Dayang Chem (Hangzhou) Co.,Ltd.
As a leading manufacturer and supplier of chemicals in China, DayangChem not only supply popular chemicals, but also DayangChem's R&D center offer custom synthesis services. DayangChem can provide different quantities of custom synthesis ch
Cas:191114-48-4
Min.Order:1 Kilogram
FOB Price: $3.0
Type:Lab/Research institutions
inquiryHangzhou JINLAN Pharm-Drugs Technology Co., Ltd
We can provide GMP validation service that complies with SFDA, FDA, WHO and EU EMPA.Excellent registration team could help us easlily to register our products in different countries.If you and your customer are interested in some products or need CMO
Cas:191114-48-4
Min.Order:1 Gram
Negotiable
Type:Manufacturers
inquiryAlity Chemical Corporation
The above product is Ality Chemical's strong item with best price, good quality and fast supply. Ality Chemical has been focusing on the research and production of this field for over 14 years. At the same time, we are always committed to providi
Xi'an Xszo Chem Co., Ltd.
1. Factory price and high quality must be guaranteed, base on 8 years of production and R&D experience2. Free samples will be provided,ensure specifications and quality are right for customer3. Customers will receive the most professional technical s
Cas:191114-48-4
Min.Order:1 Gram
FOB Price: $0.1
Type:Manufacturers
inquiryHebei Nengqian Chemical Import and Export Co., LTD
Our advantages: 1, High quality with competitive price: 1) Standard:BP/USP/EP/Enterprise standard 2) All Purity≥99% 3) We are manufacturer and can provide high quality products with factory price. 2, Fast and safe delivery 1) Parcel can be sent
Cas:191114-48-4
Min.Order:1 Kilogram
FOB Price: $9.0 / 99.0
Type:Trading Company
inquiryZhuozhou Wenxi import and Export Co., Ltd
WITH US,YOUR MONEY IN SAFE,YOUR BUSINESS IN SAFE 1)Quick Response Within 12 hours; 2)Quality Guarantee: All products are strictly tested by our QC, confirmed by QA and approved by third party lab in China, USA, Canada, Germany, UK, Italy, France et
Cas:191114-48-4
Min.Order:1 Kilogram
FOB Price: $139.0 / 210.0
Type:Trading Company
inquiryHebei Sankai Chemical Technology Co., Ltd
1. Product advantages ♦ High purity, all above 98.5%, no impurities after dissolution ♦ We will test each batch to ensure quality ♦ OEM and private brand services designed for free ♦ Various cap colors available ♦ W
Cas:191114-48-4
Min.Order:1 Metric Ton
FOB Price: $380.0 / 590.0
Type:Trading Company
inquiryHenan Sinotech Import&Export Corporation
High quality Telithromycin CAS 191114-48-4 Product Name: Telithromycin CAS: 191114-48-4 MF: C43H65N5O10 MW: 812 Packaging & Shipping 25kg carton drum (32cm*58cm)or 1kg foil bag (10cm*15cm) or by request of clients
Shanghai Upbio Tech Co.,Ltd
1.No Less 8 years exporting experience. Clients can 100% received goods 2.Lower Price with higher quality 3,Free sample 4,We are sincerely responsible for the "product quality" and "After Service" Upbio is Specialized
Cas:191114-48-4
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryBaoji Guokang Healthchem co.,ltd
Our company has been in existence for 10 years since its establishment. We have our own unique team. The company integrates independent research and development, production and sales. We have established famous brands at home and abroad. At prese
Cas:191114-48-4
Min.Order:1 Kilogram
FOB Price: $86.0 / 100.0
Type:Trading Company
inquiryQingdao Beluga Import and Export Co., LTD
Telithromycin CAS:191114-48-4 Qingdao Belugas Import and Export Co., Ltd. is a scientific and technological company integrating research and development, production and trade of chemical intermediates, specializing in high quality organic intermedia
Cas:191114-48-4
Min.Order:1 Gram
Negotiable
Type:Lab/Research institutions
inquiryShandong Hanjiang Chemical Co., Ltd.
Hello, dear friend! I'm Hansen and Allen from China. Welcome to my lookchem mall! The following is a brief introduction of our company's products and services. If you are interested in our products, please contact us by emai
Cas:191114-48-4
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryHenan Wentao Chemical Product Co., Ltd.
Henan Wentao Chemical Product Co.,Ltd is Located in Zhengzhou High-tech Development Zone with import and export license, We passed ISO 9001:2008 as well, Henan Wentao has developed more than 1000 compounds, which are widely used in the fields of prod
Cas:191114-48-4
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryAfine Chemicals Limited
Our Services 1. New Molecules R&D 2. Own test center HPLC NMR GC LC-MS 3. API and Intermediates from China reputed manufacturers 4. Documents support COA MOA MSDS DMF open part Our advantages 1. Government awarded company. Top 100 enter
Hangzhou Lingrui Chemical Co.,Ltd.
advantage: 1. The best price, satisfactory quality; 2. customers have the right to choose the delivery of parcels (EMS, DHL, FedEx, UPS); 3. customers have the right to choose from the recent effective packaging methods of their products packaging
Zibo Hangyu Biotechnology Development Co., Ltd
Zibo Hangyu Biotechnology Development Co., Ltd is a leading manufacturer and supplier of chemicals in China. We develop produce and distribute high quality pharmaceuticals, intermediates, special chemicals and OLED intermediates and other fine chemi
Cas:191114-48-4
Min.Order:10 Gram
FOB Price: $100.0
Type:Lab/Research institutions
inquiryTaiChem Taizhou Limited
Established in May 2015, TaiChem Ltd. is initially invested by a British research and development company and started by PhDs back from aboard. The company is registered in China Medical City (CMC), Taizhou, Jiangsu Province, and the production site
Henan Tianfu Chemical Co., Ltd.
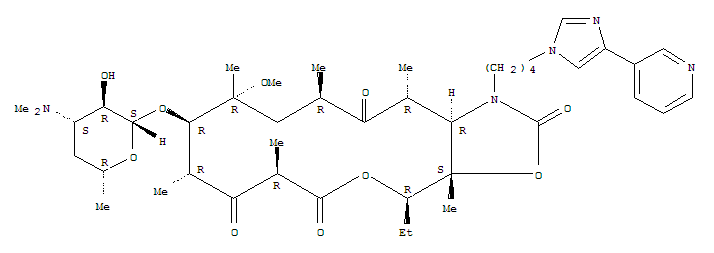
(3aS, 4R, 7R, 9R, 10R, 11R, 13R, 15R, 15aR) -4-Ethyloctahydro- 11-Methoxy-3a, 7,9,11,13,15-hexaMethyl-1- [4- [4- (3-pyridinyl) -1H-iMidazol-1-yl]
Cas:191114-48-4
Min.Order:100 Gram
Negotiable
Type:Lab/Research institutions
inquiryXiamen Jenny Chemical Technology Co., Ltd.
GMP standard, high purity, competitive price, in stock 1. Quick Response: within 6 hours after receiving your email. 2. Quality Guarantee: All products are strictly tested by our QC, confirmed by QA, and approved by a third-party lab in China, USA,
KAISA GROUP INC
1.Applied in food field.it can improve the immune system and prolong life. 2.Appliedin cosmetic field.it can improve the skin care. 3.Applied in pharmaceutical field.it can treat various dieases. 4.Our product quality assurance will make our customer
Cas:191114-48-4
Min.Order:1 Metric Ton
FOB Price: $7.0 / 8.0
Type:Trading Company
inquiryHunan chemfish Pharmaceutical co.,Ltd
Appearance:95%+ Package:R&D,Pilot run Transportation:per client require Port:Express ,Air, Sea
Bluecrystal chem-union
We are a Union of chemistry in China, consists of chemists,engineers, laboratories,factories in China. We organize surplus capacity of R&D and production as well as custom synthesis for chemical products and chemical business project. We are supp
Cas:191114-48-4
Min.Order:0
Negotiable
Type:Trading Company
inquiryHebei Mojin Biotechnology Co.,Ltd
1, High quality with competitive price:2, Fast and safe delivery3.Excellent pre-sales and after-sales service4. Well-trained and professional technologist and sales with rich experience in the field for 5-10 yearsAppearance:see detailed specification
Cas:191114-48-4
Min.Order:0
Negotiable
Type:Trading Company
inquiryGIHI CHEMICALS CO.,LIMITED
Lower price, sample is available,SDS test documents are available,large stock in warehouseAppearance:White powder Storage:Sealed and preserved Package:200/Kilograms Application:Fine chemical intermediates, used as the main raw material for the synthe
Sinoway Industrial Co., Ltd.
Why is SINOWAY:1) Specialized in pharmaceutical and healthcare industrial since 19872) ISO 9001:2015 & SGS audited supplier . 3) Accept various payment terms : T.T 30-60 days.4) We have warehouse in USA with quickly shipment .5) We can do different t
Cas:191114-48-4
Min.Order:0
Negotiable
Type:Trading Company
inquiryHangzhou Dingyan Chem Co., Ltd
R & D enterprises have their own stock in stock Package:1kg Application:pharmaceutical intermediates
Cas:191114-48-4
Min.Order:0
Negotiable
Type:Manufacturers
inquiryWuhan Fortuna Chemical Co.,Ltd
Stable stock and competitive priceAppearance:powder Storage:available Package:1KG 5KG 25KG Application:As Pharm raw material Transportation:By Courier,Sea or air
Shanghai Acmec Biochemical Technology Co., Ltd.
Acmec is a leading manufacturer and supplier of biochemical reagents and life science products. We have over 40,000 items in stock (real-time inventory) and offer discounted prices to registered members of the online store ( www.acmec.com.cn ) Appea
Cas:191114-48-4
Min.Order:1 bottle
Negotiable
Type:Lab/Research institutions
inquiryHenan Allgreen Chemical Co.,Ltd
high quality Storage:Sealed, dry, microtherm , avoid light and smell. Package:According to the demand of customer Application:Organic synthesis Transportation:by air or by sea
Aecochem Corp.
Our clients, like BASF,CHEMO,Brenntag,ASR,Evonik,Merck and etc.Appearance:COA Storage:in stock Application:MSDS/TDS
Synthetic route

