HUNAN CHEMAPI BIOLOGICAL TECHNOLOGY CO.,LTD.
Hunan Kaimir Biotechnology Co., Ltd. is a professional technology company specializing in the research and production of raw materials and intermediates. In 2017, it was recognized as a high-tech enterprise and a small technological innovation giant
Hangzhou JINLAN Pharm-Drugs Technology Co., Ltd
196618-13-0 Application:196618-13-0
Cas:196618-13-0
Min.Order:0 Metric Ton
Negotiable
Type:Other
inquiryDayang Chem (Hangzhou) Co.,Ltd.
As a leading manufacturer and supplier of chemicals in China, DayangChem not only supply popular chemicals, but also DayangChem's R&D center offer custom synthesis services. DayangChem can provide different quantities of custom synthesis ch
Cas:196618-13-0
Min.Order:1 Kilogram
FOB Price: $3.0
Type:Lab/Research institutions
inquirySimagchem Corporation
Welcome to Simagchem, your partner in China as a premier supply of bulk specialty chemicals for industry and life science. We introduce experienced quality product and exceptional JIT service with instant market intelligence in China to benefit our
Cas:196618-13-0
Min.Order:1 Metric Ton
Negotiable
Type:Manufacturers
inquiryAlity Chemical Corporation
The above product is Ality Chemical's strong item with best price, good quality and fast supply. Ality Chemical has been focusing on the research and production of this field for over 14 years. At the same time, we are always committed to providi
Xi'an Xszo Chem Co., Ltd.
1. Factory price and high quality must be guaranteed, base on 8 years of production and R&D experience2. Free samples will be provided,ensure specifications and quality are right for customer3. Customers will receive the most professional technical s
Cas:196618-13-0
Min.Order:1 Gram
FOB Price: $0.1
Type:Manufacturers
inquiryShanghai Seasonsgreen Chemical Co.,Ltd
Shanghai Seasonsgreen Chemical is a high-tech research and development, production, sale and custom synthesis set in one high-tech chemical products enterprises. Our sales and marketing division is located in Shanghai, serving international pharmaceu
Cas:196618-13-0
Min.Order:1 Kilogram
FOB Price: $1.0
Type:Manufacturers
inquiryChemwill Asia Co., Ltd.
Our main production base is located in Xuzhou industry park. We are certified both to the ISO 9001 and ISO 14001 Standards, have a safety management system in place.Our R&D team masters core technology for process-design of target building block
Hebei Nengqian Chemical Import and Export Co., LTD
Our advantages: 1. All inquiries will be replied within 12 hours. 2. Dedication to quality, supply & service. 3. Strictly on selecting raw materials. 4. Reasonable & competitive price, fast lead time. 5. Sample is available for your eva
Cas:196618-13-0
Min.Order:1 Kilogram
FOB Price: $9.0 / 99.0
Type:Trading Company
inquiryZhuozhou Wenxi import and Export Co., Ltd
WITH US,YOUR MONEY IN SAFE,YOUR BUSINESS IN SAFE 1)Quick Response Within 12 hours; 2)Quality Guarantee: All products are strictly tested by our QC, confirmed by QA and approved by third party lab in China, USA, Canada, Germany, UK, Italy, France et
Cas:196618-13-0
Min.Order:10 Gram
FOB Price: $146.0 / 176.0
Type:Trading Company
inquiryHenan Sinotech Import&Export Corporation
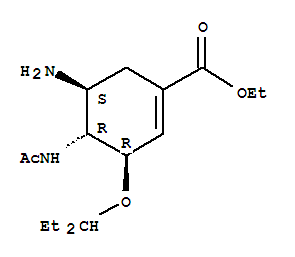
product Name Oseltamivir Synonyms Oseltamivr; ethyl (3R,4R,5S)-4-(acetylamino)-5-amino-3-(pentan-3-yloxy)cyclohex-1-ene-1-carboxylate Molecular Formula C16H28N2O4 Molecul
Qingdao Beluga Import and Export Co., LTD
Oseltamivir CAS:196618-13-0 Qingdao Belugas Import and Export Co., Ltd. is a scientific and technological company integrating research and development, production and trade of chemical intermediates, specializing in high quality organic intermediate
Cas:196618-13-0
Min.Order:1 Gram
Negotiable
Type:Lab/Research institutions
inquiryShandong Hanjiang Chemical Co., Ltd.
Hello, dear friend! I'm Hansen and Allen from China. Welcome to my lookchem mall! The following is a brief introduction of our company's products and services. If you are interested in our products, please contact us by emai
Wuhan Wonda Pharm Limited
1.high quality: quality is life. quality is the most important element for all goods. we have a lab doing research in wuhan china. hplc and nmr is available if needed. 2.reasonable price: we provide high quality products with competi
Cas:196618-13-0
Min.Order:10 Gram
Negotiable
Type:Lab/Research institutions
inquiryShanxi Ankesi Biotechnology Co., Ltd
Our Advantage 1. Rich experience We specialize in this filed for many years, our APIs exported to all over the world and and we established long friendly relations of cooperation with our clients. 2. Great quality,purity and favorable Good qual
Cas:196618-13-0
Min.Order:1 Kilogram
Negotiable
Type:Trading Company
inquiryHenan Wentao Chemical Product Co., Ltd.
