Greenutra Resource Inc
Gossypol Acetate Extract Specification: Name: Gossypol Acetate Extract Identification Standardization Asp
Cas:303-45-7
Min.Order:1 Kilogram
FOB Price: $25.0
Type:Trading Company
inquiryDayang Chem (Hangzhou) Co.,Ltd.
As a leading manufacturer and supplier of chemicals in China, DayangChem not only supply popular chemicals, but also DayangChem's R&D center offer custom synthesis services. DayangChem can provide different quantities of custom synthesis ch
Cas:303-45-7
Min.Order:1 Kilogram
FOB Price: $3.0
Type:Lab/Research institutions
inquiryXi'an Xszo Chem Co., Ltd.
1. Factory price and high quality must be guaranteed, base on 8 years of production and R&D experience2. Free samples will be provided,ensure specifications and quality are right for customer3. Customers will receive the most professional technical s
Cas:303-45-7
Min.Order:1 Gram
FOB Price: $0.1
Type:Manufacturers
inquiryHebei yanxi chemical co.,LTD.
chengdu and import and export trade co., LTD., who registered capital of 10 million yuan, nearly to $2 million, we have a pharmaceutical raw materials factory production of pharmaceutical raw materials, and a reagent r&d center, and we do res
Henan Tianfu Chemical Co., Ltd.
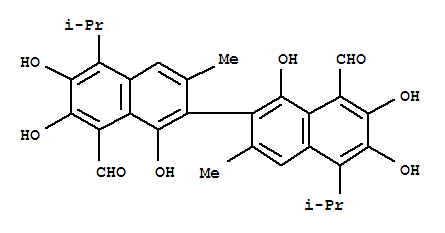
Product Name: GOSSYPOL Synonyms: 1,1',6,6',7,7'-HEXAHYDROXY-3,3'-DIMETHYL-5,5'-BIS(1-METHYLETHYL)-[2,2'-BINAPHTHALENE]-8,8'-DICARBOXALDEHYDE;2,2'-BIS[8-FORMYL-1
Cas:303-45-7
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryHebei Nengqian Chemical Import and Export Co., LTD
With our good experience, we offer detailed technical support and advice to assist customers. We communicate closely with customers to establish their quality requirements. Consistent Quality Our plant has strict quality control in each manufacturin
Cas:303-45-7
Min.Order:1 Kilogram
FOB Price: $3.0 / 10.0
Type:Trading Company
inquiryZhuozhou Wenxi import and Export Co., Ltd
WITH US,YOUR MONEY IN SAFE,YOUR BUSINESS IN SAFE 1)Quick Response Within 12 hours; 2)Quality Guarantee: All products are strictly tested by our QC, confirmed by QA and approved by third party lab in China, USA, Canada, Germany, UK, Italy, France et
Cas:303-45-7
Min.Order:1 Kilogram
FOB Price: $139.0 / 210.0
Type:Trading Company
inquiryHangzhou Sartort Biopharma Co., Ltd
Appearance:Brownish-yellow powder Storage:R.T Package:1kg/Bottle or at customers requirement Application:It has an antitumor effect and can be used as a male contraceptive Transportation:Express/Sea/Air Port:Any port in China
Cas:303-45-7
Min.Order:1 Kilogram
FOB Price: $1.0
Type:Lab/Research institutions
inquiryShanghai Upbio Tech Co.,Ltd
1.No Less 8 years exporting experience. Clients can 100% received goods 2.Lower Price with higher quality 3,Free sample 4,We are sincerely responsible for the "product quality" and "After Service" Upbio is Specialized
Cas:303-45-7
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryQingdao Beluga Import and Export Co., LTD
GOSSYPOL CAS:303-45-7 Qingdao Belugas Import and Export Co., Ltd. is a scientific and technological company integrating research and development, production and trade of chemical intermediates, specializing in high quality organic intermediates, ste
Cas:303-45-7
Min.Order:1 Gram
Negotiable
Type:Lab/Research institutions
inquiryShandong Hanjiang Chemical Co., Ltd.
Hello, dear friend! I'm Hansen and Allen from China. Welcome to my lookchem mall! The following is a brief introduction of our company's products and services. If you are interested in our products, please contact us by emai
Henan Wentao Chemical Product Co., Ltd.
Henan Wentao Chemical Product Co.,Ltd is Located in Zhengzhou High-tech Development Zone with import and export license, We passed ISO 9001:2008 as well, Henan Wentao has developed more than 1000 compounds, which are widely used in the fields of prod
Cas:303-45-7
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryChengdu Biopurify Phytochemicals Ltd.
Chengdu Biopurify Phytochemicals Ltd. is a leading company in the research, development, manufacture and marketing of High Quality Phytochemicals and Extracts(especially Active Ingredients from Traditional Chinese Medicine,Traditional Chinese Medic
Cas:303-45-7
Min.Order:0 Metric Ton
Negotiable
Type:Lab/Research institutions
inquiryHangzhou Keyingchem Co.,Ltd
Hangzhou KeyingChem Co., Ltd. exported this product to many countries and regions at best price. If you are looking for the material’s manufacturer or supplier in China, KeyingChem is your best choice. Pls contact with us freely for getting det
Hangzhou Lingrui Chemical Co.,Ltd.
advantage: 1. The best price, satisfactory quality; 2. customers have the right to choose the delivery of parcels (EMS, DHL, FedEx, UPS); 3. customers have the right to choose from the recent effective packaging methods of their products packaging
Cas:303-45-7
Min.Order:1 Gram
Negotiable
Type:Other
inquiryHangzhou J&H Chemical Co., Ltd.
J&H CHEM R&D center can offer custom synthesis according to the contract research and development services for the fine chemicals, pharmaceutical, biotechnique and some of the other chemicals. J&H CHEM has some Manufacturing base in Jia
Cas:303-45-7
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryShaanxi Cuicheng Biomedical Technology Co., Ltd.
Why Choose Us: 1. Factory direct sales, so we can provide the competitive price and high quality product base on 8 years of production and R&D experience. 2. It is available in stock for quick shipment.Products could be packaged according to cu
Shanghai Massive Chemical Technology Co., Ltd.
Massive Chemical is certified with ISO9001 and ISO14001 manufacturer for this product. We will offer all documents as requirement for the materials which includes, Certificate of Analysis, Material Safety Data Sheet, and Method of Analysis and
Zibo Hangyu Biotechnology Development Co., Ltd
Zibo Hangyu Biotechnology Development Co., Ltd is a leading manufacturer and supplier of chemicals in China. We develop produce and distribute high quality pharmaceuticals, intermediates, special chemicals and OLED intermediates and other fine chemi
Xiamen Jenny Chemical Technology Co., Ltd.
GMP standard, high purity, competitive price, in stock 1. Quick Response: within 6 hours after receiving your email. 2. Quality Guarantee: All products are strictly tested by our QC, confirmed by QA, and approved by a third-party lab in China, USA,
Hangzhou Huarong Pharm Co., Ltd.
Hangzhou Huarong Pharm Co., Ltd.established since 2006 , has been actively developing specialty products for Finished Dosages, APIs, Intermediates, and Fine chemicals markets in North America, Europe, Korea, Japan, Mid-East and all over the World. Hu
Hunan chemfish Pharmaceutical co.,Ltd
Appearance:95%+ Package:R&D,Pilot run Transportation:per client require Port:Express ,Air, Sea
shanghai Tauto Biotech Co., Ltd
The quality is guaranteed. If you find the product is wrong compared with COA, we promise 100% refund or change product. COA and HPLC will be shipped out with goods. You can also inform your analysis method and we will follow your analysi
Bluecrystal chem-union
We are a Union of chemistry in China, consists of chemists,engineers, laboratories,factories in China. We organize surplus capacity of R&D and production as well as custom synthesis for chemical products and chemical business project. We are supp
Kono Chem Co.,Ltd
high purity lowest priceAppearance:solid or liquid Storage:in sealed air resistant place Package:drum and bag Application:for pharma use Transportation:by sea or air Port:Beijing or Guangzhou
GIHI CHEMICALS CO.,LIMITED
high purity,in stock Package:25kg/drum,or as per customers'demand Application:API,or Intermediates,fine chemicals Transportation:air,sea,courier
Sinoway Industrial Co., Ltd.
Why is SINOWAY:1) Specialized in pharmaceutical and healthcare industrial since 19872) ISO 9001:2015 & SGS audited supplier . 3) Accept various payment terms : T.T 30-60 days.4) We have warehouse in USA with quickly shipment . Application:API
Shanghai Acmec Biochemical Technology Co., Ltd.
Acmec is a leading manufacturer and supplier of biochemical reagents and life science products. We have over 40,000 items in stock (real-time inventory) and offer discounted prices to registered members of the online store ( www.acmec.com.cn ) Appea
Cas:303-45-7
Min.Order:1 bottle
Negotiable
Type:Lab/Research institutions
inquiryHenan Allgreen Chemical Co.,Ltd
high quality Storage:Sealed, dry, microtherm , avoid light and smell. Package:According to the demand of customer Application:Organic synthesis Transportation:by air or by sea
Chengdu Lemeitian Pharmaceutical Technology Co,. Ltd
ADVANTAGE:1. More than 3,000 Chinese medicine reference substances / standard products available from stock, issued on the same day, multiple cities can be delivered the next day2. The products are provided with COA, HPLC, NMR, quality assurance, pac
Synthetic route
-
-
5453-04-3, 866541-93-7, 1189561-66-7
(±)-gossypol acetic acid