-
-
191114-48-4
telithromycin

| Conditions | Yield |
|---|---|
| Stage #1: 2'-hydroxy-11-amino-11-N-[4-[4-(3-pyridyl)imidazol-1-yl]butyl]-11-deoxy-5-O-desosaminyl-6-O-methylerythronolide A 11,12-cyclic carbamate With Dess-Martin periodane In dichloromethane at 20℃; for 0.5h; Stage #2: With sodium hydrogencarbonate In dichloromethane; water for 0.5h; Product distribution / selectivity; | 90% |
| Stage #1: 2'-hydroxy-11-amino-11-N-[4-[4-(3-pyridyl)imidazol-1-yl]butyl]-11-deoxy-5-O-desosaminyl-6-O-methylerythronolide A 11,12-cyclic carbamate With Dess-Martin periodane In dichloromethane at 20℃; for 0.5h; Stage #2: With sodium hydrogencarbonate In dichloromethane for 0.5h; Product distribution / selectivity; | 90% |
| With Dess-Martin periodane In dichloromethane at 20℃; for 0.5h; Product distribution / selectivity; | |
| With pyridine hydrochloride; dimethyl sulfoxide; cyclohexyl-dimethyl-aminopropylcarbodimide hydrochloride In dichloromethane at 20 - 30℃; for 6h; Product distribution / selectivity; Pfitzner-Moffatt Oxidation; | |
| Stage #1: 2'-hydroxy-11-amino-11-N-[4-[4-(3-pyridyl)imidazol-1-yl]butyl]-11-deoxy-5-O-desosaminyl-6-O-methylerythronolide A 11,12-cyclic carbamate With N-chloro-succinimide; dimethylsulfide In dichloromethane at -25 - 0℃; for 2.5h; Corey-Kim Oxidation; Stage #2: With N-ethyl-N,N-diisopropylamine In dichloromethane for 0.5h; Product distribution / selectivity; |
-
-
1001326-77-7
2',4"-di-O-bis(trimethylsilyl)-11-amino-11-N-[4-[4-(3-pyridyl)imidazol-1-yl]butyl]-11-deoxy-6-O-methylerythromycin A-11,12-cycliccarbamate