Henan Wentao Chemical Product Co.,Ltd is Located in Zhengzhou High-tech Development Zone with import and export license, We passed ISO 9001:2008 as well, Henan Wentao has developed more than 1000 compounds, which are widely used in the fields of prod
Afine Chemicals Limited
Company Introduction 1. Established in 2005, with two independent business divisions: Fine chemicals division; Pharmaceutical division. 2. Main product: Optical brightener Textile auxiliary Dye stuff Pigments
Hebei Miheng Trading Co., Ltd.
Oseltamivir 196618-13-0 Our advantage 1.Top quality: Using high quality material and establishing a strict quality control system,assigning specific persons in charge of each part of production,from raw material purchase to assembly. 2 Prof
Cas:196618-13-0
Min.Order:1 Kilogram
FOB Price: $50.0 / 100.0
Type:Trading Company
inquiryHefei Zhaobo Technology Co., Ltd.
Oseltamivir CAS 196618-13-0 Description Purity: 99% Min Application: Intermediates Appearance: Powder Package: Bag Delivery: 3-5days Our Advantage & Service 1.Top quality: Using high quality material and establishing a strict quality
Cas:196618-13-0
Min.Order:1 Kilogram
FOB Price: $50.0 / 100.0
Type:Trading Company
inquiryHangzhou J&H Chemical Co., Ltd.
J&H CHEM R&D center can offer custom synthesis according to the contract research and development services for the fine chemicals, pharmaceutical, biotechnique and some of the other chemicals. J&H CHEM has some Manufacturing base in Jia
Shaanxi Cuicheng Biomedical Technology Co., Ltd.
Why Choose Us: 1. Factory direct sales, so we can provide the competitive price and high quality product base on 8 years of production and R&D experience. 2. It is available in stock for quick shipment.Products could be packaged according to cu
Hangzhou Lingrui Chemical Co.,Ltd.
our strengths: 1: Fast and guaranteed shipment (TNT;EMS;FEDEX;DHL;UPS;EUB, special line) 2: Various payment items accepted (Btc; MoneyGram; WU) 3: Valued package (Paraffin coating; Double aluminum foil bag; Vacuum packaging) 4: Efficient delivery
Cas:196618-13-0
Min.Order:1 Gram
Negotiable
Type:Other
inquiryTaiChem Taizhou Limited
Established in May 2015, TaiChem Ltd. is initially invested by a British research and development company and started by PhDs back from aboard. The company is registered in China Medical City (CMC), Taizhou, Jiangsu Province, and the production site
Henan Tianfu Chemical Co., Ltd.
Our company was built in 2009 with an ISO certificate.In the past 5 years, we have grown up as a famous fine chemicals supplier in China and we had established stable business relationships with Samsung,LG,Merck,Thermo Fisher Scientific and so on.O
Cas:196618-13-0
Min.Order:1 Metric Ton
Negotiable
Type:Lab/Research institutions
inquiryWuhan Han Sheng New Material Technology Co.,Ltd
Our company advantages: Wuhan Han Sheng New Material Technology Co. Ltd 1. Liquid: 0.5liter/bottle, 1liter/bottle, 5liter/bottle, 25liter/box, we use corrosion-resistant chemical liquid packaging plastic barrels . and double layer metal bucket
Cas:196618-13-0
Min.Order:1 Kilogram
FOB Price: $20.0 / 60.0
Type:Trading Company
inquiryZibo Dorne chemical technology co. LTD
Product Details Grade: pharmaceutical grade Purity:99%+ ProductionCapacity: 1000 Kilogram/Month Scope of use: For scientific research only(The product must be used legally) Our Advantage 1. Best quality with competitive price. 2. Quick shipping,
Cas:196618-13-0
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryXiamen Jenny Chemical Technology Co., Ltd.
GMP standard, high purity, competitive price, in stock 1. Quick Response: within 6 hours after receiving your email. 2. Quality Guarantee: All products are strictly tested by our QC, confirmed by QA, and approved by a third-party lab in China, USA,
Siwei Development Group Ltd.
Product name: Oseltamivir Phosphate CAS No.:196618-13-0 Molecule Formula:C16H28N2O4 Molecule Weight:312.40 Purity: 99.0% Package: 25kg/drum Description: White or off-white powder Manufacture Standards:Enterprise Standard
Cas:196618-13-0
Min.Order:1 Kilogram
Negotiable
Type:Trading Company
inquiryKAISA GROUP INC
1.Applied in food field.it can improve the immune system and prolong life. 2.Appliedin cosmetic field.it can improve the skin care. 3.Applied in pharmaceutical field.it can treat various dieases. 4.Our product quality assurance will make our customer
Cas:196618-13-0
Min.Order:1 Metric Ton
FOB Price: $7.0 / 8.0
Type:Trading Company
inquiryHangzhou Zhongqi chem Co.,Ltd.
Located in Hangzhou National Hi-Tech Industrial Development Zone, zhongqichem is a technical company mainly focus on the Custom synthesis, manufacturing, sales of chemicals to various industries. Benefiting from the outstanding customer service and h
Synthetic route

