-
Name
GOSSYPOL
- EINECS 636-899-7
- CAS No. 303-45-7
- Article Data16
- CAS DataBase
- Density 1.403 g/cm3
- Solubility Soluble in 100%ethanol (25 mg/ml), DMF (25 mg/ml), acetone, DMSO (25 mM), methanol (2 mg/ml), ether, chloroform, sodium carbonate, and dilute aqueous solutions of ammonia . Insoluble in water. Gossypol is a male antifertility agent with antispermatogenic activity and has been shown to contain antitumor, anitviral, and antioxidant properties.
- Melting Point 181-183 °C
- Formula C30H30O8
- Boiling Point 707.888 °C at 760 mmHg
- Molecular Weight 518.563
- Flash Point 395.872 °C
- Transport Information
- Appearance
- Safety 22-36-36/37/39-27-26
- Risk Codes 22-40-36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi,
Xi,  Xn
Xn
- Synonyms [2,2'-Binaphthalene]-8,8'-dicarboxaldehyde,1,1',6,6',7,7'-hexahydroxy-5,5'-diisopropyl-3,3'-dimethyl- (8CI);1,1',6,6',7,7'-Hexahydroxy-5,5'-diisopropyl-3,3'-dimethyl-2,2'-binaphthalene-8,8'-dicarboxaldehyde;1,6,7,1',6',7'-Hexahydroxy-5,5'-diisopropyl-3,3'-dimethyl-[2,2']binaphthalenyl-8,8'-dicarboxaldehyde;2,2'-Bis[1,6,7-trihydroxy-3-methyl-5-isopropyl-8-aldehydonaphthyl];2,2'-Bis[8-formyl-1,6,7-trihydroxy-5-isopropyl-3-methylnaphthyl];BL 193;NSC 56817;NSC 624336;No Fertil;Pogosin;Tash 1;
- PSA 155.52000
- LogP 6.38220
Synthetic route
-
-
5453-04-3, 866541-93-7, 1189561-66-7
(±)-gossypol acetic acid

-
-
303-45-7
(rac)-gossypol

| Conditions | Yield |
|---|---|
| With iron(III) chloride In acetone at 60℃; | 85% |
| In diethyl ether; water at 25℃; for 0.166667h; Temperature; Solvent; Sonication; | 105 mg |
-
-
40817-07-0
2,3,8-trihydroxy-4-isopropyl-6-methyl-1-naphthaldehyde

-
-
303-45-7
(rac)-gossypol

| Conditions | Yield |
|---|---|
| With tert-butyl peroxyacetate In 1,2-dichloro-ethane at 80℃; for 4h; Inert atmosphere; | 76.1% |
| With tert-butyl peroxyacetate In 1,2-dichloro-ethane at 80℃; for 2.5h; Temperature; Inert atmosphere; | 52% |
| With tert-butyl peroxyacetate In 1,2-dichloro-ethane; mineral oil at 80℃; for 2.5h; Inert atmosphere; Schlenk technique; | 41% |
| With laccase of Coriolus versicolor; oxygen at 30℃; pH=5.5; aq. phosphate buffer; |

| Conditions | Yield |
|---|---|
| With hydroquinone at 180℃; under 5 - 15 Torr; for 2.5h; | 72% |
-
-
784206-41-3
apogossypol

-
-
303-45-7
(rac)-gossypol

| Conditions | Yield |
|---|---|
| With Dichloromethyl methyl ether; titanium tetrachloride In dichloromethane at 0 - 20℃; for 5h; Inert atmosphere; | 37% |
-
-
73728-76-4
4,4'-dihydroxy-5,5'-diisopropyl-7,7'-dimethyl-bis(3H-naphtho[1,8-bc]furan-3-one)

-
-
303-45-7
(rac)-gossypol

| Conditions | Yield |
|---|---|
| In water; acetone for 48h; |
-
-
17273-29-9, 38152-98-6, 125278-11-7
Gossypol

-
-
303-45-7
(rac)-gossypol

| Conditions | Yield |
|---|---|
| With hydrogenchloride In ethanol at 25℃; Kinetics; Mechanism; Thermodynamic data; other temperatures, ΔH(excit.), ΔS(excit.), ΔG(excit.); |

-
-
303-45-7
(rac)-gossypol

| Conditions | Yield |
|---|---|
| With hydrogenchloride; acetic acid In diethyl ether Heating; |

-
-
303-45-7
(rac)-gossypol

| Conditions | Yield |
|---|---|
| With hydrogenchloride; acetic acid In diethyl ether Heating; |