-
-
191114-48-4
telithromycin

| Conditions | Yield |
|---|---|
| With methanol at 20℃; for 7h; Product distribution / selectivity; Heating / reflux; | 98.9% |
-
-
306770-60-5
10-(4-dimethylamino-6-methyl-3-trimethylsilanyloxy-tetrahydro-pyran-2-yloxy)-4-ethyl-11-methoxy-3a,7,9,11,13,15-hexamethyl-1-[4-(4-pyridin-3-yl-imidazol-1-yl)-butyl]-octahydro-3,5-dioxa-1-aza-cyclopentacyclotetradecene-2,6,8,14-tetraone

-
-
191114-48-4
telithromycin

| Conditions | Yield |
|---|---|
| With methanol at 20℃; for 7h; Product distribution / selectivity; Heating / reflux; | 98.9% |

| Conditions | Yield |
|---|---|
| In methanol; water; acetonitrile at 23 - 65℃; for 38h; |
-
-
306770-59-2
2'-O-acetyl-3-de[(2,6-dideoxy-3-C-methyl-3-O-methyl-α-L-ribohexopyranosyl)oxy]-11,12-dideoxy-6-O-methyl-3-oxo-12,11-[oxycarbonyl[[4-[4-(3-pyridinyl)-1H-imidazol-1-yl]butyl]imino]]erythromycin

-
-
191114-48-4
telithromycin

| Conditions | Yield |
|---|---|
| With methanol at 20℃; for 16h; Product distribution / selectivity; | |
| With isopropyl alcohol at 20℃; for 24h; Product distribution / selectivity; | |
| With sodium hydroxide; water In ethanol at 0℃; |

-
-
191114-48-4
telithromycin

| Conditions | Yield |
|---|---|
| With methanol for 7h; Heating / reflux; |
-
-
160145-83-5
2'-O-acetyl-10,11-didehydro-11-deoxy-12-O-(1H-1-imidazoylcarbonyl)-3-O-descladinosyl-3-oxo-6-O-methyl-erythromycin A

-
-
173838-63-6
4-[4-(3-pyridyl)imidazol-1-yl]butylamine

-
-
191114-48-4
telithromycin

| Conditions | Yield |
|---|---|
| In water; acetonitrile at 60℃; Substitution; | 50% |

-
-
173838-63-6
4-[4-(3-pyridyl)imidazol-1-yl]butylamine

-
-
191114-48-4
telithromycin

| Conditions | Yield |
|---|---|
| In water; acetonitrile at 50℃; for 24h; | 72% |
-
-
51746-85-1
3-(1H-imidazol-4-yl)pyridine

-
-
191114-48-4
telithromycin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: NaH / dimethylformamide 2: aq. NH2NH2 / ethanol 3: 50 percent / acetonitrile; H2O / 60 °C View Scheme |
-
-
173838-67-0
4-(3-pyridyl)-1H-imidazol-1-butanamide phthalimide

-
-
191114-48-4
telithromycin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: aq. NH2NH2 / ethanol 2: 50 percent / acetonitrile; H2O / 60 °C View Scheme |
-
-
125637-07-2
(R)-3-hydroxy-2-methylpent-1-ene

-
-
191114-48-4
telithromycin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 9 steps 1: 1H-imidazole / N,N-dimethyl-formamide / 24 h / 20 °C 2: sodium periodate; potassium osmate(VI) dihydrate; 2,6-dimethylpyridine / water; tetrahydrofuran / 3 h 3: lithium chloride / tetrahydrofuran / 1.1 h / -20 °C 4: bis(cyclopentadienyl)titanium dichloride; 2-methylpropylmagnesium chloride 5: Dess-Martin periodane / dichloromethane; water / 0.08 h / 22 °C 6: tetrabutyl ammonium fluoride / tetrahydrofuran / 1.5 h / 0 - 23 °C 7: 130 °C 8: 1,8-diazabicyclo[5.4.0]undec-7-ene / dichloromethane / 1.5 h / -15 °C 9: acetonitrile; water; methanol / 38 h / 23 - 65 °C View Scheme | |
| Multi-step reaction with 9 steps 1: 1H-imidazole / N,N-dimethyl-formamide / 24 h / 20 °C 2: sodium periodate; potassium osmate(VI) dihydrate; 2,6-dimethylpyridine / water; tetrahydrofuran / 3 h 3: lithium chloride / tetrahydrofuran / 1.1 h / -20 °C 4: bis(cyclopentadienyl)titanium dichloride; 2-methylpropylmagnesium chloride 5: Dess-Martin periodane / dichloromethane; water / 0.08 h / 22 °C 6: tetrabutyl ammonium fluoride / tetrahydrofuran / 1.5 h / 0 - 23 °C 7: chlorobenzene / 19.17 h / 23 - 150 °C / Inert atmosphere; Sonication 8: 1,8-diazabicyclo[5.4.0]undec-7-ene / dichloromethane / 1.5 h / -15 °C 9: acetonitrile; water; methanol / 38 h / 23 - 65 °C View Scheme |
-
-
135393-98-5
(R)-tert-butyldimethyl((2-methylpent-1-en-3-yl)oxy)silane

-
-
191114-48-4
telithromycin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1: sodium periodate; potassium osmate(VI) dihydrate; 2,6-dimethylpyridine / water; tetrahydrofuran / 3 h 2: lithium chloride / tetrahydrofuran / 1.1 h / -20 °C 3: bis(cyclopentadienyl)titanium dichloride; 2-methylpropylmagnesium chloride 4: Dess-Martin periodane / dichloromethane; water / 0.08 h / 22 °C 5: tetrabutyl ammonium fluoride / tetrahydrofuran / 1.5 h / 0 - 23 °C 6: 130 °C 7: 1,8-diazabicyclo[5.4.0]undec-7-ene / dichloromethane / 1.5 h / -15 °C 8: acetonitrile; water; methanol / 38 h / 23 - 65 °C View Scheme | |
| Multi-step reaction with 8 steps 1: sodium periodate; potassium osmate(VI) dihydrate; 2,6-dimethylpyridine / water; tetrahydrofuran / 3 h 2: lithium chloride / tetrahydrofuran / 1.1 h / -20 °C 3: bis(cyclopentadienyl)titanium dichloride; 2-methylpropylmagnesium chloride 4: Dess-Martin periodane / dichloromethane; water / 0.08 h / 22 °C 5: tetrabutyl ammonium fluoride / tetrahydrofuran / 1.5 h / 0 - 23 °C 6: chlorobenzene / 19.17 h / 23 - 150 °C / Inert atmosphere; Sonication 7: 1,8-diazabicyclo[5.4.0]undec-7-ene / dichloromethane / 1.5 h / -15 °C 8: acetonitrile; water; methanol / 38 h / 23 - 65 °C View Scheme |
-
-
220018-22-4
(R)-3-((tert-butyldimethylsilyl)oxy)pentan-2-one