-
-
196618-13-0
oseltamivir

| Conditions | Yield |
|---|---|
| With 1,3-dimethylbarbituric acid; tetrakis(triphenylphosphine) palladium(0) In tetrahydrofuran at 50℃; for 2.21667h; | 88.7% |
| Stage #1: ethyl (3R,4R,5S)-4-N-acetylamino-5-N,N-diallylamino-3-(1-ethylpropoxy)-1-cyclohexene-1-carboxylate With 1,3-dimethylbarbituric acid; palladium diacetate; triphenylphosphine In ethanol at 35℃; for 2h; Inert atmosphere; Stage #2: With phosphoric acid In acetone for 2h; | 83.69% |
| With tetrakis(triphenylphosphine) palladium(0); 1,3-dimethylbarbituric acid |
-
-
367252-68-4
(3R,4R,5S)-ethyl 4-acetamido-5-((tert-butoxycarbonyl)amino)-3-(pentan-3-yloxy)cyclohex-1-enecarboxylate

-
-
196618-13-0
oseltamivir

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid In dichloromethane at 20℃; for 4h; Inert atmosphere; | 92% |
| Stage #1: (3R,4R,5S)-ethyl 4-acetamido-5-((tert-butoxycarbonyl)amino)-3-(pentan-3-yloxy)cyclohex-1-enecarboxylate With hydrogenchloride; water In ethanol at 50℃; for 1h; Stage #2: With sodium hydrogencarbonate In water | 90% |
| With hydrogenchloride In tetrahydrofuran; ethyl acetate for 0.05h; | 13.8 mg |
-
-
312904-18-0
ethyl (3R,4R,5S)-4-N-acetylamino-5-N-allylamino-3-(1-ethylpropoxy)-1-cyclohexene-1-carboxylate

-
-
196618-13-0
oseltamivir

| Conditions | Yield |
|---|---|
| With ethanolamine; palladium 10% on activated carbon In ethanol at 20℃; for 3h; Heating / reflux; | 70% |
| With palladium on activated charcoal; ethanolamine In ethanol for 3h; Heating; | |
| With ethanolamine; palladium on activated charcoal In ethanol for 3h; Heating; |

-
-
196618-13-0
oseltamivir

| Conditions | Yield |
|---|---|
| Stage #1: (1S,2R,3S,4R,5S)-4-acetylamino-3-(1-ethylpropoxy)-5-nitro-2-p-tolylsulfanylcyclohexanecarboxylic acid ethyl ester With chloro-trimethyl-silane; zinc In ethanol at 23 - 70℃; for 2h; Inert atmosphere; Stage #2: With ammonia In ethanol at 0℃; for 0.166667h; Stage #3: With potassium carbonate In ethanol at 23℃; for 6h; | 85% |
| Stage #1: (1S,2R,3S,4R,5S)-4-acetylamino-3-(1-ethylpropoxy)-5-nitro-2-p-tolylsulfanylcyclohexanecarboxylic acid ethyl ester With chloro-trimethyl-silane; zinc In ethanol at 70℃; for 2h; Inert atmosphere; Stage #2: With ammonia In ethanol at 0℃; for 0.166667h; Inert atmosphere; Stage #3: With potassium carbonate In ethanol at 23℃; for 9h; Inert atmosphere; | 81% |
| Stage #1: (1S,2R,3S,4R,5S)-4-acetylamino-3-(1-ethylpropoxy)-5-nitro-2-p-tolylsulfanylcyclohexanecarboxylic acid ethyl ester With chloro-trimethyl-silane; zinc In ethanol at 70℃; for 2h; Stage #2: With ammonia; potassium carbonate In ethanol at 20℃; for 6h; | 80% |
-
-
1041262-68-3
(3R,4R,5S)-4-acetamido-5-(1,3-dioxoisoindol-2-yl)-3-(pentyl-3-yloxy)cyclohexyl-ethyl-1-ene-1-carboxylate

-
-
196618-13-0
oseltamivir

| Conditions | Yield |
|---|---|
| With hydrazine In ethanol at 68℃; for 10h; | 100% |
| With hydrazine In ethanol at 68℃; for 10h; Inert atmosphere; | 100% |
| With hydrazine In ethanol at 68℃; for 11h; Schlenk technique; Inert atmosphere; | 86% |

-
-
196618-13-0
oseltamivir

| Conditions | Yield |
|---|---|
| With pyrrolidine; tetrakis(triphenylphosphine) palladium(0) In ethanol for 0.5h; | 95.1% |
| With 1,3-dimethylbarbituric acid; triphenylphosphine; palladium on activated charcoal In ethanol; water at 80℃; for 1h; |
-
-
1197396-47-6
(3R,4R,5S)-ethyl-4-(N-acetylacetamide)-5-(1,3-dioxoisoindolin-2-yl)-3-(3-pentyloxy)cyclohex-1-ene carboxylate