-
-
303-45-7
(rac)-gossypol

| Conditions | Yield |
|---|---|
| With iron(III) chloride In acetone at 60℃; | 85% |
| In diethyl ether; water at 25℃; for 0.166667h; Temperature; Solvent; Sonication; | 105 mg |
-
-
40817-07-0
2,3,8-trihydroxy-4-isopropyl-6-methyl-1-naphthaldehyde

-
-
303-45-7
(rac)-gossypol

| Conditions | Yield |
|---|---|
| With tert-butyl peroxyacetate In 1,2-dichloro-ethane at 80℃; for 4h; Inert atmosphere; | 76.1% |
| With tert-butyl peroxyacetate In 1,2-dichloro-ethane at 80℃; for 2.5h; Temperature; Inert atmosphere; | 52% |
| With tert-butyl peroxyacetate In 1,2-dichloro-ethane; mineral oil at 80℃; for 2.5h; Inert atmosphere; Schlenk technique; | 41% |
| With laccase of Coriolus versicolor; oxygen at 30℃; pH=5.5; aq. phosphate buffer; |

| Conditions | Yield |
|---|---|
| With hydroquinone at 180℃; under 5 - 15 Torr; for 2.5h; | 72% |
-
-
784206-41-3
apogossypol

-
-
303-45-7
(rac)-gossypol

| Conditions | Yield |
|---|---|
| With Dichloromethyl methyl ether; titanium tetrachloride In dichloromethane at 0 - 20℃; for 5h; Inert atmosphere; | 37% |
-
-
73728-76-4
4,4'-dihydroxy-5,5'-diisopropyl-7,7'-dimethyl-bis(3H-naphtho[1,8-bc]furan-3-one)

-
-
303-45-7
(rac)-gossypol

| Conditions | Yield |
|---|---|
| In water; acetone for 48h; |
-
-
17273-29-9, 38152-98-6, 125278-11-7
Gossypol

-
-
303-45-7
(rac)-gossypol

| Conditions | Yield |
|---|---|
| With hydrogenchloride In ethanol at 25℃; Kinetics; Mechanism; Thermodynamic data; other temperatures, ΔH(excit.), ΔS(excit.), ΔG(excit.); |

-
-
303-45-7
(rac)-gossypol

| Conditions | Yield |
|---|---|
| With hydrogenchloride; acetic acid In diethyl ether Heating; |

-
-
303-45-7
(rac)-gossypol

| Conditions | Yield |
|---|---|
| With hydrogenchloride; acetic acid In diethyl ether Heating; |

-
-
303-45-7
(rac)-gossypol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: silver(l) oxide / methanol / 3 h / 20 °C 2: potassium carbonate / acetone / 5 h / 20 °C 3: dichloromethane / 0.5 h / 20 °C 4: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 5: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 6: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 7: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 7 steps 1: silver(l) oxide / 2 h / Reflux 2: potassium carbonate / acetone / 5 h / 20 °C 3: dichloromethane / 0.5 h / 20 °C 4: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 5: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 6: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 7: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 8 steps 1: silver(l) oxide / methanol / 3 h / 20 °C 2: potassium carbonate / acetone / 5 h / 20 °C 3: dichloromethane / 0.5 h / 20 °C 4: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 5: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 6: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 7: hydrogenchloride / water; acetonitrile / 5 h / 20 °C 8: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 8 steps 1: silver(l) oxide / 2 h / Reflux 2: potassium carbonate / acetone / 5 h / 20 °C 3: dichloromethane / 0.5 h / 20 °C 4: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 5: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 6: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 7: hydrogenchloride / water; acetonitrile / 5 h / 20 °C 8: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme |

-
-
303-45-7
(rac)-gossypol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: potassium carbonate / acetone / 5 h / 20 °C 2: dichloromethane / 0.5 h / 20 °C 3: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 4: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 5: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 6: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 7 steps 1: potassium carbonate / acetone / 5 h / 20 °C 2: dichloromethane / 0.5 h / 20 °C 3: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 4: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 5: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 6: hydrogenchloride / water; acetonitrile / 5 h / 20 °C 7: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme |

-
-
303-45-7
(rac)-gossypol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: dichloromethane / 0.5 h / 20 °C 2: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 3: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 4: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 5: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 6 steps 1: dichloromethane / 0.5 h / 20 °C 2: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 3: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 4: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 5: hydrogenchloride / water; acetonitrile / 5 h / 20 °C 6: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 4 steps 1.1: trifluoroacetic acid / dichloromethane / 1 h / 20 °C / Inert atmosphere; Schlenk technique 1.2: 20 °C / Inert atmosphere; Schlenk technique 2.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C / Inert atmosphere; Schlenk technique 3.1: boron tribromide / dichloromethane / 10 h / -78 - -10 °C / Inert atmosphere; Schlenk technique 4.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane; mineral oil / 2.5 h / 80 °C / Inert atmosphere; Schlenk technique View Scheme | |
| Multi-step reaction with 4 steps 1: trifluoroacetic acid / dichloromethane / 1 h / 20 °C 2: 2-Iodobenzoic acid / dimethyl sulfoxide / 12 h 3: boron tribromide / dichloromethane / 10 h / -78 - -10 °C 4: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 2.5 h / 80 °C / Inert atmosphere View Scheme |