-
-
191114-48-4
telithromycin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: lithium chloride / tetrahydrofuran / 1.1 h / -20 °C 2: bis(cyclopentadienyl)titanium dichloride; 2-methylpropylmagnesium chloride 3: Dess-Martin periodane / dichloromethane; water / 0.08 h / 22 °C 4: tetrabutyl ammonium fluoride / tetrahydrofuran / 1.5 h / 0 - 23 °C 5: 130 °C 6: 1,8-diazabicyclo[5.4.0]undec-7-ene / dichloromethane / 1.5 h / -15 °C 7: acetonitrile; water; methanol / 38 h / 23 - 65 °C View Scheme | |
| Multi-step reaction with 7 steps 1: lithium chloride / tetrahydrofuran / 1.1 h / -20 °C 2: bis(cyclopentadienyl)titanium dichloride; 2-methylpropylmagnesium chloride 3: Dess-Martin periodane / dichloromethane; water / 0.08 h / 22 °C 4: tetrabutyl ammonium fluoride / tetrahydrofuran / 1.5 h / 0 - 23 °C 5: chlorobenzene / 19.17 h / 23 - 150 °C / Inert atmosphere; Sonication 6: 1,8-diazabicyclo[5.4.0]undec-7-ene / dichloromethane / 1.5 h / -15 °C 7: acetonitrile; water; methanol / 38 h / 23 - 65 °C View Scheme |

-
-
191114-48-4
telithromycin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 10 steps 1.1: magnesium bromide ethyl etherate / dichloromethane / 0.17 h / -78 - -10 °C 1.2: 12 h / -78 °C 2.1: silver trifluoromethanesulfonate / dichloromethane; toluene / 1 h / 0 °C / Molecular sieve 3.1: hydrogen fluoride / acetonitrile / 12 h / 20 °C 4.1: Dess-Martin periodane / dichloromethane; water / 0.5 h / 20 °C 5.1: bis(cyclopentadienyl)titanium dichloride; 2-methylpropylmagnesium chloride 6.1: Dess-Martin periodane / dichloromethane; water / 0.08 h / 22 °C 7.1: tetrabutyl ammonium fluoride / tetrahydrofuran / 1.5 h / 0 - 23 °C 8.1: 130 °C 9.1: 1,8-diazabicyclo[5.4.0]undec-7-ene / dichloromethane / 1.5 h / -15 °C 10.1: acetonitrile; water; methanol / 38 h / 23 - 65 °C View Scheme | |
| Multi-step reaction with 10 steps 1.1: magnesium bromide ethyl etherate / dichloromethane / 0.17 h / -78 - -10 °C 1.2: 12 h / -78 °C 2.1: silver trifluoromethanesulfonate / dichloromethane; toluene / 1 h / 0 °C / Molecular sieve 3.1: hydrogen fluoride / acetonitrile / 12 h / 20 °C 4.1: Dess-Martin periodane / dichloromethane; water / 0.5 h / 20 °C 5.1: bis(cyclopentadienyl)titanium dichloride; 2-methylpropylmagnesium chloride 6.1: Dess-Martin periodane / dichloromethane; water / 0.08 h / 22 °C 7.1: tetrabutyl ammonium fluoride / tetrahydrofuran / 1.5 h / 0 - 23 °C 8.1: chlorobenzene / 19.17 h / 23 - 150 °C / Inert atmosphere; Sonication 9.1: 1,8-diazabicyclo[5.4.0]undec-7-ene / dichloromethane / 1.5 h / -15 °C 10.1: acetonitrile; water; methanol / 38 h / 23 - 65 °C View Scheme |

-
-
191114-48-4
telithromycin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: Dess-Martin periodane / dichloromethane; water / 0.08 h / 22 °C 2: tetrabutyl ammonium fluoride / tetrahydrofuran / 1.5 h / 0 - 23 °C 3: 130 °C 4: 1,8-diazabicyclo[5.4.0]undec-7-ene / dichloromethane / 1.5 h / -15 °C 5: acetonitrile; water; methanol / 38 h / 23 - 65 °C View Scheme | |
| Multi-step reaction with 5 steps 1: Dess-Martin periodane / dichloromethane; water / 0.08 h / 22 °C 2: tetrabutyl ammonium fluoride / tetrahydrofuran / 1.5 h / 0 - 23 °C 3: chlorobenzene / 19.17 h / 23 - 150 °C / Inert atmosphere; Sonication 4: 1,8-diazabicyclo[5.4.0]undec-7-ene / dichloromethane / 1.5 h / -15 °C 5: acetonitrile; water; methanol / 38 h / 23 - 65 °C View Scheme |

-
-
191114-48-4
telithromycin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: tetrabutyl ammonium fluoride / tetrahydrofuran / 1.5 h / 0 - 23 °C 2: 130 °C 3: 1,8-diazabicyclo[5.4.0]undec-7-ene / dichloromethane / 1.5 h / -15 °C 4: acetonitrile; water; methanol / 38 h / 23 - 65 °C View Scheme | |
| Multi-step reaction with 4 steps 1: tetrabutyl ammonium fluoride / tetrahydrofuran / 1.5 h / 0 - 23 °C 2: chlorobenzene / 19.17 h / 23 - 150 °C / Inert atmosphere; Sonication 3: 1,8-diazabicyclo[5.4.0]undec-7-ene / dichloromethane / 1.5 h / -15 °C 4: acetonitrile; water; methanol / 38 h / 23 - 65 °C View Scheme |

-
-
191114-48-4
telithromycin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: chlorobenzene / 19.17 h / 23 - 150 °C / Inert atmosphere; Sonication 2: 1,8-diazabicyclo[5.4.0]undec-7-ene / dichloromethane / 1.5 h / -15 °C 3: acetonitrile; water; methanol / 38 h / 23 - 65 °C View Scheme | |
| Multi-step reaction with 3 steps 1: 130 °C 2: 1,8-diazabicyclo[5.4.0]undec-7-ene / dichloromethane / 1.5 h / -15 °C 3: acetonitrile; water; methanol / 38 h / 23 - 65 °C View Scheme |

