
-
177834-92-3
eletriptan hydrobromide

| Conditions | Yield |
|---|---|
| With hydrogen; 5% Pd(II)/C(eggshell) In methanol; water at 20 - 25℃; under 3800.26 Torr; Product distribution / selectivity; | |
| With hydrogen; 5% Pd(II)/C(eggshell) In water at 20 - 50℃; Product distribution / selectivity; Autoclave; |

-
-
177834-92-3
eletriptan hydrobromide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: triethylamine / acetonitrile / 25 - 83 °C 2.1: triethylamine / palladium diacetate; tris-(o-tolyl)phosphine / acetonitrile / 25 - 35 °C 2.2: 25 - 83 °C 3.1: potassium carbonate; methanol / 25 - 35 °C 3.2: 25 - 30 °C 4.1: hydrogen / 5% Pd(II)/C(eggshell) / water; acetone / 25 - 35 °C / Inert atmosphere 5.1: sodium hydroxide / ethyl acetate; water / 0.33 h / 25 - 30 °C / pH 7 - 10 5.2: 10.5 h / 20 - 25 °C View Scheme | |
| Multi-step reaction with 4 steps 1.1: triethylamine / acetonitrile / 25 - 83 °C 2.1: triethylamine / palladium diacetate; tris-(o-tolyl)phosphine / acetonitrile / 25 - 35 °C 2.2: 25 - 83 °C 3.1: potassium carbonate; methanol / 25 - 35 °C 3.2: 25 - 35 °C / Inert atmosphere 4.1: sodium hydroxide / ethyl acetate; water / 0.33 h / 25 - 30 °C / pH 7 - 10 4.2: 10.5 h / 20 - 25 °C View Scheme |
-
-
180637-88-1
(R)-1-acetyl-5-[2-(phenylsulfonyl)ethyenyl]-3-(N-methylpyrrolidin-2-ylmethyl)-1H-indole

-
-
177834-92-3
eletriptan hydrobromide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: potassium carbonate; methanol / 25 - 35 °C 1.2: 25 - 30 °C 2.1: hydrogen / 5% Pd(II)/C(eggshell) / water; acetone / 25 - 35 °C / Inert atmosphere 3.1: sodium hydroxide / ethyl acetate; water / 0.33 h / 25 - 30 °C / pH 7 - 10 3.2: 10.5 h / 20 - 25 °C View Scheme | |
| Multi-step reaction with 2 steps 1.1: potassium carbonate; methanol / 25 - 35 °C 1.2: 25 - 35 °C / Inert atmosphere 2.1: sodium hydroxide / ethyl acetate; water / 0.33 h / 25 - 30 °C / pH 7 - 10 2.2: 10.5 h / 20 - 25 °C View Scheme | |
| Multi-step reaction with 3 steps 1.1: water; sodium carbonate; methanol / 2 h / 25 - 30 °C 2.1: hydrogen; methanesulfonic acid / 5%-palladium/activated carbon / acetone / 20 °C / 2585.81 Torr 2.2: 0.5 h / 25 - 30 °C 3.1: hydrogen bromide / dichloromethane; water; ethylene glycol / 0.5 h / 5 - 10 °C / Inert atmosphere View Scheme |

-
-
177834-92-3
eletriptan hydrobromide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: triethylamine / palladium diacetate; tris-(o-tolyl)phosphine / acetonitrile / 25 - 35 °C 1.2: 25 - 83 °C 2.1: potassium carbonate; methanol / 25 - 35 °C 2.2: 25 - 30 °C 3.1: hydrogen / 5% Pd(II)/C(eggshell) / water; acetone / 25 - 35 °C / Inert atmosphere 4.1: sodium hydroxide / ethyl acetate; water / 0.33 h / 25 - 30 °C / pH 7 - 10 4.2: 10.5 h / 20 - 25 °C View Scheme | |
| Multi-step reaction with 3 steps 1.1: triethylamine / palladium diacetate; tris-(o-tolyl)phosphine / acetonitrile / 25 - 35 °C 1.2: 25 - 83 °C 2.1: potassium carbonate; methanol / 25 - 35 °C 2.2: 25 - 35 °C / Inert atmosphere 3.1: sodium hydroxide / ethyl acetate; water / 0.33 h / 25 - 30 °C / pH 7 - 10 3.2: 10.5 h / 20 - 25 °C View Scheme |
-
-
1261286-61-6
(R)-5-(2-phenylsulphonylethyl)-3-(N-methylpyrrolidine-2-yl-methyl)-1H-indole methanesulphonate