-
-
196618-13-0
oseltamivir

| Conditions | Yield |
|---|---|
| Stage #1: (3R,4R,5S)-ethyl-4-(N-acetylacetamide)-5-(1,3-dioxoisoindolin-2-yl)-3-(3-pentyloxy)cyclohex-1-ene carboxylate With hydrazine hydrate In ethanol at 50℃; for 14h; Inert atmosphere; Stage #2: With hydrogenchloride; water In ethanol Stage #3: With ammonium hydroxide In water pH=11; | 96% |

-
-
196618-13-0
oseltamivir

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid In isopropyl alcohol at 0 - 30℃; for 7h; Solvent; Reagent/catalyst; Temperature; | 96% |
-
-
204255-06-1
oseltamivir

-
-
196618-13-0
oseltamivir

| Conditions | Yield |
|---|---|
| With hydrogen at 20℃; under 380.026 Torr; for 3h; | 98.5% |
| With tributylphosphine; acetic acid In ethanol; water at 5 - 25℃; for 5.5 - 9h; | 97% |
| With triphenylphosphine In tetrahydrofuran; water at 50℃; for 10h; | 96% |

-
-
196618-13-0
oseltamivir

| Conditions | Yield |
|---|---|
| With potassium carbonate In ethanol at 23℃; for 4h; retro Michael reaction; | 91% |
| With potassium carbonate In ethanol at 23℃; for 4h; Product distribution / selectivity; | 91% |
| Stage #1: (1S,2R,3S,4R,5S)-ethyl 4-acetamido-5-amino-3-(pentan-3-yloxy)-2-(p-tolylthio)cyclohexanecarboxylate With ammonia In ethanol at 0℃; for 0.166667h; Inert atmosphere; Stage #2: With potassium carbonate In ethanol at 23℃; for 6h; Inert atmosphere; | 45.2 mg |
-
-
204255-11-8
oseltamivir phosphate

-
-
196618-13-0
oseltamivir

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In water at 20℃; for 0.0833333h; Inert atmosphere; | 98% |
| Stage #1: oseltamivir phosphate In dichloromethane; water at 25 - 30℃; for 0.166667h; Stage #2: With ammonia In dichloromethane; water pH=9 - 10; Stage #3: In n-heptane at 25 - 30℃; for 1h; Purification / work up; | |
| Stage #1: oseltamivir phosphate In dichloromethane; water at 25 - 30℃; for 0.166667h; Stage #2: With ammonia In dichloromethane; water pH=9 - 10; Stage #3: In n-heptane; toluene at 20 - 30℃; for 3.5h; Purification / work up; | |
| In dichloromethane; water at 20℃; for 0.75h; |
-
-
651324-07-1
ethyl (3R,4R,5S)-4-N-acetyl(1,1-dimethylethyl)amino-5-N,N-diallylamino-3-(1-ethylpropoxy)-1-cyclohexene-1-carboxylate

-
-
196618-13-0
oseltamivir

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: hydrogenchloride / water; ethanol / 13 - 25 °C 2: trifluoroacetic acid / 1.5 h / 50 °C 3: 1,3-dimethylbarbituric acid; triphenylphosphine; palladium diacetate / ethanol / 2 h / 35 °C / Sealed tube; Inert atmosphere View Scheme | |
| Multi-step reaction with 2 steps 1: trifluoroacetic acid / dichloromethane / 1 h / 50 °C 2: 1,3-dimethylbarbituric acid; palladium diacetate; triphenylphosphine / ethanol / 2 h / 20 - 25 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 2 steps 1: 1,3-dimethylbarbituric acid; palladium diacetate; triphenylphosphine / ethanol / 2 h / 20 - 25 °C / Inert atmosphere 2: trifluoroacetic acid / dichloromethane / 2 h / 35 - 40 °C View Scheme | |
| With 1,3-dimethylbarbituric acid; palladium diacetate; triphenylphosphine In ethanol; water at 50℃; |

-
-
196618-13-0
oseltamivir

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid In dichloromethane at 35 - 40℃; for 2h; | |
| With trifluoroacetic acid In dichloromethane at 50℃; for 2h; Reagent/catalyst; Solvent; |
-
-
891831-22-4
tert-butyl [(1S,5R,6R)-6-acetylamino-3-cyano-5-(1-ethylpropoxy)cyclohex-3-en-1-yl]carbamate

-
-
196618-13-0
oseltamivir

| Conditions | Yield |
|---|---|
| Stage #1: tert-butyl [(1S,5R,6R)-6-acetylamino-3-cyano-5-(1-ethylpropoxy)cyclohex-3-en-1-yl]carbamate With hydrogenchloride In ethanol at 60℃; for 4h; Stage #2: With water In ethanol at 4℃; for 3h; | 53% |

-
A
-
1402431-91-7
ethyl (3R,4R,5R)-4-acetylamino-5-amino-3-(pentan-3-yloxy)cyclohex-1-ene-1-carboxylate

-
B
-
196618-13-0
oseltamivir

| Conditions | Yield |
|---|---|
| With chloro-trimethyl-silane; ethanol; zinc In tetrahydrofuran; toluene at 70℃; for 2h; Flow reactor; | A n/a B n/a |
-
-
1362750-80-8
C19H35N2O4P