-
-
191114-48-4
telithromycin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 9 steps 1: silver trifluoromethanesulfonate / dichloromethane; toluene / 1 h / 0 °C / Molecular sieve 2: hydrogen fluoride / acetonitrile / 12 h / 20 °C 3: Dess-Martin periodane / dichloromethane; water / 0.5 h / 20 °C 4: bis(cyclopentadienyl)titanium dichloride; 2-methylpropylmagnesium chloride 5: Dess-Martin periodane / dichloromethane; water / 0.08 h / 22 °C 6: tetrabutyl ammonium fluoride / tetrahydrofuran / 1.5 h / 0 - 23 °C 7: 130 °C 8: 1,8-diazabicyclo[5.4.0]undec-7-ene / dichloromethane / 1.5 h / -15 °C 9: acetonitrile; water; methanol / 38 h / 23 - 65 °C View Scheme | |
| Multi-step reaction with 9 steps 1: silver trifluoromethanesulfonate / dichloromethane; toluene / 1 h / 0 °C / Molecular sieve 2: hydrogen fluoride / acetonitrile / 12 h / 20 °C 3: Dess-Martin periodane / dichloromethane; water / 0.5 h / 20 °C 4: bis(cyclopentadienyl)titanium dichloride; 2-methylpropylmagnesium chloride 5: Dess-Martin periodane / dichloromethane; water / 0.08 h / 22 °C 6: tetrabutyl ammonium fluoride / tetrahydrofuran / 1.5 h / 0 - 23 °C 7: chlorobenzene / 19.17 h / 23 - 150 °C / Inert atmosphere; Sonication 8: 1,8-diazabicyclo[5.4.0]undec-7-ene / dichloromethane / 1.5 h / -15 °C 9: acetonitrile; water; methanol / 38 h / 23 - 65 °C View Scheme |

-
-
191114-48-4
telithromycin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1: hydrogen fluoride / acetonitrile / 12 h / 20 °C 2: Dess-Martin periodane / dichloromethane; water / 0.5 h / 20 °C 3: bis(cyclopentadienyl)titanium dichloride; 2-methylpropylmagnesium chloride 4: Dess-Martin periodane / dichloromethane; water / 0.08 h / 22 °C 5: tetrabutyl ammonium fluoride / tetrahydrofuran / 1.5 h / 0 - 23 °C 6: 130 °C 7: 1,8-diazabicyclo[5.4.0]undec-7-ene / dichloromethane / 1.5 h / -15 °C 8: acetonitrile; water; methanol / 38 h / 23 - 65 °C View Scheme | |
| Multi-step reaction with 8 steps 1: hydrogen fluoride / acetonitrile / 12 h / 20 °C 2: Dess-Martin periodane / dichloromethane; water / 0.5 h / 20 °C 3: bis(cyclopentadienyl)titanium dichloride; 2-methylpropylmagnesium chloride 4: Dess-Martin periodane / dichloromethane; water / 0.08 h / 22 °C 5: tetrabutyl ammonium fluoride / tetrahydrofuran / 1.5 h / 0 - 23 °C 6: chlorobenzene / 19.17 h / 23 - 150 °C / Inert atmosphere; Sonication 7: 1,8-diazabicyclo[5.4.0]undec-7-ene / dichloromethane / 1.5 h / -15 °C 8: acetonitrile; water; methanol / 38 h / 23 - 65 °C View Scheme |

-
-
191114-48-4
telithromycin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: Dess-Martin periodane / dichloromethane; water / 0.5 h / 20 °C 2: bis(cyclopentadienyl)titanium dichloride; 2-methylpropylmagnesium chloride 3: Dess-Martin periodane / dichloromethane; water / 0.08 h / 22 °C 4: tetrabutyl ammonium fluoride / tetrahydrofuran / 1.5 h / 0 - 23 °C 5: 130 °C 6: 1,8-diazabicyclo[5.4.0]undec-7-ene / dichloromethane / 1.5 h / -15 °C 7: acetonitrile; water; methanol / 38 h / 23 - 65 °C View Scheme | |
| Multi-step reaction with 7 steps 1: Dess-Martin periodane / dichloromethane; water / 0.5 h / 20 °C 2: bis(cyclopentadienyl)titanium dichloride; 2-methylpropylmagnesium chloride 3: Dess-Martin periodane / dichloromethane; water / 0.08 h / 22 °C 4: tetrabutyl ammonium fluoride / tetrahydrofuran / 1.5 h / 0 - 23 °C 5: chlorobenzene / 19.17 h / 23 - 150 °C / Inert atmosphere; Sonication 6: 1,8-diazabicyclo[5.4.0]undec-7-ene / dichloromethane / 1.5 h / -15 °C 7: acetonitrile; water; methanol / 38 h / 23 - 65 °C View Scheme |

-
-
191114-48-4
telithromycin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: bis(cyclopentadienyl)titanium dichloride; 2-methylpropylmagnesium chloride 2: Dess-Martin periodane / dichloromethane; water / 0.08 h / 22 °C 3: tetrabutyl ammonium fluoride / tetrahydrofuran / 1.5 h / 0 - 23 °C 4: 130 °C 5: 1,8-diazabicyclo[5.4.0]undec-7-ene / dichloromethane / 1.5 h / -15 °C 6: acetonitrile; water; methanol / 38 h / 23 - 65 °C View Scheme | |
| Multi-step reaction with 6 steps 1: bis(cyclopentadienyl)titanium dichloride; 2-methylpropylmagnesium chloride 2: Dess-Martin periodane / dichloromethane; water / 0.08 h / 22 °C 3: tetrabutyl ammonium fluoride / tetrahydrofuran / 1.5 h / 0 - 23 °C 4: chlorobenzene / 19.17 h / 23 - 150 °C / Inert atmosphere; Sonication 5: 1,8-diazabicyclo[5.4.0]undec-7-ene / dichloromethane / 1.5 h / -15 °C 6: acetonitrile; water; methanol / 38 h / 23 - 65 °C View Scheme |
-
-
26735-86-4
tert-butyl 2-methyl-3-oxopentanoate