-
-
177834-92-3
eletriptan hydrobromide

| Conditions | Yield |
|---|---|
| Stage #1: (R)-5-(2-phenylsulphonylethyl)-3-(N-methylpyrrolidine-2-yl-methyl)-1H-indole methanesulphonate With sodium hydroxide In water; ethyl acetate at 25 - 30℃; for 0.333333h; pH=7 - 10; Stage #2: With hydrogen bromide In water; acetone at 20 - 25℃; for 10.5h; Product distribution / selectivity; |
-
-
143322-57-0
(R)-5-bromo-3-(N-methylpyrrolidine-2-ylmethyl)-1H-indole

-
-
177834-92-3
eletriptan hydrobromide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1.1: isopropyl alcohol; water / 4 h / 20 - 35 °C 2.1: potassium hydroxide / toluene; water / 0.25 h / 21 - 25 °C 2.2: 4 h / 100 °C 3.1: triethylamine / palladium diacetate; tris-(o-tolyl)phosphine / N,N-dimethyl-formamide / 5 h / 115 °C 4.1: potassium carbonate; water / methanol 5.1: hydrogen bromide / water / pH 1.5 6.1: hydrogen / 5% Pd(II)/C(eggshell) / water / 20 - 50 °C / Autoclave View Scheme | |
| Multi-step reaction with 5 steps 1.1: N,N-dimethyl-formamide / 95 - 100 °C 1.2: 0 - 5 °C / pH 8 - 9 2.1: N-ethyl-N,N-diisopropylamine / palladium diacetate; tris-(o-tolyl)phosphine / N,N-dimethyl-formamide / 90 - 95 °C / Inert atmosphere 3.1: water; sodium carbonate; methanol / 2 h / 25 - 30 °C 4.1: hydrogen; methanesulfonic acid / 5%-palladium/activated carbon / acetone / 20 °C / 2585.81 Torr 4.2: 0.5 h / 25 - 30 °C 5.1: hydrogen bromide / dichloromethane; water; ethylene glycol / 0.5 h / 5 - 10 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 3 steps 1.1: triethylamine / N,N-dimethyl-formamide / 2 h / 90 - 100 °C 1.2: 3 h / Reflux 2.1: water; sodium carbonate; methanol / 2 h / 25 - 30 °C 3.1: hydrogen; methanesulfonic acid / 5%-palladium/activated carbon / acetone 3.3: 0.5 h / 20 °C View Scheme |
-
-
143322-56-9
(R)-3-[(N-benzyloxycarbonylpyrrolidin-2-yl)carbonyl]-5-bromo-1H-indole

-
-
177834-92-3
eletriptan hydrobromide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1.1: lithium aluminium tetrahydride / tetrahydrofuran / 3.5 h / 60 °C / Reflux 2.1: isopropyl alcohol; water / 4 h / 20 - 35 °C 3.1: potassium hydroxide / toluene; water / 0.25 h / 21 - 25 °C 3.2: 4 h / 100 °C 4.1: triethylamine / palladium diacetate; tris-(o-tolyl)phosphine / N,N-dimethyl-formamide / 5 h / 115 °C 5.1: potassium carbonate; water / methanol 6.1: hydrogen bromide / water / pH 1.5 7.1: hydrogen / 5% Pd(II)/C(eggshell) / water / 20 - 50 °C / Autoclave View Scheme | |
| Multi-step reaction with 3 steps 1.1: lithium aluminium tetrahydride / tetrahydrofuran / 39 h / 25 °C / 760.05 Torr / Inert atmosphere; Reflux 2.1: triethylamine / palladium diacetate; tris-(o-tolyl)phosphine / acetonitrile / 75 - 80 °C 3.1: hydrogen / palladium 10% on activated carbon / methanol / 40 °C / 3677.86 Torr 3.2: 5 h / 25 - 70 °C View Scheme |

-
-
177834-92-3
eletriptan hydrobromide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: potassium carbonate; water / methanol 2: hydrogen bromide / water / pH 1.5 3: hydrogen / 5% Pd(II)/C(eggshell) / water / 20 - 50 °C / Autoclave View Scheme |
-
-
6404-31-5
Z-D-proline