-
-
196618-13-0
oseltamivir

| Conditions | Yield |
|---|---|
| Stage #1: C19H35N2O4P With hydrogenchloride; water In dichloromethane; toluene at 20℃; for 4h; Stage #2: With sodium hydrogencarbonate In dichloromethane; water; toluene pH=10; | 53.2 mg |
-
-
1443055-49-9
(3R,4R,5S)-ethyl 4-acetylamino-3-(pentan-3-yloxy)-5-(2,2,2-trichloroacetamido)cyclohex-1-enecarboxylate

-
-
196618-13-0
oseltamivir

| Conditions | Yield |
|---|---|
| Stage #1: (3R,4R,5S)-ethyl 4-acetylamino-3-(pentan-3-yloxy)-5-(2,2,2-trichloroacetamido)cyclohex-1-enecarboxylate With caesium carbonate In dimethyl sulfoxide at 80℃; for 0.25h; Stage #2: With acetic acid In water; dimethyl sulfoxide at 80℃; for 0.333333h; |
-
-
949164-64-1
ethyl (3R,4R,5S)-4-acetamido-5-nitro-3-(pentan-3-yloxy)cyclohex-1-ene-1-carboxylate

-
-
196618-13-0
oseltamivir

| Conditions | Yield |
|---|---|
| With acetic acid; zinc at 20℃; for 34h; Inert atmosphere; | 81% |
| Stage #1: ethyl (3R,4R,5S)-4-acetamido-5-nitro-3-(pentan-3-yloxy)cyclohex-1-ene-1-carboxylate With acetic acid; zinc In ethanol at 70℃; Inert atmosphere; Reflux; Stage #2: With ammonium hydroxide In ethanol; water at 20℃; pH=8; Inert atmosphere; | 71% |
| With hydrogenchloride In water at 50℃; for 1h; Inert atmosphere; | |
| With chloro-trimethyl-silane; acetic acid; zinc In diethyl ether for 8h; Reflux; |
-
-
927395-63-9
(3R,4R,5S)-4-acetylamino-5-benzyloxycarbonylamino-1-cyano-3-(1-ethylpropoxy)cyclohexene

-
-
64-17-5
ethanol

-
-
196618-13-0
oseltamivir

| Conditions | Yield |
|---|---|
| Stage #1: (3R,4R,5S)-4-acetylamino-5-benzyloxycarbonylamino-1-cyano-3-(1-ethylpropoxy)cyclohexene; ethanol With hydrogenchloride at 20℃; for 24h; Stage #2: With water In ethanol at 20℃; for 2h; Stage #3: With ammonium hydroxide In ethanol at 20℃; for 10h; | 74% |
-
-
1196490-17-1
ethyl (3R,4R,5R,6R)-4-acetamido-5-azido-6-(methylsulfonyloxy)-3-(pentan-3-yloxy)cyclohex-1-enecarboxylate

-
A
-
1196490-21-7
C16H27NO4

-
B
-
1052063-39-4
ethyl (3S,4R,5R)-4-acetamido-3-amino-5-(pentan-3-yloxy)-cyclohex-1-enecarboxylate

-
C
-
196618-13-0
oseltamivir

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In tetrahydrofuran; ethanol at -30 - 20℃; |

-
-
204254-96-6
(1S,5R,6S)-5-(pentan-3-yloxy)-7-oxabicyclo[4.1.0]hept-3-ene-3-carboxylic acid ethyl ester

-
-
196618-13-0
oseltamivir

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1.1: 97 percent / MgBr2*OEt2 / acetonitrile; various solvent(s) / 16 h / 55 °C 2.1: ethanolamine; Pd/C / ethanol / 3 h / Heating 2.2: 77 percent / H2SO4 3.1: various solvent(s) 4.1: Et3N / various solvent(s) 5.1: various solvent(s) / 15 h / 111 - 112 °C / 2625.21 - 3375.27 Torr 6.1: AcOH; MeSO3H / various solvent(s) / 15 h / 20 °C 7.1: NH2CH2CH2OH; Pd/C / ethanol / 3 h / Heating View Scheme | |
| Multi-step reaction with 7 steps 1.1: MgBr2*OEt2 / acetonitrile; various solvent(s) / Heating 1.2: H2NCH2CH2OH / Pd/C / ethanol / 3 h / Heating 2.1: 6 h / 100 °C / 750.08 - 3750.38 Torr 3.1: Et3N / ethyl acetate / 0 - 20 °C 4.1: MeSO3H / ethanol / 2.67 h / Heating 5.1: ethyl acetate / 6 h / 112 °C / 750.08 - 4500.45 Torr 6.1: AcOH; MeSO3H / various solvent(s) / 0 - 20 °C 7.1: H2NCH2CH2OH / Pd/C / ethanol / 3 h / Heating View Scheme | |
| Multi-step reaction with 7 steps 1.1: MgBr2*OEt2 / acetonitrile; various solvent(s) / Heating 1.2: H2NCH2CH2OH / Pd/C / ethanol / 3 h / Heating 2.1: 6 h / 100 °C / 750.08 - 3750.38 Torr 3.1: Et3N / ethyl acetate / 0 - 20 °C 4.1: MeSO3H / ethanol / 2.67 h / Heating 5.1: ethyl acetate / 6 h / 112 °C / 750.08 - 4500.45 Torr 6.1: AcOH; MeSO3H / various solvent(s) / 0 - 20 °C 7.1: HOCH2CH2OH / Pd/C / ethanol / Heating View Scheme |
-
-
312904-12-4
ethyl (3R,4S,5R)-5-amino-3-(1-ethylpropoxy)-4-hydroxy-1-cyclohexene-1-carboxylate