-
-
191114-48-4
telithromycin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 12 steps 1.1: acetic anhydride; sulfuric acid / 5 h / 0 - 20 °C 2.1: n-butyllithium; N-ethyl-N,N-diisopropylamine / tetrahydrofuran / 1 h / -78 - 0 °C 2.2: 3 h / -78 - 20 °C 3.1: magnesium bromide ethyl etherate / dichloromethane / 0.17 h / -78 - -10 °C 3.2: 12 h / -78 °C 4.1: silver trifluoromethanesulfonate / dichloromethane; toluene / 1 h / 0 °C / Molecular sieve 5.1: hydrogen fluoride / acetonitrile / 12 h / 20 °C 6.1: Dess-Martin periodane / dichloromethane; water / 0.5 h / 20 °C 7.1: bis(cyclopentadienyl)titanium dichloride; 2-methylpropylmagnesium chloride 8.1: Dess-Martin periodane / dichloromethane; water / 0.08 h / 22 °C 9.1: tetrabutyl ammonium fluoride / tetrahydrofuran / 1.5 h / 0 - 23 °C 10.1: 130 °C 11.1: 1,8-diazabicyclo[5.4.0]undec-7-ene / dichloromethane / 1.5 h / -15 °C 12.1: acetonitrile; water; methanol / 38 h / 23 - 65 °C View Scheme | |
| Multi-step reaction with 12 steps 1.1: acetic anhydride; sulfuric acid / 5 h / 0 - 20 °C 2.1: n-butyllithium; N-ethyl-N,N-diisopropylamine / tetrahydrofuran / 1 h / -78 - 0 °C 2.2: 3 h / -78 - 20 °C 3.1: magnesium bromide ethyl etherate / dichloromethane / 0.17 h / -78 - -10 °C 3.2: 12 h / -78 °C 4.1: silver trifluoromethanesulfonate / dichloromethane; toluene / 1 h / 0 °C / Molecular sieve 5.1: hydrogen fluoride / acetonitrile / 12 h / 20 °C 6.1: Dess-Martin periodane / dichloromethane; water / 0.5 h / 20 °C 7.1: bis(cyclopentadienyl)titanium dichloride; 2-methylpropylmagnesium chloride 8.1: Dess-Martin periodane / dichloromethane; water / 0.08 h / 22 °C 9.1: tetrabutyl ammonium fluoride / tetrahydrofuran / 1.5 h / 0 - 23 °C 10.1: chlorobenzene / 19.17 h / 23 - 150 °C / Inert atmosphere; Sonication 11.1: 1,8-diazabicyclo[5.4.0]undec-7-ene / dichloromethane / 1.5 h / -15 °C 12.1: acetonitrile; water; methanol / 38 h / 23 - 65 °C View Scheme |

-
-
191114-48-4
telithromycin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 12 steps 1.1: potassium hydride / diethyl ether; mineral oil / 1 h / 0 °C 1.2: 2 h / 20 °C 2.1: periodic acid / ethyl acetate 3.1: magnesium bromide ethyl etherate / dichloromethane / 0.17 h / -78 - -10 °C 3.2: 12 h / -78 °C 4.1: silver trifluoromethanesulfonate / dichloromethane; toluene / 1 h / 0 °C / Molecular sieve 5.1: hydrogen fluoride / acetonitrile / 12 h / 20 °C 6.1: Dess-Martin periodane / dichloromethane; water / 0.5 h / 20 °C 7.1: bis(cyclopentadienyl)titanium dichloride; 2-methylpropylmagnesium chloride 8.1: Dess-Martin periodane / dichloromethane; water / 0.08 h / 22 °C 9.1: tetrabutyl ammonium fluoride / tetrahydrofuran / 1.5 h / 0 - 23 °C 10.1: 130 °C 11.1: 1,8-diazabicyclo[5.4.0]undec-7-ene / dichloromethane / 1.5 h / -15 °C 12.1: acetonitrile; water; methanol / 38 h / 23 - 65 °C View Scheme | |
| Multi-step reaction with 12 steps 1.1: potassium hydride / diethyl ether; mineral oil / 1 h / 0 °C 1.2: 2 h / 20 °C 2.1: periodic acid / ethyl acetate 3.1: magnesium bromide ethyl etherate / dichloromethane / 0.17 h / -78 - -10 °C 3.2: 12 h / -78 °C 4.1: silver trifluoromethanesulfonate / dichloromethane; toluene / 1 h / 0 °C / Molecular sieve 5.1: hydrogen fluoride / acetonitrile / 12 h / 20 °C 6.1: Dess-Martin periodane / dichloromethane; water / 0.5 h / 20 °C 7.1: bis(cyclopentadienyl)titanium dichloride; 2-methylpropylmagnesium chloride 8.1: Dess-Martin periodane / dichloromethane; water / 0.08 h / 22 °C 9.1: tetrabutyl ammonium fluoride / tetrahydrofuran / 1.5 h / 0 - 23 °C 10.1: chlorobenzene / 19.17 h / 23 - 150 °C / Inert atmosphere; Sonication 11.1: 1,8-diazabicyclo[5.4.0]undec-7-ene / dichloromethane / 1.5 h / -15 °C 12.1: acetonitrile; water; methanol / 38 h / 23 - 65 °C View Scheme |
-
-
135425-66-0
6-ethyl-2,2,5-trimethyl-4H-1,3-dioxin-4-one