-
-
177834-92-3
eletriptan hydrobromide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 9 steps 1.1: oxalyl dichloride / N,N-dimethyl-formamide; toluene / 2.5 h / 20 - 25 °C 2.1: methylmagnesium chloride / tert-butyl methyl ether; tetrahydrofuran / 0.75 h / 20 °C 2.2: 0.5 h / 3 - 20 °C 3.1: lithium aluminium tetrahydride / tetrahydrofuran / 3.5 h / 60 °C / Reflux 4.1: isopropyl alcohol; water / 4 h / 20 - 35 °C 5.1: potassium hydroxide / toluene; water / 0.25 h / 21 - 25 °C 5.2: 4 h / 100 °C 6.1: triethylamine / palladium diacetate; tris-(o-tolyl)phosphine / N,N-dimethyl-formamide / 5 h / 115 °C 7.1: potassium carbonate; water / methanol 8.1: hydrogen bromide / water / pH 1.5 9.1: hydrogen / 5% Pd(II)/C(eggshell) / water / 20 - 50 °C / Autoclave View Scheme | |
| Multi-step reaction with 5 steps 1.1: oxalyl dichloride / N,N-dimethyl-formamide / dichloromethane / 25 - 30 °C 2.1: ethylmagnesium bromide / diethyl ether / 2.25 h / 25 °C / 760.05 Torr / Reflux 2.2: 1 h / -30 °C 2.3: 0.17 h / -30 - 30 °C 3.1: lithium aluminium tetrahydride / tetrahydrofuran / 39 h / 25 °C / 760.05 Torr / Inert atmosphere; Reflux 4.1: triethylamine / palladium diacetate; tris-(o-tolyl)phosphine / acetonitrile / 75 - 80 °C 5.1: hydrogen / palladium 10% on activated carbon / methanol / 40 °C / 3677.86 Torr 5.2: 5 h / 25 - 70 °C View Scheme |
-
-
61350-62-7
N-benzyloxycarbonyl-D-proline acid chloride

-
-
177834-92-3
eletriptan hydrobromide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1.1: methylmagnesium chloride / tert-butyl methyl ether; tetrahydrofuran / 0.75 h / 20 °C 1.2: 0.5 h / 3 - 20 °C 2.1: lithium aluminium tetrahydride / tetrahydrofuran / 3.5 h / 60 °C / Reflux 3.1: isopropyl alcohol; water / 4 h / 20 - 35 °C 4.1: potassium hydroxide / toluene; water / 0.25 h / 21 - 25 °C 4.2: 4 h / 100 °C 5.1: triethylamine / palladium diacetate; tris-(o-tolyl)phosphine / N,N-dimethyl-formamide / 5 h / 115 °C 6.1: potassium carbonate; water / methanol 7.1: hydrogen bromide / water / pH 1.5 8.1: hydrogen / 5% Pd(II)/C(eggshell) / water / 20 - 50 °C / Autoclave View Scheme | |
| Multi-step reaction with 4 steps 1.1: ethylmagnesium bromide / diethyl ether / 2.25 h / 25 °C / 760.05 Torr / Reflux 1.2: 1 h / -30 °C 1.3: 0.17 h / -30 - 30 °C 2.1: lithium aluminium tetrahydride / tetrahydrofuran / 39 h / 25 °C / 760.05 Torr / Inert atmosphere; Reflux 3.1: triethylamine / palladium diacetate; tris-(o-tolyl)phosphine / acetonitrile / 75 - 80 °C 4.1: hydrogen / palladium 10% on activated carbon / methanol / 40 °C / 3677.86 Torr 4.2: 5 h / 25 - 70 °C View Scheme |
-
-
205369-12-6
(R)-1-acetyl-5-bromo-3-(N-methylpyrrolidin-2-ylmethyl)-1H-indole