-
-
303-45-7
(rac)-gossypol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 2: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 3: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 4 steps 1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 2: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 3: hydrogenchloride / water; acetonitrile / 5 h / 20 °C 4: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 3 steps 1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C / Inert atmosphere; Schlenk technique 2: boron tribromide / dichloromethane / 10 h / -78 - -10 °C / Inert atmosphere; Schlenk technique 3: tert-butyl peroxyacetate / 1,2-dichloro-ethane; mineral oil / 2.5 h / 80 °C / Inert atmosphere; Schlenk technique View Scheme | |
| Multi-step reaction with 3 steps 1: 2-Iodobenzoic acid / dimethyl sulfoxide / 12 h 2: boron tribromide / dichloromethane / 10 h / -78 - -10 °C 3: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 2.5 h / 80 °C / Inert atmosphere View Scheme |
-
-
50399-96-7
4-isopropyl-2,3,8-trimethoxy-6-methyl-1-naphthaldehyde

-
-
303-45-7
(rac)-gossypol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 2: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 3 steps 1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 2: hydrogenchloride / water; acetonitrile / 5 h / 20 °C 3: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 2 steps 1: boron tribromide / dichloromethane / 10 h / -78 - -10 °C / Inert atmosphere; Schlenk technique 2: tert-butyl peroxyacetate / 1,2-dichloro-ethane; mineral oil / 2.5 h / 80 °C / Inert atmosphere; Schlenk technique View Scheme | |
| Multi-step reaction with 2 steps 1: boron tribromide / dichloromethane / 10 h / -78 - -10 °C 2: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 2.5 h / 80 °C / Inert atmosphere View Scheme |
-
-
1256723-09-7
4-hydroxy-5-isopropyl-7-methylnaphtho[1,8-bc]furan-3-one

-
-
303-45-7
(rac)-gossypol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: hydrogenchloride / water; acetonitrile / 5 h / 20 °C 2: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 17 steps 1.1: acetic acid; bromine / 20.25 h / 20 °C / Cooling with ice 2.1: potassium carbonate / acetone / 15 h / 30 °C 3.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 6 h / 20 °C 3.2: 2 h / Reflux 4.1: potassium carbonate / acetone / 12 h / 20 °C 5.1: n-butyllithium / tetrahydrofuran / 0.5 h / -78 °C / Inert atmosphere 5.2: 10 h / -78 - -30 °C / Inert atmosphere 6.1: potassium tert-butylate / tetrahydrofuran / 3 h / 20 °C 7.1: acetic anhydride; sodium acetate / 2 h / Reflux 8.1: sodium hydroxide / methanol; water / Reflux 9.1: potassium carbonate / acetone / 12 h / 20 °C 10.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 10.2: 10 h / 20 °C 11.1: silver(l) oxide / methanol / 3 h / 20 °C 12.1: potassium carbonate / acetone / 5 h / 20 °C 13.1: dichloromethane / 0.5 h / 20 °C 14.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 15.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 16.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 17.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 17 steps 1.1: acetic acid; bromine / 20.25 h / 20 °C / Cooling with ice 2.1: potassium carbonate / acetone / 15 h / 30 °C 3.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 6 h / 20 °C 3.2: 2 h / Reflux 4.1: potassium carbonate / acetone / 12 h / 20 °C 5.1: n-butyllithium / tetrahydrofuran / 0.5 h / -78 °C / Inert atmosphere 5.2: 10 h / -78 - -30 °C / Inert atmosphere 6.1: potassium tert-butylate / tetrahydrofuran / 3 h / 20 °C 7.1: acetic anhydride; sodium acetate / 2 h / Reflux 8.1: sodium hydroxide / methanol; water / Reflux 9.1: potassium carbonate / acetone / 12 h / 20 °C 10.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 10.2: 10 h / 20 °C 11.1: silver(l) oxide / 2 h / Reflux 12.1: potassium carbonate / acetone / 5 h / 20 °C 13.1: dichloromethane / 0.5 h / 20 °C 14.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 15.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 16.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 17.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 18 steps 1.1: acetic acid; bromine / 20.25 h / 20 °C / Cooling with ice 2.1: potassium carbonate / acetone / 15 h / 30 °C 3.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 6 h / 20 °C 3.2: 2 h / Reflux 4.1: potassium carbonate / acetone / 12 h / 20 °C 5.1: n-butyllithium / tetrahydrofuran / 0.5 h / -78 °C / Inert atmosphere 5.2: 10 h / -78 - -30 °C / Inert atmosphere 6.1: potassium tert-butylate / tetrahydrofuran / 3 h / 20 °C 7.1: acetic anhydride; sodium acetate / 2 h / Reflux 8.1: sodium hydroxide / methanol; water / Reflux 9.1: potassium carbonate / acetone / 12 h / 20 °C 10.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 10.2: 10 h / 20 °C 11.1: silver(l) oxide / methanol / 3 h / 20 °C 12.1: potassium carbonate / acetone / 5 h / 20 °C 13.1: dichloromethane / 0.5 h / 20 °C 14.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 15.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 16.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 17.1: hydrogenchloride / water; acetonitrile / 5 h / 20 °C 18.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 18 steps 1.1: acetic acid; bromine / 20.25 h / 20 °C / Cooling with ice 2.1: potassium carbonate / acetone / 15 h / 30 °C 3.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 6 h / 20 °C 3.2: 2 h / Reflux 4.1: potassium carbonate / acetone / 12 h / 20 °C 5.1: n-butyllithium / tetrahydrofuran / 0.5 h / -78 °C / Inert atmosphere 5.2: 10 h / -78 - -30 °C / Inert atmosphere 6.1: potassium tert-butylate / tetrahydrofuran / 3 h / 20 °C 7.1: acetic anhydride; sodium acetate / 2 h / Reflux 8.1: sodium hydroxide / methanol; water / Reflux 9.1: potassium carbonate / acetone / 12 h / 20 °C 10.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 10.2: 10 h / 20 °C 11.1: silver(l) oxide / 2 h / Reflux 12.1: potassium carbonate / acetone / 5 h / 20 °C 13.1: dichloromethane / 0.5 h / 20 °C 14.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 15.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 16.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 17.1: hydrogenchloride / water; acetonitrile / 5 h / 20 °C 18.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 18 steps 1.1: tin(IV) chloride; triethylamine / toluene / 0.33 h / 20 °C / Inert atmosphere 1.2: 8 h / 100 °C / Inert atmosphere 2.1: acetic acid; bromine / 20.25 h / 20 °C / Cooling with ice 3.1: potassium carbonate / acetone / 15 h / 30 °C 4.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 6 h / 20 °C 4.2: 2 h / Reflux 5.1: potassium carbonate / acetone / 12 h / 20 °C 6.1: n-butyllithium / tetrahydrofuran / 0.5 h / -78 °C / Inert atmosphere 6.2: 10 h / -78 - -30 °C / Inert atmosphere 7.1: potassium tert-butylate / tetrahydrofuran / 3 h / 20 °C 8.1: acetic anhydride; sodium acetate / 2 h / Reflux 9.1: sodium hydroxide / methanol; water / Reflux 10.1: potassium carbonate / acetone / 12 h / 20 °C 11.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 11.2: 10 h / 20 °C 12.1: silver(l) oxide / methanol / 3 h / 20 °C 13.1: potassium carbonate / acetone / 5 h / 20 °C 14.1: dichloromethane / 0.5 h / 20 °C 15.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 16.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 17.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 18.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 18 steps 1.1: tin(IV) chloride; triethylamine / toluene / 0.33 h / 20 °C / Inert atmosphere 1.2: 8 h / 100 °C / Inert atmosphere 2.1: acetic acid; bromine / 20.25 h / 20 °C / Cooling with ice 3.1: potassium carbonate / acetone / 15 h / 30 °C 4.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 6 h / 20 °C 4.2: 2 h / Reflux 5.1: potassium carbonate / acetone / 12 h / 20 °C 6.1: n-butyllithium / tetrahydrofuran / 0.5 h / -78 °C / Inert atmosphere 6.2: 10 h / -78 - -30 °C / Inert atmosphere 7.1: potassium tert-butylate / tetrahydrofuran / 3 h / 20 °C 8.1: acetic anhydride; sodium acetate / 2 h / Reflux 9.1: sodium hydroxide / methanol; water / Reflux 10.1: potassium carbonate / acetone / 12 h / 20 °C 11.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 11.2: 10 h / 20 °C 12.1: silver(l) oxide / 2 h / Reflux 13.1: potassium carbonate / acetone / 5 h / 20 °C 14.1: dichloromethane / 0.5 h / 20 °C 15.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 16.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 17.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 18.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 19 steps 1.1: tin(IV) chloride; triethylamine / toluene / 0.33 h / 20 °C / Inert atmosphere 1.2: 8 h / 100 °C / Inert atmosphere 2.1: acetic acid; bromine / 20.25 h / 20 °C / Cooling with ice 3.1: potassium carbonate / acetone / 15 h / 30 °C 4.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 6 h / 20 °C 4.2: 2 h / Reflux 5.1: potassium carbonate / acetone / 12 h / 20 °C 6.1: n-butyllithium / tetrahydrofuran / 0.5 h / -78 °C / Inert atmosphere 6.2: 10 h / -78 - -30 °C / Inert atmosphere 7.1: potassium tert-butylate / tetrahydrofuran / 3 h / 20 °C 8.1: acetic anhydride; sodium acetate / 2 h / Reflux 9.1: sodium hydroxide / methanol; water / Reflux 10.1: potassium carbonate / acetone / 12 h / 20 °C 11.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 11.2: 10 h / 20 °C 12.1: silver(l) oxide / methanol / 3 h / 20 °C 13.1: potassium carbonate / acetone / 5 h / 20 °C 14.1: dichloromethane / 0.5 h / 20 °C 15.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 16.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 17.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 18.1: hydrogenchloride / water; acetonitrile / 5 h / 20 °C 19.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 19 steps 1.1: tin(IV) chloride; triethylamine / toluene / 0.33 h / 20 °C / Inert atmosphere 1.2: 8 h / 100 °C / Inert atmosphere 2.1: acetic acid; bromine / 20.25 h / 20 °C / Cooling with ice 3.1: potassium carbonate / acetone / 15 h / 30 °C 4.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 6 h / 20 °C 4.2: 2 h / Reflux 5.1: potassium carbonate / acetone / 12 h / 20 °C 6.1: n-butyllithium / tetrahydrofuran / 0.5 h / -78 °C / Inert atmosphere 6.2: 10 h / -78 - -30 °C / Inert atmosphere 7.1: potassium tert-butylate / tetrahydrofuran / 3 h / 20 °C 8.1: acetic anhydride; sodium acetate / 2 h / Reflux 9.1: sodium hydroxide / methanol; water / Reflux 10.1: potassium carbonate / acetone / 12 h / 20 °C 11.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 11.2: 10 h / 20 °C 12.1: silver(l) oxide / 2 h / Reflux 13.1: potassium carbonate / acetone / 5 h / 20 °C 14.1: dichloromethane / 0.5 h / 20 °C 15.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 16.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 17.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 18.1: hydrogenchloride / water; acetonitrile / 5 h / 20 °C 19.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme |

-
-
303-45-7
(rac)-gossypol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 16 steps 1.1: potassium carbonate / acetone / 15 h / 30 °C 2.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 6 h / 20 °C 2.2: 2 h / Reflux 3.1: potassium carbonate / acetone / 12 h / 20 °C 4.1: n-butyllithium / tetrahydrofuran / 0.5 h / -78 °C / Inert atmosphere 4.2: 10 h / -78 - -30 °C / Inert atmosphere 5.1: potassium tert-butylate / tetrahydrofuran / 3 h / 20 °C 6.1: acetic anhydride; sodium acetate / 2 h / Reflux 7.1: sodium hydroxide / methanol; water / Reflux 8.1: potassium carbonate / acetone / 12 h / 20 °C 9.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 9.2: 10 h / 20 °C 10.1: silver(l) oxide / methanol / 3 h / 20 °C 11.1: potassium carbonate / acetone / 5 h / 20 °C 12.1: dichloromethane / 0.5 h / 20 °C 13.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 14.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 15.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 16.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 16 steps 1.1: potassium carbonate / acetone / 15 h / 30 °C 2.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 6 h / 20 °C 2.2: 2 h / Reflux 3.1: potassium carbonate / acetone / 12 h / 20 °C 4.1: n-butyllithium / tetrahydrofuran / 0.5 h / -78 °C / Inert atmosphere 4.2: 10 h / -78 - -30 °C / Inert atmosphere 5.1: potassium tert-butylate / tetrahydrofuran / 3 h / 20 °C 6.1: acetic anhydride; sodium acetate / 2 h / Reflux 7.1: sodium hydroxide / methanol; water / Reflux 8.1: potassium carbonate / acetone / 12 h / 20 °C 9.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 9.2: 10 h / 20 °C 10.1: silver(l) oxide / 2 h / Reflux 11.1: potassium carbonate / acetone / 5 h / 20 °C 12.1: dichloromethane / 0.5 h / 20 °C 13.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 14.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 15.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 16.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 17 steps 1.1: potassium carbonate / acetone / 15 h / 30 °C 2.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 6 h / 20 °C 2.2: 2 h / Reflux 3.1: potassium carbonate / acetone / 12 h / 20 °C 4.1: n-butyllithium / tetrahydrofuran / 0.5 h / -78 °C / Inert atmosphere 4.2: 10 h / -78 - -30 °C / Inert atmosphere 5.1: potassium tert-butylate / tetrahydrofuran / 3 h / 20 °C 6.1: acetic anhydride; sodium acetate / 2 h / Reflux 7.1: sodium hydroxide / methanol; water / Reflux 8.1: potassium carbonate / acetone / 12 h / 20 °C 9.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 9.2: 10 h / 20 °C 10.1: silver(l) oxide / methanol / 3 h / 20 °C 11.1: potassium carbonate / acetone / 5 h / 20 °C 12.1: dichloromethane / 0.5 h / 20 °C 13.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 14.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 15.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 16.1: hydrogenchloride / water; acetonitrile / 5 h / 20 °C 17.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 17 steps 1.1: potassium carbonate / acetone / 15 h / 30 °C 2.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 6 h / 20 °C 2.2: 2 h / Reflux 3.1: potassium carbonate / acetone / 12 h / 20 °C 4.1: n-butyllithium / tetrahydrofuran / 0.5 h / -78 °C / Inert atmosphere 4.2: 10 h / -78 - -30 °C / Inert atmosphere 5.1: potassium tert-butylate / tetrahydrofuran / 3 h / 20 °C 6.1: acetic anhydride; sodium acetate / 2 h / Reflux 7.1: sodium hydroxide / methanol; water / Reflux 8.1: potassium carbonate / acetone / 12 h / 20 °C 9.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 9.2: 10 h / 20 °C 10.1: silver(l) oxide / 2 h / Reflux 11.1: potassium carbonate / acetone / 5 h / 20 °C 12.1: dichloromethane / 0.5 h / 20 °C 13.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 14.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 15.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 16.1: hydrogenchloride / water; acetonitrile / 5 h / 20 °C 17.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme |

-
-
303-45-7
(rac)-gossypol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 10 steps 1.1: potassium carbonate / acetone / 12 h / 20 °C 2.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 2.2: 10 h / 20 °C 3.1: silver(l) oxide / methanol / 3 h / 20 °C 4.1: potassium carbonate / acetone / 5 h / 20 °C 5.1: dichloromethane / 0.5 h / 20 °C 6.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 7.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 8.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 9.1: hydrogenchloride / water; acetonitrile / 5 h / 20 °C 10.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 10 steps 1.1: potassium carbonate / acetone / 12 h / 20 °C 2.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 2.2: 10 h / 20 °C 3.1: silver(l) oxide / 2 h / Reflux 4.1: potassium carbonate / acetone / 5 h / 20 °C 5.1: dichloromethane / 0.5 h / 20 °C 6.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 7.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 8.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 9.1: hydrogenchloride / water; acetonitrile / 5 h / 20 °C 10.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 9 steps 1.1: potassium carbonate / acetone / 12 h / 20 °C 2.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 2.2: 10 h / 20 °C 3.1: silver(l) oxide / 2 h / Reflux 4.1: potassium carbonate / acetone / 5 h / 20 °C 5.1: dichloromethane / 0.5 h / 20 °C 6.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 7.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 8.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 9.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 9 steps 1.1: potassium carbonate / acetone / 12 h / 20 °C 2.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 2.2: 10 h / 20 °C 3.1: silver(l) oxide / methanol / 3 h / 20 °C 4.1: potassium carbonate / acetone / 5 h / 20 °C 5.1: dichloromethane / 0.5 h / 20 °C 6.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 7.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 8.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 9.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme |

-
-
303-45-7
(rac)-gossypol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 15 steps 1.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 6 h / 20 °C 1.2: 2 h / Reflux 2.1: potassium carbonate / acetone / 12 h / 20 °C 3.1: n-butyllithium / tetrahydrofuran / 0.5 h / -78 °C / Inert atmosphere 3.2: 10 h / -78 - -30 °C / Inert atmosphere 4.1: potassium tert-butylate / tetrahydrofuran / 3 h / 20 °C 5.1: acetic anhydride; sodium acetate / 2 h / Reflux 6.1: sodium hydroxide / methanol; water / Reflux 7.1: potassium carbonate / acetone / 12 h / 20 °C 8.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 8.2: 10 h / 20 °C 9.1: silver(l) oxide / methanol / 3 h / 20 °C 10.1: potassium carbonate / acetone / 5 h / 20 °C 11.1: dichloromethane / 0.5 h / 20 °C 12.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 13.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 14.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 15.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 15 steps 1.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 6 h / 20 °C 1.2: 2 h / Reflux 2.1: potassium carbonate / acetone / 12 h / 20 °C 3.1: n-butyllithium / tetrahydrofuran / 0.5 h / -78 °C / Inert atmosphere 3.2: 10 h / -78 - -30 °C / Inert atmosphere 4.1: potassium tert-butylate / tetrahydrofuran / 3 h / 20 °C 5.1: acetic anhydride; sodium acetate / 2 h / Reflux 6.1: sodium hydroxide / methanol; water / Reflux 7.1: potassium carbonate / acetone / 12 h / 20 °C 8.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 8.2: 10 h / 20 °C 9.1: silver(l) oxide / 2 h / Reflux 10.1: potassium carbonate / acetone / 5 h / 20 °C 11.1: dichloromethane / 0.5 h / 20 °C 12.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 13.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 14.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 15.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 16 steps 1.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 6 h / 20 °C 1.2: 2 h / Reflux 2.1: potassium carbonate / acetone / 12 h / 20 °C 3.1: n-butyllithium / tetrahydrofuran / 0.5 h / -78 °C / Inert atmosphere 3.2: 10 h / -78 - -30 °C / Inert atmosphere 4.1: potassium tert-butylate / tetrahydrofuran / 3 h / 20 °C 5.1: acetic anhydride; sodium acetate / 2 h / Reflux 6.1: sodium hydroxide / methanol; water / Reflux 7.1: potassium carbonate / acetone / 12 h / 20 °C 8.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 8.2: 10 h / 20 °C 9.1: silver(l) oxide / methanol / 3 h / 20 °C 10.1: potassium carbonate / acetone / 5 h / 20 °C 11.1: dichloromethane / 0.5 h / 20 °C 12.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 13.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 14.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 15.1: hydrogenchloride / water; acetonitrile / 5 h / 20 °C 16.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 16 steps 1.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 6 h / 20 °C 1.2: 2 h / Reflux 2.1: potassium carbonate / acetone / 12 h / 20 °C 3.1: n-butyllithium / tetrahydrofuran / 0.5 h / -78 °C / Inert atmosphere 3.2: 10 h / -78 - -30 °C / Inert atmosphere 4.1: potassium tert-butylate / tetrahydrofuran / 3 h / 20 °C 5.1: acetic anhydride; sodium acetate / 2 h / Reflux 6.1: sodium hydroxide / methanol; water / Reflux 7.1: potassium carbonate / acetone / 12 h / 20 °C 8.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 8.2: 10 h / 20 °C 9.1: silver(l) oxide / 2 h / Reflux 10.1: potassium carbonate / acetone / 5 h / 20 °C 11.1: dichloromethane / 0.5 h / 20 °C 12.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 13.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 14.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 15.1: hydrogenchloride / water; acetonitrile / 5 h / 20 °C 16.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme |

-
-
303-45-7
(rac)-gossypol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 2: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 3: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 4: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 5 steps 1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 2: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 3: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 4: hydrogenchloride / water; acetonitrile / 5 h / 20 °C 5: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme |

-
-
303-45-7
(rac)-gossypol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 14 steps 1.1: potassium carbonate / acetone / 12 h / 20 °C 2.1: n-butyllithium / tetrahydrofuran / 0.5 h / -78 °C / Inert atmosphere 2.2: 10 h / -78 - -30 °C / Inert atmosphere 3.1: potassium tert-butylate / tetrahydrofuran / 3 h / 20 °C 4.1: acetic anhydride; sodium acetate / 2 h / Reflux 5.1: sodium hydroxide / methanol; water / Reflux 6.1: potassium carbonate / acetone / 12 h / 20 °C 7.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 7.2: 10 h / 20 °C 8.1: silver(l) oxide / methanol / 3 h / 20 °C 9.1: potassium carbonate / acetone / 5 h / 20 °C 10.1: dichloromethane / 0.5 h / 20 °C 11.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 12.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 13.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 14.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 14 steps 1.1: potassium carbonate / acetone / 12 h / 20 °C 2.1: n-butyllithium / tetrahydrofuran / 0.5 h / -78 °C / Inert atmosphere 2.2: 10 h / -78 - -30 °C / Inert atmosphere 3.1: potassium tert-butylate / tetrahydrofuran / 3 h / 20 °C 4.1: acetic anhydride; sodium acetate / 2 h / Reflux 5.1: sodium hydroxide / methanol; water / Reflux 6.1: potassium carbonate / acetone / 12 h / 20 °C 7.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 7.2: 10 h / 20 °C 8.1: silver(l) oxide / 2 h / Reflux 9.1: potassium carbonate / acetone / 5 h / 20 °C 10.1: dichloromethane / 0.5 h / 20 °C 11.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 12.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 13.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 14.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 15 steps 1.1: potassium carbonate / acetone / 12 h / 20 °C 2.1: n-butyllithium / tetrahydrofuran / 0.5 h / -78 °C / Inert atmosphere 2.2: 10 h / -78 - -30 °C / Inert atmosphere 3.1: potassium tert-butylate / tetrahydrofuran / 3 h / 20 °C 4.1: acetic anhydride; sodium acetate / 2 h / Reflux 5.1: sodium hydroxide / methanol; water / Reflux 6.1: potassium carbonate / acetone / 12 h / 20 °C 7.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 7.2: 10 h / 20 °C 8.1: silver(l) oxide / methanol / 3 h / 20 °C 9.1: potassium carbonate / acetone / 5 h / 20 °C 10.1: dichloromethane / 0.5 h / 20 °C 11.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 12.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 13.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 14.1: hydrogenchloride / water; acetonitrile / 5 h / 20 °C 15.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 15 steps 1.1: potassium carbonate / acetone / 12 h / 20 °C 2.1: n-butyllithium / tetrahydrofuran / 0.5 h / -78 °C / Inert atmosphere 2.2: 10 h / -78 - -30 °C / Inert atmosphere 3.1: potassium tert-butylate / tetrahydrofuran / 3 h / 20 °C 4.1: acetic anhydride; sodium acetate / 2 h / Reflux 5.1: sodium hydroxide / methanol; water / Reflux 6.1: potassium carbonate / acetone / 12 h / 20 °C 7.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 7.2: 10 h / 20 °C 8.1: silver(l) oxide / 2 h / Reflux 9.1: potassium carbonate / acetone / 5 h / 20 °C 10.1: dichloromethane / 0.5 h / 20 °C 11.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 12.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 13.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 14.1: hydrogenchloride / water; acetonitrile / 5 h / 20 °C 15.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme |

-
-
303-45-7
(rac)-gossypol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 13 steps 1.1: n-butyllithium / tetrahydrofuran / 0.5 h / -78 °C / Inert atmosphere 1.2: 10 h / -78 - -30 °C / Inert atmosphere 2.1: potassium tert-butylate / tetrahydrofuran / 3 h / 20 °C 3.1: acetic anhydride; sodium acetate / 2 h / Reflux 4.1: sodium hydroxide / methanol; water / Reflux 5.1: potassium carbonate / acetone / 12 h / 20 °C 6.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 6.2: 10 h / 20 °C 7.1: silver(l) oxide / methanol / 3 h / 20 °C 8.1: potassium carbonate / acetone / 5 h / 20 °C 9.1: dichloromethane / 0.5 h / 20 °C 10.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 11.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 12.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 13.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 13 steps 1.1: n-butyllithium / tetrahydrofuran / 0.5 h / -78 °C / Inert atmosphere 1.2: 10 h / -78 - -30 °C / Inert atmosphere 2.1: potassium tert-butylate / tetrahydrofuran / 3 h / 20 °C 3.1: acetic anhydride; sodium acetate / 2 h / Reflux 4.1: sodium hydroxide / methanol; water / Reflux 5.1: potassium carbonate / acetone / 12 h / 20 °C 6.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 6.2: 10 h / 20 °C 7.1: silver(l) oxide / 2 h / Reflux 8.1: potassium carbonate / acetone / 5 h / 20 °C 9.1: dichloromethane / 0.5 h / 20 °C 10.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 11.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 12.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 13.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 14 steps 1.1: n-butyllithium / tetrahydrofuran / 0.5 h / -78 °C / Inert atmosphere 1.2: 10 h / -78 - -30 °C / Inert atmosphere 2.1: potassium tert-butylate / tetrahydrofuran / 3 h / 20 °C 3.1: acetic anhydride; sodium acetate / 2 h / Reflux 4.1: sodium hydroxide / methanol; water / Reflux 5.1: potassium carbonate / acetone / 12 h / 20 °C 6.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 6.2: 10 h / 20 °C 7.1: silver(l) oxide / methanol / 3 h / 20 °C 8.1: potassium carbonate / acetone / 5 h / 20 °C 9.1: dichloromethane / 0.5 h / 20 °C 10.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 11.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 12.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 13.1: hydrogenchloride / water; acetonitrile / 5 h / 20 °C 14.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 14 steps 1.1: n-butyllithium / tetrahydrofuran / 0.5 h / -78 °C / Inert atmosphere 1.2: 10 h / -78 - -30 °C / Inert atmosphere 2.1: potassium tert-butylate / tetrahydrofuran / 3 h / 20 °C 3.1: acetic anhydride; sodium acetate / 2 h / Reflux 4.1: sodium hydroxide / methanol; water / Reflux 5.1: potassium carbonate / acetone / 12 h / 20 °C 6.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 6.2: 10 h / 20 °C 7.1: silver(l) oxide / 2 h / Reflux 8.1: potassium carbonate / acetone / 5 h / 20 °C 9.1: dichloromethane / 0.5 h / 20 °C 10.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 11.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 12.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 13.1: hydrogenchloride / water; acetonitrile / 5 h / 20 °C 14.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme |

-
-
303-45-7
(rac)-gossypol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 12 steps 1.1: potassium tert-butylate / tetrahydrofuran / 3 h / 20 °C 2.1: acetic anhydride; sodium acetate / 2 h / Reflux 3.1: sodium hydroxide / methanol; water / Reflux 4.1: potassium carbonate / acetone / 12 h / 20 °C 5.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 5.2: 10 h / 20 °C 6.1: silver(l) oxide / methanol / 3 h / 20 °C 7.1: potassium carbonate / acetone / 5 h / 20 °C 8.1: dichloromethane / 0.5 h / 20 °C 9.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 10.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 11.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 12.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 12 steps 1.1: potassium tert-butylate / tetrahydrofuran / 3 h / 20 °C 2.1: acetic anhydride; sodium acetate / 2 h / Reflux 3.1: sodium hydroxide / methanol; water / Reflux 4.1: potassium carbonate / acetone / 12 h / 20 °C 5.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 5.2: 10 h / 20 °C 6.1: silver(l) oxide / 2 h / Reflux 7.1: potassium carbonate / acetone / 5 h / 20 °C 8.1: dichloromethane / 0.5 h / 20 °C 9.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 10.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 11.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 12.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 13 steps 1.1: potassium tert-butylate / tetrahydrofuran / 3 h / 20 °C 2.1: acetic anhydride; sodium acetate / 2 h / Reflux 3.1: sodium hydroxide / methanol; water / Reflux 4.1: potassium carbonate / acetone / 12 h / 20 °C 5.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 5.2: 10 h / 20 °C 6.1: silver(l) oxide / methanol / 3 h / 20 °C 7.1: potassium carbonate / acetone / 5 h / 20 °C 8.1: dichloromethane / 0.5 h / 20 °C 9.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 10.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 11.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 12.1: hydrogenchloride / water; acetonitrile / 5 h / 20 °C 13.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 13 steps 1.1: potassium tert-butylate / tetrahydrofuran / 3 h / 20 °C 2.1: acetic anhydride; sodium acetate / 2 h / Reflux 3.1: sodium hydroxide / methanol; water / Reflux 4.1: potassium carbonate / acetone / 12 h / 20 °C 5.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 5.2: 10 h / 20 °C 6.1: silver(l) oxide / 2 h / Reflux 7.1: potassium carbonate / acetone / 5 h / 20 °C 8.1: dichloromethane / 0.5 h / 20 °C 9.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 10.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 11.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 12.1: hydrogenchloride / water; acetonitrile / 5 h / 20 °C 13.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme |

-
-
303-45-7
(rac)-gossypol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 11 steps 1.1: acetic anhydride; sodium acetate / 2 h / Reflux 2.1: sodium hydroxide / methanol; water / Reflux 3.1: potassium carbonate / acetone / 12 h / 20 °C 4.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 4.2: 10 h / 20 °C 5.1: silver(l) oxide / methanol / 3 h / 20 °C 6.1: potassium carbonate / acetone / 5 h / 20 °C 7.1: dichloromethane / 0.5 h / 20 °C 8.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 9.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 10.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 11.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 11 steps 1.1: acetic anhydride; sodium acetate / 2 h / Reflux 2.1: sodium hydroxide / methanol; water / Reflux 3.1: potassium carbonate / acetone / 12 h / 20 °C 4.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 4.2: 10 h / 20 °C 5.1: silver(l) oxide / 2 h / Reflux 6.1: potassium carbonate / acetone / 5 h / 20 °C 7.1: dichloromethane / 0.5 h / 20 °C 8.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 9.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 10.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 11.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 12 steps 1.1: acetic anhydride; sodium acetate / 2 h / Reflux 2.1: sodium hydroxide / methanol; water / Reflux 3.1: potassium carbonate / acetone / 12 h / 20 °C 4.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 4.2: 10 h / 20 °C 5.1: silver(l) oxide / methanol / 3 h / 20 °C 6.1: potassium carbonate / acetone / 5 h / 20 °C 7.1: dichloromethane / 0.5 h / 20 °C 8.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 9.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 10.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 11.1: hydrogenchloride / water; acetonitrile / 5 h / 20 °C 12.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 12 steps 1.1: acetic anhydride; sodium acetate / 2 h / Reflux 2.1: sodium hydroxide / methanol; water / Reflux 3.1: potassium carbonate / acetone / 12 h / 20 °C 4.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 4.2: 10 h / 20 °C 5.1: silver(l) oxide / 2 h / Reflux 6.1: potassium carbonate / acetone / 5 h / 20 °C 7.1: dichloromethane / 0.5 h / 20 °C 8.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 9.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 10.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 11.1: hydrogenchloride / water; acetonitrile / 5 h / 20 °C 12.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme |

-
-
303-45-7
(rac)-gossypol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 10 steps 1.1: sodium hydroxide / methanol; water / Reflux 2.1: potassium carbonate / acetone / 12 h / 20 °C 3.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 3.2: 10 h / 20 °C 4.1: silver(l) oxide / methanol / 3 h / 20 °C 5.1: potassium carbonate / acetone / 5 h / 20 °C 6.1: dichloromethane / 0.5 h / 20 °C 7.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 8.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 9.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 10.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 10 steps 1.1: sodium hydroxide / methanol; water / Reflux 2.1: potassium carbonate / acetone / 12 h / 20 °C 3.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 3.2: 10 h / 20 °C 4.1: silver(l) oxide / 2 h / Reflux 5.1: potassium carbonate / acetone / 5 h / 20 °C 6.1: dichloromethane / 0.5 h / 20 °C 7.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 8.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 9.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 10.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 11 steps 1.1: sodium hydroxide / methanol; water / Reflux 2.1: potassium carbonate / acetone / 12 h / 20 °C 3.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 3.2: 10 h / 20 °C 4.1: silver(l) oxide / methanol / 3 h / 20 °C 5.1: potassium carbonate / acetone / 5 h / 20 °C 6.1: dichloromethane / 0.5 h / 20 °C 7.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 8.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 9.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 10.1: hydrogenchloride / water; acetonitrile / 5 h / 20 °C 11.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 11 steps 1.1: sodium hydroxide / methanol; water / Reflux 2.1: potassium carbonate / acetone / 12 h / 20 °C 3.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 3.2: 10 h / 20 °C 4.1: silver(l) oxide / 2 h / Reflux 5.1: potassium carbonate / acetone / 5 h / 20 °C 6.1: dichloromethane / 0.5 h / 20 °C 7.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 8.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 9.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 10.1: hydrogenchloride / water; acetonitrile / 5 h / 20 °C 11.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme |

-
-
303-45-7
(rac)-gossypol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 1.2: 10 h / 20 °C 2.1: silver(l) oxide / 2 h / Reflux 3.1: potassium carbonate / acetone / 5 h / 20 °C 4.1: dichloromethane / 0.5 h / 20 °C 5.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 6.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 7.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 8.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 8 steps 1.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 1.2: 10 h / 20 °C 2.1: silver(l) oxide / methanol / 3 h / 20 °C 3.1: potassium carbonate / acetone / 5 h / 20 °C 4.1: dichloromethane / 0.5 h / 20 °C 5.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 6.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 7.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 8.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 9 steps 1.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 1.2: 10 h / 20 °C 2.1: silver(l) oxide / 2 h / Reflux 3.1: potassium carbonate / acetone / 5 h / 20 °C 4.1: dichloromethane / 0.5 h / 20 °C 5.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 6.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 7.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 8.1: hydrogenchloride / water; acetonitrile / 5 h / 20 °C 9.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 9 steps 1.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 1.2: 10 h / 20 °C 2.1: silver(l) oxide / methanol / 3 h / 20 °C 3.1: potassium carbonate / acetone / 5 h / 20 °C 4.1: dichloromethane / 0.5 h / 20 °C 5.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 6.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 7.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 8.1: hydrogenchloride / water; acetonitrile / 5 h / 20 °C 9.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme |

-
-
303-45-7
(rac)-gossypol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 13 steps 1.1: sodium tetrahydroborate; methanol / 0.5 h / 0 °C / Inert atmosphere; Schlenk technique 2.1: sodium hydride / tetrahydrofuran; mineral oil / 0.75 h / 0 °C / Inert atmosphere; Schlenk technique 2.2: 14 h / 0 - 20 °C / Inert atmosphere; Schlenk technique 3.1: palladium dichloride; copper(l) chloride; oxygen / water; N,N-dimethyl-formamide / 32 h / 20 °C / Schlenk technique 4.1: potassium tert-butylate / tetrahydrofuran / 0.75 h / 0 - 20 °C / Inert atmosphere; Schlenk technique 4.2: 2 h / 20 °C / Inert atmosphere; Schlenk technique 5.1: 2,3-dicyano-5,6-dichloro-p-benzoquinone / dichloromethane; water / 4 h / 20 °C / Inert atmosphere; Schlenk technique 6.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C / Inert atmosphere; Schlenk technique 7.1: 2,6-di-tert-butyl-4-methyl-phenol / chlorobenzene / 13 h / 160 °C / Schlenk technique; Inert atmosphere 8.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C / Inert atmosphere; Schlenk technique 9.1: potassium carbonate / acetonitrile / 20 h / 80 °C / Inert atmosphere; Schlenk technique 10.1: trifluoroacetic acid / dichloromethane / 1 h / 20 °C / Inert atmosphere; Schlenk technique 10.2: 20 °C / Inert atmosphere; Schlenk technique 11.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C / Inert atmosphere; Schlenk technique 12.1: boron tribromide / dichloromethane / 10 h / -78 - -10 °C / Inert atmosphere; Schlenk technique 13.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane; mineral oil / 2.5 h / 80 °C / Inert atmosphere; Schlenk technique View Scheme |

-
-
303-45-7
(rac)-gossypol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: potassium carbonate / acetonitrile / 20 h / 80 °C / Inert atmosphere; Schlenk technique 2.1: trifluoroacetic acid / dichloromethane / 1 h / 20 °C / Inert atmosphere; Schlenk technique 2.2: 20 °C / Inert atmosphere; Schlenk technique 3.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C / Inert atmosphere; Schlenk technique 4.1: boron tribromide / dichloromethane / 10 h / -78 - -10 °C / Inert atmosphere; Schlenk technique 5.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane; mineral oil / 2.5 h / 80 °C / Inert atmosphere; Schlenk technique View Scheme | |
| Multi-step reaction with 5 steps 1: potassium carbonate / acetonitrile / 4 h / 80 °C 2: trifluoroacetic acid / dichloromethane / 1 h / 20 °C 3: 2-Iodobenzoic acid / dimethyl sulfoxide / 12 h 4: boron tribromide / dichloromethane / 10 h / -78 - -10 °C 5: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 2.5 h / 80 °C / Inert atmosphere View Scheme |

| Conditions | Yield |
|---|---|
| In methanol for 5h; Ambient temperature; | 100% |
-
-
303-45-7
(rac)-gossypol