-
-
191114-48-4
telithromycin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 11 steps 1.1: n-butyllithium; N-ethyl-N,N-diisopropylamine / tetrahydrofuran / 1 h / -78 - 0 °C 1.2: 3 h / -78 - 20 °C 2.1: magnesium bromide ethyl etherate / dichloromethane / 0.17 h / -78 - -10 °C 2.2: 12 h / -78 °C 3.1: silver trifluoromethanesulfonate / dichloromethane; toluene / 1 h / 0 °C / Molecular sieve 4.1: hydrogen fluoride / acetonitrile / 12 h / 20 °C 5.1: Dess-Martin periodane / dichloromethane; water / 0.5 h / 20 °C 6.1: bis(cyclopentadienyl)titanium dichloride; 2-methylpropylmagnesium chloride 7.1: Dess-Martin periodane / dichloromethane; water / 0.08 h / 22 °C 8.1: tetrabutyl ammonium fluoride / tetrahydrofuran / 1.5 h / 0 - 23 °C 9.1: 130 °C 10.1: 1,8-diazabicyclo[5.4.0]undec-7-ene / dichloromethane / 1.5 h / -15 °C 11.1: acetonitrile; water; methanol / 38 h / 23 - 65 °C View Scheme | |
| Multi-step reaction with 11 steps 1.1: n-butyllithium; N-ethyl-N,N-diisopropylamine / tetrahydrofuran / 1 h / -78 - 0 °C 1.2: 3 h / -78 - 20 °C 2.1: magnesium bromide ethyl etherate / dichloromethane / 0.17 h / -78 - -10 °C 2.2: 12 h / -78 °C 3.1: silver trifluoromethanesulfonate / dichloromethane; toluene / 1 h / 0 °C / Molecular sieve 4.1: hydrogen fluoride / acetonitrile / 12 h / 20 °C 5.1: Dess-Martin periodane / dichloromethane; water / 0.5 h / 20 °C 6.1: bis(cyclopentadienyl)titanium dichloride; 2-methylpropylmagnesium chloride 7.1: Dess-Martin periodane / dichloromethane; water / 0.08 h / 22 °C 8.1: tetrabutyl ammonium fluoride / tetrahydrofuran / 1.5 h / 0 - 23 °C 9.1: chlorobenzene / 19.17 h / 23 - 150 °C / Inert atmosphere; Sonication 10.1: 1,8-diazabicyclo[5.4.0]undec-7-ene / dichloromethane / 1.5 h / -15 °C 11.1: acetonitrile; water; methanol / 38 h / 23 - 65 °C View Scheme |

-
-
191114-48-4
telithromycin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 10 steps 1.1: magnesium bromide ethyl etherate / dichloromethane / 0.17 h / -78 - -10 °C 1.2: 12 h / -78 °C 2.1: silver trifluoromethanesulfonate / dichloromethane; toluene / 1 h / 0 °C / Molecular sieve 3.1: hydrogen fluoride / acetonitrile / 12 h / 20 °C 4.1: Dess-Martin periodane / dichloromethane; water / 0.5 h / 20 °C 5.1: bis(cyclopentadienyl)titanium dichloride; 2-methylpropylmagnesium chloride 6.1: Dess-Martin periodane / dichloromethane; water / 0.08 h / 22 °C 7.1: tetrabutyl ammonium fluoride / tetrahydrofuran / 1.5 h / 0 - 23 °C 8.1: 130 °C 9.1: 1,8-diazabicyclo[5.4.0]undec-7-ene / dichloromethane / 1.5 h / -15 °C 10.1: acetonitrile; water; methanol / 38 h / 23 - 65 °C View Scheme | |
| Multi-step reaction with 10 steps 1.1: magnesium bromide ethyl etherate / dichloromethane / 0.17 h / -78 - -10 °C 1.2: 12 h / -78 °C 2.1: silver trifluoromethanesulfonate / dichloromethane; toluene / 1 h / 0 °C / Molecular sieve 3.1: hydrogen fluoride / acetonitrile / 12 h / 20 °C 4.1: Dess-Martin periodane / dichloromethane; water / 0.5 h / 20 °C 5.1: bis(cyclopentadienyl)titanium dichloride; 2-methylpropylmagnesium chloride 6.1: Dess-Martin periodane / dichloromethane; water / 0.08 h / 22 °C 7.1: tetrabutyl ammonium fluoride / tetrahydrofuran / 1.5 h / 0 - 23 °C 8.1: chlorobenzene / 19.17 h / 23 - 150 °C / Inert atmosphere; Sonication 9.1: 1,8-diazabicyclo[5.4.0]undec-7-ene / dichloromethane / 1.5 h / -15 °C 10.1: acetonitrile; water; methanol / 38 h / 23 - 65 °C View Scheme |

-
-
191114-48-4
telithromycin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: bis(cyclopentadienyl)titanium dichloride; 2-methylpropylmagnesium chloride 2: Dess-Martin periodane / dichloromethane; water / 0.08 h / 22 °C 3: tetrabutyl ammonium fluoride / tetrahydrofuran / 1.5 h / 0 - 23 °C 4: 130 °C 5: 1,8-diazabicyclo[5.4.0]undec-7-ene / dichloromethane / 1.5 h / -15 °C 6: acetonitrile; water; methanol / 38 h / 23 - 65 °C View Scheme | |
| Multi-step reaction with 6 steps 1: bis(cyclopentadienyl)titanium dichloride; 2-methylpropylmagnesium chloride 2: Dess-Martin periodane / dichloromethane; water / 0.08 h / 22 °C 3: tetrabutyl ammonium fluoride / tetrahydrofuran / 1.5 h / 0 - 23 °C 4: chlorobenzene / 19.17 h / 23 - 150 °C / Inert atmosphere; Sonication 5: 1,8-diazabicyclo[5.4.0]undec-7-ene / dichloromethane / 1.5 h / -15 °C 6: acetonitrile; water; methanol / 38 h / 23 - 65 °C View Scheme |

-
-
191114-48-4
telithromycin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 11 steps 1.1: periodic acid / ethyl acetate 2.1: magnesium bromide ethyl etherate / dichloromethane / 0.17 h / -78 - -10 °C 2.2: 12 h / -78 °C 3.1: silver trifluoromethanesulfonate / dichloromethane; toluene / 1 h / 0 °C / Molecular sieve 4.1: hydrogen fluoride / acetonitrile / 12 h / 20 °C 5.1: Dess-Martin periodane / dichloromethane; water / 0.5 h / 20 °C 6.1: bis(cyclopentadienyl)titanium dichloride; 2-methylpropylmagnesium chloride 7.1: Dess-Martin periodane / dichloromethane; water / 0.08 h / 22 °C 8.1: tetrabutyl ammonium fluoride / tetrahydrofuran / 1.5 h / 0 - 23 °C 9.1: 130 °C 10.1: 1,8-diazabicyclo[5.4.0]undec-7-ene / dichloromethane / 1.5 h / -15 °C 11.1: acetonitrile; water; methanol / 38 h / 23 - 65 °C View Scheme | |
| Multi-step reaction with 11 steps 1.1: periodic acid / ethyl acetate 2.1: magnesium bromide ethyl etherate / dichloromethane / 0.17 h / -78 - -10 °C 2.2: 12 h / -78 °C 3.1: silver trifluoromethanesulfonate / dichloromethane; toluene / 1 h / 0 °C / Molecular sieve 4.1: hydrogen fluoride / acetonitrile / 12 h / 20 °C 5.1: Dess-Martin periodane / dichloromethane; water / 0.5 h / 20 °C 6.1: bis(cyclopentadienyl)titanium dichloride; 2-methylpropylmagnesium chloride 7.1: Dess-Martin periodane / dichloromethane; water / 0.08 h / 22 °C 8.1: tetrabutyl ammonium fluoride / tetrahydrofuran / 1.5 h / 0 - 23 °C 9.1: chlorobenzene / 19.17 h / 23 - 150 °C / Inert atmosphere; Sonication 10.1: 1,8-diazabicyclo[5.4.0]undec-7-ene / dichloromethane / 1.5 h / -15 °C 11.1: acetonitrile; water; methanol / 38 h / 23 - 65 °C View Scheme |
-
-
152235-55-7
2',4''-di-O-acetyl-6-O-methylerythromycin A