-
-
196618-13-0
oseltamivir

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: various solvent(s) 2: Et3N / various solvent(s) 3: various solvent(s) / 15 h / 111 - 112 °C / 2625.21 - 3375.27 Torr 4: AcOH; MeSO3H / various solvent(s) / 15 h / 20 °C 5: NH2CH2CH2OH; Pd/C / ethanol / 3 h / Heating View Scheme | |
| Multi-step reaction with 6 steps 1: 6 h / 100 °C / 750.08 - 3750.38 Torr 2: Et3N / ethyl acetate / 0 - 20 °C 3: MeSO3H / ethanol / 2.67 h / Heating 4: ethyl acetate / 6 h / 112 °C / 750.08 - 4500.45 Torr 5: AcOH; MeSO3H / various solvent(s) / 0 - 20 °C 6: H2NCH2CH2OH / Pd/C / ethanol / 3 h / Heating View Scheme | |
| Multi-step reaction with 6 steps 1: 6 h / 100 °C / 750.08 - 3750.38 Torr 2: Et3N / ethyl acetate / 0 - 20 °C 3: MeSO3H / ethanol / 2.67 h / Heating 4: ethyl acetate / 6 h / 112 °C / 750.08 - 4500.45 Torr 5: AcOH; MeSO3H / various solvent(s) / 0 - 20 °C 6: HOCH2CH2OH / Pd/C / ethanol / Heating View Scheme | |
| Multi-step reaction with 6 steps 1: acetonitrile / 1 h / 35 °C 2: pyridine / chloroform / 3 h / -5 °C 3: pyridine / tert-butyl methyl ether / 10 h / 80 °C 4: hydrogen; palladium on activated charcoal / ethanol / 8 h / 35 °C / 760.05 Torr 5: tert-butyl methyl ether / 2 h / 40 °C 6: trifluoroacetic acid / isopropyl alcohol / 7 h / 0 - 30 °C View Scheme |
-
-
584-02-1
2-pentanol

-
-
196618-13-0
oseltamivir

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: BF3*Et2O / 2 h / 70 - 75 °C 2: pyridine, (dimethylamino)pyridine / 18 h / Ambient temperature 3: Ph3P, H2O / tetrahydrofuran / 10 h / 50 °C View Scheme | |
| Multi-step reaction with 4 steps 1: boron trifluoride diethyl etherate / 75 °C / Inert atmosphere 2: pyridine; dmap / 1 h / 150 °C / Inert atmosphere; Microwave irradiation 3: tetrabutyl ammonium fluoride / tetrahydrofuran / 0.5 h / 20 °C / Inert atmosphere 4: hydrazine / ethanol / 10 h / 68 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 4 steps 1: boron trifluoride diethyl etherate / 75 °C / Inert atmosphere 2: pyridine; dmap / 1 h / 150 °C / Inert atmosphere; Microwave irradiation 3: tetrabutyl ammonium fluoride / tetrahydrofuran / 0.5 h / 20 °C / Inert atmosphere 4: hydrazine / ethanol / 10 h / 68 °C / Inert atmosphere View Scheme |
-
-
1158724-89-0
C24H34N2O6

-
-
196618-13-0
oseltamivir

| Conditions | Yield |
|---|---|
| With hydrogenchloride In 1,4-dioxane; ethanol at 20℃; for 24h; | 83% |
-
-
1141364-90-0
2-(1-ethylpropoxy)acetaldehyde