-
-
177834-92-3
eletriptan hydrobromide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: triethylamine / palladium diacetate; tris-(o-tolyl)phosphine / N,N-dimethyl-formamide / 5 h / 115 °C 2: potassium carbonate; water / methanol 3: hydrogen bromide / water / pH 1.5 4: hydrogen / 5% Pd(II)/C(eggshell) / water / 20 - 50 °C / Autoclave View Scheme | |
| Multi-step reaction with 4 steps 1.1: N-ethyl-N,N-diisopropylamine / palladium diacetate; tris-(o-tolyl)phosphine / N,N-dimethyl-formamide / 90 - 95 °C / Inert atmosphere 2.1: water; sodium carbonate; methanol / 2 h / 25 - 30 °C 3.1: hydrogen; methanesulfonic acid / 5%-palladium/activated carbon / acetone / 20 °C / 2585.81 Torr 3.2: 0.5 h / 25 - 30 °C 4.1: hydrogen bromide / dichloromethane; water; ethylene glycol / 0.5 h / 5 - 10 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 3 steps 1.1: N-ethyl-N,N-diisopropylamine / palladium diacetate; tris-(o-tolyl)phosphine / N,N-dimethyl-formamide / 90 - 95 °C / Inert atmosphere 2.1: water; sodium carbonate; methanol / 2 h / 25 - 30 °C 3.1: hydrogen; methanesulfonic acid / 5%-palladium/activated carbon / acetone 3.3: 0.5 h / 20 °C View Scheme |
-
-
1196663-29-2
5-bromo-3-{[(2R)-1-methylpyrrolidin-2-yl]methyl}-1H-indole ethanedioate

-
-
177834-92-3
eletriptan hydrobromide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: potassium hydroxide / toluene; water / 0.25 h / 21 - 25 °C 1.2: 4 h / 100 °C 2.1: triethylamine / palladium diacetate; tris-(o-tolyl)phosphine / N,N-dimethyl-formamide / 5 h / 115 °C 3.1: potassium carbonate; water / methanol 4.1: hydrogen bromide / water / pH 1.5 5.1: hydrogen / 5% Pd(II)/C(eggshell) / water / 20 - 50 °C / Autoclave View Scheme |

-
-
177834-92-3
eletriptan hydrobromide

| Conditions | Yield |
|---|---|
| Stage #1: (R)-5-(2-Benzenesulphonylethenyl)-3-(N-methylpyrrolidin-2-ylmethyl)-1H-indole With hydrogen; Raney nickel In methanol under 517.162 - 775.743 Torr; for 5h; Industry scale; Stage #2: With hydrogen bromide In water; ethyl acetate for 0.5h; Industry scale; Stage #3: In toluene Reflux; Industry scale; | |
| Multi-step reaction with 2 steps 1: hydrogen bromide / water / pH 1.5 2: hydrogen / 5% Pd(II)/C(eggshell) / water / 20 - 50 °C / Autoclave View Scheme | |
| Multi-step reaction with 2 steps 1.1: methanesulfonic acid / acetone; water / 0.08 h 1.2: 6 h / 30 °C / 2250.23 Torr 2.1: hydrogen bromide / water; ethanol / 6 h / 20 °C View Scheme |
-
-
180637-89-2
(R)-5-[(2-phenylsulfonyl)ethenyl]-3-(N-methylpyrrolidine-2-ylmethyl)-1H-indole

-
-
177834-92-3
eletriptan hydrobromide

| Conditions | Yield |
|---|---|
| Stage #1: (R)-5-[(2-phenylsulfonyl)ethenyl]-3-(N-methylpyrrolidine-2-ylmethyl)-1H-indole With methanesulfonic acid; hydrogen; 5%-palladium/activated carbon In acetone Stage #2: With ammonia In dichloromethane; water Stage #3: With hydrogen bromide In dichloromethane; water at 20℃; for 0.5h; | |
| Multi-step reaction with 2 steps 1.1: hydrogen; methanesulfonic acid / 5%-palladium/activated carbon / acetone / 20 °C / 2585.81 Torr 1.2: 0.5 h / 25 - 30 °C 2.1: hydrogen bromide / dichloromethane; water; ethylene glycol / 0.5 h / 5 - 10 °C / Inert atmosphere View Scheme | |
| With 10% Pd/C; hydrogen bromide In water; acetone at 25 - 30℃; | |
| Multi-step reaction with 2 steps 1: methanesulfonic acid; 5%-palladium/activated carbon; hydrogen / water; acetone / 0.08 h / 25 - 35 °C / 3800.26 - 4560.31 Torr 2: hydrogen bromide / water; isopropyl alcohol / 4 - 5 h / 25 - 35 °C View Scheme |
-
-
5535-48-8
PVS