-
-
191114-48-4
telithromycin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: pyridine / dichloromethane / 8.5 h / -5 - 20 °C 2: water; hydrogenchloride / ethanol / 6 h / 20 °C 3: phosphorus pentoxide; dimethyl sulfoxide / dichloromethane / 4 h / 20 °C 4: sodium hexamethyldisilazane / tetrahydrofuran / 4 h / 0 - 20 °C 5: water; acetonitrile / 24 h / 50 °C View Scheme |
-
-
81103-11-9
clarithromycin

-
-
191114-48-4
telithromycin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: dichloromethane / 6 h / 20 °C 2: pyridine / dichloromethane / 8.5 h / -5 - 20 °C 3: water; hydrogenchloride / ethanol / 6 h / 20 °C 4: phosphorus pentoxide; dimethyl sulfoxide / dichloromethane / 4 h / 20 °C 5: sodium hexamethyldisilazane / tetrahydrofuran / 4 h / 0 - 20 °C 6: water; acetonitrile / 24 h / 50 °C View Scheme |

-
-
191114-48-4
telithromycin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: water; hydrogenchloride / ethanol / 6 h / 20 °C 2: phosphorus pentoxide; dimethyl sulfoxide / dichloromethane / 4 h / 20 °C 3: sodium hexamethyldisilazane / tetrahydrofuran / 4 h / 0 - 20 °C 4: water; acetonitrile / 24 h / 50 °C View Scheme |
-
-
191114-48-4
telithromycin

-
-
255918-52-6
3’-N-desmethyltelithromycin

| Conditions | Yield |
|---|---|
| With N-iodo-succinimide In acetonitrile at 0 - 20℃; | 68% |
| Stage #1: telithromycin With N-iodo-succinimide In acetonitrile at 0 - 20℃; Stage #2: With water; sodium thiosulfate In acetonitrile | 68% |
| With N-iodo-succinimide In acetonitrile at 0 - 20℃; Inert atmosphere; | 68% |
| With N-iodo-succinimide In acetonitrile |
-
-
191114-48-4
telithromycin

| Conditions | Yield |
|---|---|
| Stage #1: telithromycin With oxalyl dichloride; dimethyl sulfoxide; triethylamine In dichloromethane at -78 - 20℃; Swern oxidation; Stage #2: With methanol; silica gel at 65℃; | 55% |
| Stage #1: telithromycin With oxalyl dichloride; dimethyl sulfoxide In dichloromethane at -50℃; for 0.5h; Stage #2: With triethylamine In dichloromethane at -50 - 20℃; Stage #3: With methanol at 20℃; | 44% |

| Conditions | Yield |
|---|---|
| With triethylamine In chloroform at 0 - 20℃; for 5h; |
-
-
191114-48-4
telithromycin

-
-
1384954-33-9
C43H65N5O9

| Conditions | Yield |
|---|---|
| Stage #1: telithromycin With 1,1'-Thiocarbonyldiimidazole at 20℃; Stage #2: With 2,2'-azobis(isobutyronitrile); tri-n-butyl-tin hydride |
-
-
191114-48-4
telithromycin

-
-
1384954-36-2
C46H71N5O10

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: N-iodo-succinimide / acetonitrile 2: sodium cyanoborohydride View Scheme |
-
-
191114-48-4
telithromycin

-
-
1384954-35-1
C46H71N5O9

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: N-iodo-succinimide / acetonitrile 2: sodium cyanoborohydride 3: 1,1'-Thiocarbonyldiimidazole / 20 °C View Scheme |
-
-
191114-48-4
telithromycin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: oxalyl dichloride; dimethyl sulfoxide / dichloromethane / 0.5 h / -50 °C 1.2: -50 - 20 °C 1.3: 20 °C 2.1: 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; dmap / dichloromethane View Scheme |
-
-
191114-48-4
telithromycin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: oxalyl dichloride; dimethyl sulfoxide / dichloromethane / 0.5 h / -50 °C 1.2: -50 - 20 °C 1.3: 20 °C 2.1: 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; dmap / dichloromethane 3.1: trifluoroacetic acid; water / 1 h / 0 °C 4.1: trifluoroacetic acid; dimethylsulfide / 1 h / 0 °C View Scheme |
-
-
191114-48-4
telithromycin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: oxalyl dichloride; dimethyl sulfoxide / dichloromethane / 0.5 h / -50 °C 1.2: -50 - 20 °C 1.3: 20 °C 2.1: 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; dmap / dichloromethane 3.1: trifluoroacetic acid; water / 1 h / 0 °C 4.1: trifluoroacetic acid; dimethylsulfide / 1 h / 0 °C 5.1: sodium tris(acetoxy)borohydride / tetrahydrofuran; water / 1 h View Scheme |
-
-
191114-48-4
telithromycin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: oxalyl dichloride; dimethyl sulfoxide / dichloromethane / 0.5 h / -50 °C 1.2: -50 - 20 °C 1.3: 20 °C 2.1: palladium diacetate; triphenylphosphine / tetrahydrofuran / 24 h / Inert atmosphere; Reflux View Scheme |
-
-
191114-48-4
telithromycin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: oxalyl dichloride; dimethyl sulfoxide / dichloromethane / 0.5 h / -50 °C 1.2: -50 - 20 °C 1.3: 20 °C 2.1: 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; dmap / dichloromethane 3.1: trifluoroacetic acid; water / 1 h / 0 °C View Scheme |
Related products
Raw Materials
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View





 Xi
Xi