-
-
196618-13-0
oseltamivir

| Conditions | Yield |
|---|---|
| Multi-step reaction with 9 steps 1.1: (R)-2-(diphenyl(trimethylsilyloxy)methyl)pyrrolidine; chloroacetic acid / toluene 2.1: caesium carbonate / 4 h / 0 - 20 °C 3.1: -15 °C 4.1: trifluoroacetic acid / toluene / 4 h / 20 °C 5.1: oxalyl dichloride; N,N-dimethyl-formamide / toluene / 0.33 h / 20 °C 6.1: trimethylsilylazide / toluene / 0.33 h / 20 °C 7.1: acetic acid / 48 h / 20 °C 8.1: chloro-trimethyl-silane; zinc / ethanol / 70 °C 8.2: 0 °C 9.1: potassium carbonate / 20 °C View Scheme | |
| Multi-step reaction with 5 steps 1.1: (R)-2-(diphenyl(trimethylsilyloxy)methyl)pyrrolidine; chloroacetic acid / toluene / 6 h / 23 °C / Inert atmosphere 1.2: 2 h / 23 °C / Inert atmosphere 2.1: trifluoroacetic acid / toluene / 4 h / 23 °C / Inert atmosphere 3.1: oxalyl dichloride; N,N-dimethyl-formamide / toluene / 0.5 h / 23 °C / Inert atmosphere 4.1: pyridine; trimethylsilylazide / N,N-dimethyl-formamide / 20 °C / Microflow reaction; Inert atmosphere 4.2: 110 °C / Microflow reaction; Inert atmosphere 5.1: chloro-trimethyl-silane; zinc / ethanol / 2 h / 70 °C / Inert atmosphere 5.2: 0.17 h / 0 °C / Inert atmosphere 5.3: 9 h / 23 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 3 steps 1: (2S)-2-{diphenyl[(trimethylsilyl)oxy]methyl}pyrrolidine; chloroacetic acid / dimethyl sulfoxide / 20 °C 2: 1,8-diazabicyclo[5.4.0]undec-7-ene; lithium chloride / acetonitrile / 70 °C / Inert atmosphere 3: acetic acid; zinc / 34 h / 20 °C / Inert atmosphere View Scheme |
-
-
1226768-14-4
N-((2R,3S)-1-nitro-4-oxo-3-(pentan-3-yloxy)butan-2-yl)acetamide

-
-
196618-13-0
oseltamivir

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: caesium carbonate / 3 h / 0 °C 2: 36 h / -15 °C 3: chloro-trimethyl-silane; zinc / 2 h / -15 - 70 °C 4: ammonia; potassium carbonate / 14 h / 20 °C View Scheme | |
| Multi-step reaction with 4 steps 1.1: caesium carbonate / 3 h / 0 °C 1.2: 1 h 2.1: 36 h / -15 °C 3.1: chloro-trimethyl-silane; zinc / 2 h / -15 - 70 °C 4.1: ammonia; potassium carbonate / 14 h / 20 °C View Scheme | |
| Multi-step reaction with 5 steps 1: potassium carbonate / acetonitrile / 3 h / 0 - 20 °C 2: zinc; acetic acid / ethanol / 0.5 h / 70 °C / Inert atmosphere 3: acetonitrile / 4 h / 20 °C 4: lithium chloride / dimethyl sulfoxide / 2 h / 190 °C / Inert atmosphere 5: trifluoroacetic acid / dichloromethane / 4 h / 20 °C / Inert atmosphere View Scheme |
-
-
891831-22-4
tert-butyl [(1S,5R,6R)-6-acetylamino-3-cyano-5-(1-ethylpropoxy)cyclohex-3-en-1-yl]carbamate

-
-
64-17-5
ethanol

-
-
196618-13-0
oseltamivir

| Conditions | Yield |
|---|---|
| Stage #1: tert-butyl [(1S,5R,6R)-6-acetylamino-3-cyano-5-(1-ethylpropoxy)cyclohex-3-en-1-yl]carbamate; ethanol With hydrogenchloride at 20℃; for 24h; Stage #2: With water at 4 - 20℃; for 7.5h; | 60% |
-
-
312904-15-7
ethyl (3R,4R,5S)-5-N-allylamino-4-amino-3-(1-ethylpropoxy)-1-cyclohexene-1-carboxylate

-
-
196618-13-0
oseltamivir

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: AcOH; MeSO3H / various solvent(s) / 15 h / 20 °C 2: NH2CH2CH2OH; Pd/C / ethanol / 3 h / Heating View Scheme | |
| Multi-step reaction with 2 steps 1: AcOH; MeSO3H / various solvent(s) / 0 - 20 °C 2: H2NCH2CH2OH / Pd/C / ethanol / 3 h / Heating View Scheme | |
| Multi-step reaction with 2 steps 1: AcOH; MeSO3H / various solvent(s) / 0 - 20 °C 2: HOCH2CH2OH / Pd/C / ethanol / Heating View Scheme |

-
-
144-55-8
sodium hydrogencarbonate

-
-
107-11-9
1-amino-2-propene

-
A
-
196618-13-0
oseltamivir

-
B
-
312904-15-7
ethyl (3R,4R,5S)-5-N-allylamino-4-amino-3-(1-ethylpropoxy)-1-cyclohexene-1-carboxylate

| Conditions | Yield |
|---|---|
| With sodium sulfate In ethyl acetate |

-
-
1132659-99-4
(3R,4S,5R)-4-acetylamino-3-(1-ethyl-propoxy)-5-methanesulfonyloxy-cyclohex-1-enecarboxylic acid ethyl ester

-
-
196618-13-0
oseltamivir

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: sodium azide / N,N-dimethyl-formamide / 8 h / 90 °C 2: water; triphenylphosphine / tetrahydrofuran / 8 h / Reflux View Scheme | |
| Multi-step reaction with 2 steps 1: sodium azide / N,N-dimethyl-formamide / 3 h / 80 °C 2: hydrogen / ethanol / 20 °C View Scheme | |
| Multi-step reaction with 2 steps 1.1: sodium azide / ethanol; water / 8 h / Reflux 2.1: triphenylphosphine / tetrahydrofuran / 4 h / 20 °C 2.2: Staudinger Azide Reduction / 24 h / 20 °C View Scheme |
-
-
207857-15-6
1,3-di(tert-butyloxycarbonyl)-2-(trifluoromethylsulfonyl)guanidine