-
-
177834-92-3
eletriptan hydrobromide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: triethylamine / N,N-dimethyl-formamide / 2 h / 90 - 100 °C 1.2: 3 h / Reflux 2.1: water; sodium carbonate; methanol / 2 h / 25 - 30 °C 3.1: hydrogen; methanesulfonic acid / 5%-palladium/activated carbon / acetone / 20 °C / 2585.81 Torr 3.2: 0.5 h / 25 - 30 °C 4.1: hydrogen bromide / dichloromethane; water; ethylene glycol / 0.5 h / 5 - 10 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 3 steps 1.1: triethylamine / N,N-dimethyl-formamide / 2 h / 90 - 100 °C 1.2: 3 h / Reflux 2.1: water; sodium carbonate; methanol / 2 h / 25 - 30 °C 3.1: hydrogen; methanesulfonic acid / 5%-palladium/activated carbon / acetone 3.3: 0.5 h / 20 °C View Scheme |
-
-
105370-80-7
(R)-pyrrolidine-1,2-dicarboxylic acid 1-phenyl ester

-
-
177834-92-3
eletriptan hydrobromide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1.1: N,N-dimethyl-formamide; thionyl chloride / dichloromethane / 3 h / Reflux 2.1: ethylmagnesium bromide; zinc(II) chloride / dichloromethane; tetrahydrofuran / 5 - 30 °C 2.2: -10 - 25 °C 3.1: sodium bis(2-methoxyethoxy)aluminium dihydride / toluene / 10 - 50 °C / Inert atmosphere; Darkness 3.2: 0 - 35 °C / pH 12 - 13 4.1: N,N-dimethyl-formamide / 95 - 100 °C 4.2: 0 - 5 °C / pH 8 - 9 5.1: N-ethyl-N,N-diisopropylamine / palladium diacetate; tris-(o-tolyl)phosphine / N,N-dimethyl-formamide / 90 - 95 °C / Inert atmosphere 6.1: water; sodium carbonate; methanol / 2 h / 25 - 30 °C 7.1: hydrogen; methanesulfonic acid / 5%-palladium/activated carbon / acetone / 20 °C / 2585.81 Torr 7.2: 0.5 h / 25 - 30 °C 8.1: hydrogen bromide / dichloromethane; water; ethylene glycol / 0.5 h / 5 - 10 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 6 steps 1.1: N,N-dimethyl-formamide; thionyl chloride / dichloromethane / 3 h / Reflux 2.1: ethylmagnesium bromide; zinc(II) chloride / dichloromethane; tetrahydrofuran / 5 - 30 °C 2.2: -10 - 25 °C 3.1: sodium bis(2-methoxyethoxy)aluminium dihydride / toluene / 10 - 50 °C / Inert atmosphere; Darkness 3.2: 0 - 35 °C / pH 12 - 13 4.1: triethylamine / N,N-dimethyl-formamide / 2 h / 90 - 100 °C 4.2: 3 h / Reflux 5.1: water; sodium carbonate; methanol / 2 h / 25 - 30 °C 6.1: hydrogen; methanesulfonic acid / 5%-palladium/activated carbon / acetone 6.3: 0.5 h / 20 °C View Scheme | |
| Multi-step reaction with 7 steps 1.1: N,N-dimethyl-formamide; thionyl chloride / dichloromethane / 3 h / Reflux 2.1: ethylmagnesium bromide; zinc(II) chloride / dichloromethane; tetrahydrofuran / 5 - 30 °C 2.2: -10 - 25 °C 3.1: sodium bis(2-methoxyethoxy)aluminium dihydride / toluene / 10 - 50 °C / Inert atmosphere; Darkness 3.2: 0 - 35 °C / pH 12 - 13 4.1: triethylamine / N,N-dimethyl-formamide / 2 h / 90 - 100 °C 4.2: 3 h / Reflux 5.1: water; sodium carbonate; methanol / 2 h / 25 - 30 °C 6.1: hydrogen; methanesulfonic acid / 5%-palladium/activated carbon / acetone / 20 °C / 2585.81 Torr 6.2: 0.5 h / 25 - 30 °C 7.1: hydrogen bromide / dichloromethane; water; ethylene glycol / 0.5 h / 5 - 10 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 7 steps 1.1: N,N-dimethyl-formamide; thionyl chloride / dichloromethane / 3 h / Reflux 2.1: ethylmagnesium bromide; zinc(II) chloride / dichloromethane; tetrahydrofuran / 5 - 30 °C 2.2: -10 - 25 °C 3.1: sodium bis(2-methoxyethoxy)aluminium dihydride / toluene / 10 - 50 °C / Inert atmosphere; Darkness 3.2: 0 - 35 °C / pH 12 - 13 4.1: N,N-dimethyl-formamide / 95 - 100 °C 4.2: 0 - 5 °C / pH 8 - 9 5.1: N-ethyl-N,N-diisopropylamine / palladium diacetate; tris-(o-tolyl)phosphine / N,N-dimethyl-formamide / 90 - 95 °C / Inert atmosphere 6.1: water; sodium carbonate; methanol / 2 h / 25 - 30 °C 7.1: hydrogen; methanesulfonic acid / 5%-palladium/activated carbon / acetone 7.3: 0.5 h / 20 °C View Scheme |
-
-
1353991-67-9
(R)-2-chlorocarbonylpyrrolidine-1-carboxylic acid phenyl ester