-
-
5619-09-0, 7524-52-9, 14907-27-8, 26988-71-6, 41222-70-2, 67557-19-1, 109492-62-8
methyl tryptophanate hydrochloride

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol at 45℃; for 2h; Resolution of racemate; | A 99.4% B 95% |


| Conditions | Yield |
|---|---|
| With acid In ethanol for 3h; Heating; | 98.7% |

| Conditions | Yield |
|---|---|
| at 20℃; | 98% |
-
-
303-45-7
(rac)-gossypol

-
-
14907-27-8, 5619-09-0, 7524-52-9, 26988-71-6, 41222-70-2, 67557-19-1, 109492-62-8
(R)-(+)-tryptophan methyl ester hydrochloride

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol Reflux; | 98% |
-
-
303-45-7
(rac)-gossypol

-
-
7524-52-9, 26988-71-6, 5619-09-0, 14907-27-8, 41222-70-2, 67557-19-1, 109492-62-8
(S)-tryptophan methyl ester hydrochloride

-
-
132303-09-4
C54H54N4O10

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol Reflux; | 98% |
-
-
303-45-7
(rac)-gossypol

-
-
7524-52-9, 26988-71-6, 5619-09-0, 14907-27-8, 41222-70-2, 67557-19-1, 109492-62-8
(S)-tryptophan methyl ester hydrochloride

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol at 20℃; for 0.5h; | A 97% B 94% |

-
-
303-45-7
(rac)-gossypol

-
-
75-04-7
ethylamine

-
-
307976-02-9
1,6,7,1',6',7'-hexahydroxy-5,5'-diisopropyl-3,3'-dimethyl-[2,2']binaphthyl-8,8'-dicarbaldehyde bis-ethylimine

| Conditions | Yield |
|---|---|
| In diethyl ether; isopropyl alcohol at 20 - 45℃; for 2.08333h; Condensation; | 96% |
| With isopropyl alcohol |

| Conditions | Yield |
|---|---|
| In diethyl ether; isopropyl alcohol at 20 - 45℃; for 2.08333h; Condensation; | 96% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol for 3h; Heating; | 95.8% |

| Conditions | Yield |
|---|---|
| In ethanol for 3h; Heating; | 95.31% |
-
-
303-45-7
(rac)-gossypol

-
-
784206-41-3
apogossypol

| Conditions | Yield |
|---|---|
| Stage #1: (rac)-gossypol With sodium hydroxide; acetic acid In water at 90℃; for 3.5h; darkness; Stage #2: With sulfuric acid In water at 0℃; | 95% |
| Stage #1: (rac)-gossypol With water; sodium hydroxide at 90℃; for 3.5h; Inert atmosphere; Darkness; Stage #2: With sulfuric acid In water Cooling with ice; | 95% |
| Stage #1: (rac)-gossypol With sodium hydroxide In water at 90℃; for 3.5h; Inert atmosphere; Darkness; Stage #2: With sulfuric acid In water | 95% |

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide Condensation; | 95% |
-
-
303-45-7
(rac)-gossypol

-
-
850480-50-1
4-hydroxynon-2-enal

| Conditions | Yield |
|---|---|
| With zinc(II) chloride In acetonitrile at 60℃; for 5h; Reagent/catalyst; Solvent; Temperature; | 93.5% |

| Conditions | Yield |
|---|---|
| Stage #1: 1.3-propanedithiol; (rac)-gossypol In chloroform at 20℃; for 1h; Stage #2: With boron trifluoride diethyl etherate In chloroform at 20℃; for 15h; | 93% |

| Conditions | Yield |
|---|---|
| In methanol at 20℃; for 3h; Inert atmosphere; | 92.9% |
-
-
303-45-7
(rac)-gossypol

-
-
17460-45-6, 23707-23-5, 23739-93-7, 23743-51-3, 26108-75-8, 59042-16-9, 124516-07-0, 124580-56-9
tetra-acetyl glucosamine

| Conditions | Yield |
|---|---|
| In methanol at 20℃; for 3h; Inert atmosphere; | 92.6% |

| Conditions | Yield |
|---|---|
| With acid In ethanol for 3h; Heating; | 92.3% |

| Conditions | Yield |
|---|---|
| In diethyl ether; isopropyl alcohol at 20 - 45℃; for 2.08333h; Condensation; | 92% |

| Conditions | Yield |
|---|---|
| In diethyl ether; isopropyl alcohol at 20 - 45℃; for 2.08333h; Condensation; | 92% |

| Conditions | Yield |
|---|---|
| In ethanol for 3h; Heating; | 91.9% |
-
-
303-45-7
(rac)-gossypol

-
-
86133-36-0
N-Aminoanabasine

-
-
86133-35-9
5,5'-Diisopropyl-3,3'-dimethyl-8,8'-bis-{[(Z)-3,4,5,6-tetrahydro-2H-[2,3']bipyridinyl-1-ylimino]-methyl}-[2,2']binaphthalenyl-1,6,7,1',6',7'-hexaol

| Conditions | Yield |
|---|---|
| In ethanol Heating; | 91% |
-
-
124-22-1
n-Dodecylamine

-
-
303-45-7
(rac)-gossypol

-
-
5463-57-0
1,6,7,1',6',7'-hexahydroxy-5,5'-diisopropyl-3,3'-dimethyl-[2,2']binaphthyl-8,8'-dicarbaldehyde bis-dodecylimine

| Conditions | Yield |
|---|---|
| In diethyl ether; isopropyl alcohol at 20 - 45℃; for 2.08333h; Condensation; | 91% |

| Conditions | Yield |
|---|---|
| Stage #1: (rac)-gossypol With iron(III) chloride; acetic acid In water; acetone at 100℃; for 2h; Stage #2: With sulfuric acid In diethyl ether; water | 91% |
| With iron(III) chloride In acetic acid; acetone at 60 - 70℃; Oxidation; | 49% |
| With iron(III) chloride; acetic acid In acetone at 70℃; for 0.75h; |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In methanol at 20℃; for 3h; pH=7.4; Inert atmosphere; | 90.8% |

| Conditions | Yield |
|---|---|
| In ethanol for 3h; Heating; | 90.17% |
-
-
60835-71-4
4'-aminobenzo-15-crown-5-ether

-
-
303-45-7
(rac)-gossypol

| Conditions | Yield |
|---|---|
| In ethanol for 6h; Heating; | 90.1% |
-
-
123-00-2
4-(3-Aminopropyl)morpholine

-
-
303-45-7
(rac)-gossypol

-
-
104757-46-2
5,5'-Diisopropyl-3,3'-dimethyl-8,8'-bis-{[(Z)-3-morpholin-4-yl-propylimino]-methyl}-[2,2']binaphthalenyl-1,6,7,1',6',7'-hexaol

| Conditions | Yield |
|---|---|
| In isopropyl alcohol Heating; | 90% |

| Conditions | Yield |
|---|---|
| In diethyl ether; isopropyl alcohol at 20 - 45℃; for 2.08333h; Condensation; | 90% |

| Conditions | Yield |
|---|---|
| In diethyl ether; isopropyl alcohol at 20 - 45℃; for 2.08333h; Condensation; | 90% |
Related products
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View
 Xi,
Xi,  Xn
Xn