-
-
196618-13-0
oseltamivir

-
-
208720-81-4
(3R,4R,5S)-5-[[(1Z)-[[(tert-butoxy)carbonyl]amino]([[(tert-butoxy)carbonyl]imino])methyl]amino]-4-acetylamino-3-(pentan-3-yloxy)cyclohex-1-ene-1-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane | 100% |
-
-
196618-13-0
oseltamivir

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol at 90℃; for 2h; | 99% |
| Stage #1: oseltamivir With potassium hydroxide; water In tetrahydrofuran at 0 - 20℃; for 1h; Stage #2: With Amberlite IR-120 In tetrahydrofuran pH=5; | 88% |
| Stage #1: oseltamivir With potassium hydroxide In methanol; water Stage #2: In methanol; water for 0.0833333h; | 79% |
-
-
196618-13-0
oseltamivir

-
-
204255-06-1
oseltamivir

| Conditions | Yield |
|---|---|
| With fluorosulfonyl azide; potassium hydrogencarbonate In tert-butyl methyl ether; water; N,N-dimethyl-formamide at 20℃; for 0.5h; | 99% |
-
-
196618-13-0
oseltamivir

-
-
204255-11-8
oseltamivir phosphate

| Conditions | Yield |
|---|---|
| With phosphoric acid In ethanol at 50℃; for 0.0166667h; Sonication; | 98% |
| With phosphoric acid In ethanol at -18.8℃; for 17h; | 89.9% |
| With phosphoric acid In ethanol at 50 - 55℃; | 88.6% |
-
-
75-89-8
2,2,2-trifluoroethanol

-
-
196618-13-0
oseltamivir

| Conditions | Yield |
|---|---|
| With copper(l) iodide; 9-azabicyclo<3.3.1>nonane-N-oxyl; oxygen; 4,4'-di-tert-butyl-2,2'-bipyridine In acetonitrile at 20℃; under 760.051 Torr; for 4h; Sealed tube; | 90% |
-
-
145013-05-4
tert-butyl N-({[(tert-butoxy)carbonyl]amino}methanethioyl)carbamate

-
-
196618-13-0
oseltamivir

| Conditions | Yield |
|---|---|
| With triethylamine; mercury dichloride In N,N-dimethyl-formamide at 0℃; | 89% |
| With triethylamine In N,N-dimethyl-formamide at 0 - 20℃; | 89% |
| With triethylamine; mercury dichloride In N,N-dimethyl-formamide at 20℃; for 10h; | 177 mg |

| Conditions | Yield |
|---|---|
| In ethanol; water at 50 - 55℃; for 1.5 - 2h; | 88% |
-
-
19350-66-4
2,6-dimethyl-1,4-dihydropyridine-3,4,5-tricarboxylic acid 3,5-diethyl ester

-
-
196618-13-0
oseltamivir

| Conditions | Yield |
|---|---|
| Stage #1: 2,6-dimethyl-1,4-dihydropyridine-3,4,5-tricarboxylic acid 3,5-diethyl ester With triethylamine; isobutyl chloroformate In dichloromethane at 0 - 20℃; for 0.5h; Inert atmosphere; Stage #2: oseltamivir In dichloromethane at 20℃; Inert atmosphere; | 87% |
-
-
34619-03-9
tert-butyldicarbonate

-
-
196618-13-0
oseltamivir

-
-
367252-68-4
(3R,4R,5S)-ethyl 4-acetamido-5-((tert-butoxycarbonyl)amino)-3-(pentan-3-yloxy)cyclohex-1-enecarboxylate

| Conditions | Yield |
|---|---|
| With triethylamine In methanol at 0 - 20℃; for 18h; | 86% |
| With triethylamine In methanol at 0 - 20℃; for 18h; | 86% |
-
-
67443-38-3
5-bromo-2-chloro-3-nitropyridine

-
-
196618-13-0
oseltamivir

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 60℃; | 82% |
-
-
196618-13-0
oseltamivir

| Conditions | Yield |
|---|---|
| With phosphoric acid In ethanol; water at 65 - 75℃; for 8h; pH=4; Temperature; pH-value; | 81.9% |

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; for 2h; | 80% |
| With triethylamine In dichloromethane at 0℃; |

-
-
196618-13-0
oseltamivir

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 2h; | 75% |
-
-
64-19-7
acetic acid

-
-
196618-13-0
oseltamivir

-
-
1191921-01-3
(3R,4R,5S)-4-acetylamino-5-acetylamino-3-(1-ethylpropoxy)-1-cyclohexene-1-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate at 20℃; | 74.5% |
| With N-ethyl-N,N-diisopropylamine; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate In dichloromethane at 20℃; |
Related products
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View