-
-
177834-92-3
eletriptan hydrobromide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1.1: ethylmagnesium bromide; zinc(II) chloride / dichloromethane; tetrahydrofuran / 5 - 30 °C 1.2: -10 - 25 °C 2.1: sodium bis(2-methoxyethoxy)aluminium dihydride / toluene / 10 - 50 °C / Inert atmosphere; Darkness 2.2: 0 - 35 °C / pH 12 - 13 3.1: N,N-dimethyl-formamide / 95 - 100 °C 3.2: 0 - 5 °C / pH 8 - 9 4.1: N-ethyl-N,N-diisopropylamine / palladium diacetate; tris-(o-tolyl)phosphine / N,N-dimethyl-formamide / 90 - 95 °C / Inert atmosphere 5.1: water; sodium carbonate; methanol / 2 h / 25 - 30 °C 6.1: hydrogen; methanesulfonic acid / 5%-palladium/activated carbon / acetone / 20 °C / 2585.81 Torr 6.2: 0.5 h / 25 - 30 °C 7.1: hydrogen bromide / dichloromethane; water; ethylene glycol / 0.5 h / 5 - 10 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 5 steps 1.1: ethylmagnesium bromide; zinc(II) chloride / dichloromethane; tetrahydrofuran / 5 - 30 °C 1.2: -10 - 25 °C 2.1: sodium bis(2-methoxyethoxy)aluminium dihydride / toluene / 10 - 50 °C / Inert atmosphere; Darkness 2.2: 0 - 35 °C / pH 12 - 13 3.1: triethylamine / N,N-dimethyl-formamide / 2 h / 90 - 100 °C 3.2: 3 h / Reflux 4.1: water; sodium carbonate; methanol / 2 h / 25 - 30 °C 5.1: hydrogen; methanesulfonic acid / 5%-palladium/activated carbon / acetone 5.3: 0.5 h / 20 °C View Scheme | |
| Multi-step reaction with 6 steps 1.1: ethylmagnesium bromide; zinc(II) chloride / dichloromethane; tetrahydrofuran / 5 - 30 °C 1.2: -10 - 25 °C 2.1: sodium bis(2-methoxyethoxy)aluminium dihydride / toluene / 10 - 50 °C / Inert atmosphere; Darkness 2.2: 0 - 35 °C / pH 12 - 13 3.1: triethylamine / N,N-dimethyl-formamide / 2 h / 90 - 100 °C 3.2: 3 h / Reflux 4.1: water; sodium carbonate; methanol / 2 h / 25 - 30 °C 5.1: hydrogen; methanesulfonic acid / 5%-palladium/activated carbon / acetone / 20 °C / 2585.81 Torr 5.2: 0.5 h / 25 - 30 °C 6.1: hydrogen bromide / dichloromethane; water; ethylene glycol / 0.5 h / 5 - 10 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 6 steps 1.1: ethylmagnesium bromide; zinc(II) chloride / dichloromethane; tetrahydrofuran / 5 - 30 °C 1.2: -10 - 25 °C 2.1: sodium bis(2-methoxyethoxy)aluminium dihydride / toluene / 10 - 50 °C / Inert atmosphere; Darkness 2.2: 0 - 35 °C / pH 12 - 13 3.1: N,N-dimethyl-formamide / 95 - 100 °C 3.2: 0 - 5 °C / pH 8 - 9 4.1: N-ethyl-N,N-diisopropylamine / palladium diacetate; tris-(o-tolyl)phosphine / N,N-dimethyl-formamide / 90 - 95 °C / Inert atmosphere 5.1: water; sodium carbonate; methanol / 2 h / 25 - 30 °C 6.1: hydrogen; methanesulfonic acid / 5%-palladium/activated carbon / acetone 6.3: 0.5 h / 20 °C View Scheme |
-
-
1353991-68-0
(R)-2-(5-bromo-1H-indole-3-carbonyl)pyrrolidine-1-carboxylic acid phenyl ester

-
-
177834-92-3
eletriptan hydrobromide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1.1: sodium bis(2-methoxyethoxy)aluminium dihydride / toluene / 10 - 50 °C / Inert atmosphere; Darkness 1.2: 0 - 35 °C / pH 12 - 13 2.1: N,N-dimethyl-formamide / 95 - 100 °C 2.2: 0 - 5 °C / pH 8 - 9 3.1: N-ethyl-N,N-diisopropylamine / palladium diacetate; tris-(o-tolyl)phosphine / N,N-dimethyl-formamide / 90 - 95 °C / Inert atmosphere 4.1: water; sodium carbonate; methanol / 2 h / 25 - 30 °C 5.1: hydrogen; methanesulfonic acid / 5%-palladium/activated carbon / acetone / 20 °C / 2585.81 Torr 5.2: 0.5 h / 25 - 30 °C 6.1: hydrogen bromide / dichloromethane; water; ethylene glycol / 0.5 h / 5 - 10 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 4 steps 1.1: sodium bis(2-methoxyethoxy)aluminium dihydride / toluene / 10 - 50 °C / Inert atmosphere; Darkness 1.2: 0 - 35 °C / pH 12 - 13 2.1: triethylamine / N,N-dimethyl-formamide / 2 h / 90 - 100 °C 2.2: 3 h / Reflux 3.1: water; sodium carbonate; methanol / 2 h / 25 - 30 °C 4.1: hydrogen; methanesulfonic acid / 5%-palladium/activated carbon / acetone 4.3: 0.5 h / 20 °C View Scheme | |
| Multi-step reaction with 5 steps 1.1: sodium bis(2-methoxyethoxy)aluminium dihydride / toluene / 10 - 50 °C / Inert atmosphere; Darkness 1.2: 0 - 35 °C / pH 12 - 13 2.1: N,N-dimethyl-formamide / 95 - 100 °C 2.2: 0 - 5 °C / pH 8 - 9 3.1: N-ethyl-N,N-diisopropylamine / palladium diacetate; tris-(o-tolyl)phosphine / N,N-dimethyl-formamide / 90 - 95 °C / Inert atmosphere 4.1: water; sodium carbonate; methanol / 2 h / 25 - 30 °C 5.1: hydrogen; methanesulfonic acid / 5%-palladium/activated carbon / acetone 5.3: 0.5 h / 20 °C View Scheme | |
| Multi-step reaction with 5 steps 1.1: sodium bis(2-methoxyethoxy)aluminium dihydride / toluene / 10 - 50 °C / Inert atmosphere; Darkness 1.2: 0 - 35 °C / pH 12 - 13 2.1: triethylamine / N,N-dimethyl-formamide / 2 h / 90 - 100 °C 2.2: 3 h / Reflux 3.1: water; sodium carbonate; methanol / 2 h / 25 - 30 °C 4.1: hydrogen; methanesulfonic acid / 5%-palladium/activated carbon / acetone / 20 °C / 2585.81 Torr 4.2: 0.5 h / 25 - 30 °C 5.1: hydrogen bromide / dichloromethane; water; ethylene glycol / 0.5 h / 5 - 10 °C / Inert atmosphere View Scheme |
-
-
5535-48-8
PVS

-
-
143322-57-0
(R)-5-bromo-3-(N-methylpyrrolidine-2-ylmethyl)-1H-indole

-
-
177834-92-3
eletriptan hydrobromide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: triethylamine / palladium diacetate; tris-(o-tolyl)phosphine / acetonitrile / 75 - 80 °C 2.1: hydrogen / palladium 10% on activated carbon / methanol / 40 °C / 3677.86 Torr 2.2: 5 h / 25 - 70 °C View Scheme |

-
-
177834-92-3
eletriptan hydrobromide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: trifluoroacetic acid / dichloromethane / 4 h / 20 °C 2.1: methanesulfonic acid / acetone; water / 0.08 h 2.2: 6 h / 30 °C / 2250.23 Torr 3.1: hydrogen bromide / water; ethanol / 6 h / 20 °C View Scheme |

-
-
177834-92-3
eletriptan hydrobromide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: tris-(o-tolyl)phosphine; triethylamine; palladium diacetate / acetonitrile / 15 h / Inert atmosphere; Reflux 2.1: trifluoroacetic acid / dichloromethane / 4 h / 20 °C 3.1: methanesulfonic acid / acetone; water / 0.08 h 3.2: 6 h / 30 °C / 2250.23 Torr 4.1: hydrogen bromide / water; ethanol / 6 h / 20 °C View Scheme |
-
-
177834-92-3
eletriptan hydrobromide

-
-
1408337-90-5
4-methyl-8-[2-(phenylsulfonyl)ethyl]-1,2,3,5,10,10a-hexahydropyrrolidino[3,2-b]indol-4-ium

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide In water; acetonitrile at 80 - 85℃; for 0.75h; | 60% |
-
-
177834-92-3
eletriptan hydrobromide

-
-
273211-28-2
3-(N-methyl-2(R)-pyrrolidinylmethyl)-5-(2-phenylsulphonylethyl)-1H-indole hydrobromide monohydrate

| Conditions | Yield |
|---|---|
| With water at 60℃; under 20 Torr; for 16h; Product distribution / selectivity; |
Eletriptan hydrobromide Chemical Properties
Molecule structure of Eletriptan hydrobromide (CAS NO.177834-92-3):
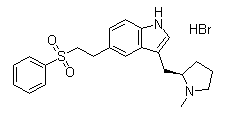
IUPAC Name: 5-[2-(Benzenesulfonyl)ethyl]-3-[[(2R)-1-methylpyrrolidin-2-yl]methyl]-1H-indole hydrobromide
Molecular Weight: 463.43098 g/mol
Molecular Formula: C22H27BrN2O2S
Flash Point: 337.2 °C
Enthalpy of Vaporization: 96.44 kJ/mol
Boiling Point: 633.9 °C at 760 mmHg
Vapour Pressure: 1.58E-16 mmHg at 25 °C
H-Bond Donor: 1
H-Bond Acceptor: 3
Rotatable Bond Count: 6
Exact Mass: 462.097661
MonoIsotopic Mass: 462.097661
Topological Polar Surface Area: 53.2
Heavy Atom Count: 28
Canonical SMILES: CN1CCCC1CC2=CNC3=C2C=C(C=C3)CCS(=O)(=O)C4=CC=CC=C4.Br
Isomeric SMILES: CN1CCC[C@@H]1CC2=CNC3=C2C=C(C=C3)CCS(=O)(=O)C4=CC=CC=C4.Br
InChI: InChI=1S/C22H26N2O2S.BrH/c1-24-12-5-6-19(24)15-18-16-23-22-10-9-17(14-21(18)22)11-13-27(25,26)20-7-3-2-4-8-20;/h2-4,7-10,14,16,19,23H,5-6,11-13,15H2,1H3;1H/t19-;/m1./s1
InChIKey of Eletriptan hydrobromide (CAS NO.177834-92-3): UTINOWOSWSPFLJ-FSRHSHDFSA-N
Eletriptan hydrobromide Specification
Eletriptan hydrobromide (CAS NO.177834-92-3) is also named as Eletriptan ; (R)-3-((1-Methyl-2-pyrrolidinyl)methyl)-5-(2-(phenylsulfonyl)ethyl)-1H-indole monohydrobromide ; 3-(((R)-1-Methyl-2-pyrrolidinyl)methyl)-5-(2-(phenylsulfonyl)ethyl)indole, monohydrobromide ; Relpax ; UK 116044-04 ; UNII-M41W832TA3 ; 1H-Indole, 3-(((2R)-1-methyl-2-yrrolidinyl))methyl)-5-(2-(phenylsulfonyl)ethyl)-, monohydrobromide .
Related Products
- Eletriptan
- Eletriptan hydrobromide
- 17783-50-5
- 177839-85-9
- 177842-14-7
- 17784-60-0
- 177854-95-4
- 17786-43-5
- 17787-40-5
- 17787-72-3
- 17788-94-2
- 17789-14-9
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View