-
-
303-45-7
(rac)-gossypol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: silver(l) oxide / methanol / 3 h / 20 °C 2: potassium carbonate / acetone / 5 h / 20 °C 3: dichloromethane / 0.5 h / 20 °C 4: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 5: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 6: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 7: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 7 steps 1: silver(l) oxide / 2 h / Reflux 2: potassium carbonate / acetone / 5 h / 20 °C 3: dichloromethane / 0.5 h / 20 °C 4: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 5: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 6: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 7: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 8 steps 1: silver(l) oxide / methanol / 3 h / 20 °C 2: potassium carbonate / acetone / 5 h / 20 °C 3: dichloromethane / 0.5 h / 20 °C 4: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 5: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 6: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 7: hydrogenchloride / water; acetonitrile / 5 h / 20 °C 8: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 8 steps 1: silver(l) oxide / 2 h / Reflux 2: potassium carbonate / acetone / 5 h / 20 °C 3: dichloromethane / 0.5 h / 20 °C 4: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 5: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 6: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 7: hydrogenchloride / water; acetonitrile / 5 h / 20 °C 8: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme |

-
-
303-45-7
(rac)-gossypol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: potassium carbonate / acetone / 5 h / 20 °C 2: dichloromethane / 0.5 h / 20 °C 3: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 4: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 5: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 6: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 7 steps 1: potassium carbonate / acetone / 5 h / 20 °C 2: dichloromethane / 0.5 h / 20 °C 3: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 4: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 5: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 6: hydrogenchloride / water; acetonitrile / 5 h / 20 °C 7: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme |

-
-
303-45-7
(rac)-gossypol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: dichloromethane / 0.5 h / 20 °C 2: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 3: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 4: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 5: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 6 steps 1: dichloromethane / 0.5 h / 20 °C 2: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 3: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 4: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 5: hydrogenchloride / water; acetonitrile / 5 h / 20 °C 6: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 4 steps 1.1: trifluoroacetic acid / dichloromethane / 1 h / 20 °C / Inert atmosphere; Schlenk technique 1.2: 20 °C / Inert atmosphere; Schlenk technique 2.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C / Inert atmosphere; Schlenk technique 3.1: boron tribromide / dichloromethane / 10 h / -78 - -10 °C / Inert atmosphere; Schlenk technique 4.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane; mineral oil / 2.5 h / 80 °C / Inert atmosphere; Schlenk technique View Scheme | |
| Multi-step reaction with 4 steps 1: trifluoroacetic acid / dichloromethane / 1 h / 20 °C 2: 2-Iodobenzoic acid / dimethyl sulfoxide / 12 h 3: boron tribromide / dichloromethane / 10 h / -78 - -10 °C 4: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 2.5 h / 80 °C / Inert atmosphere View Scheme |

-
-
303-45-7
(rac)-gossypol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 2: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 3: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 4 steps 1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 2: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 3: hydrogenchloride / water; acetonitrile / 5 h / 20 °C 4: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 3 steps 1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C / Inert atmosphere; Schlenk technique 2: boron tribromide / dichloromethane / 10 h / -78 - -10 °C / Inert atmosphere; Schlenk technique 3: tert-butyl peroxyacetate / 1,2-dichloro-ethane; mineral oil / 2.5 h / 80 °C / Inert atmosphere; Schlenk technique View Scheme | |
| Multi-step reaction with 3 steps 1: 2-Iodobenzoic acid / dimethyl sulfoxide / 12 h 2: boron tribromide / dichloromethane / 10 h / -78 - -10 °C 3: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 2.5 h / 80 °C / Inert atmosphere View Scheme |
-
-
50399-96-7
4-isopropyl-2,3,8-trimethoxy-6-methyl-1-naphthaldehyde

-
-
303-45-7
(rac)-gossypol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 2: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 3 steps 1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 2: hydrogenchloride / water; acetonitrile / 5 h / 20 °C 3: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 2 steps 1: boron tribromide / dichloromethane / 10 h / -78 - -10 °C / Inert atmosphere; Schlenk technique 2: tert-butyl peroxyacetate / 1,2-dichloro-ethane; mineral oil / 2.5 h / 80 °C / Inert atmosphere; Schlenk technique View Scheme | |
| Multi-step reaction with 2 steps 1: boron tribromide / dichloromethane / 10 h / -78 - -10 °C 2: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 2.5 h / 80 °C / Inert atmosphere View Scheme |
-
-
1256723-09-7
4-hydroxy-5-isopropyl-7-methylnaphtho[1,8-bc]furan-3-one

-
-
303-45-7
(rac)-gossypol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: hydrogenchloride / water; acetonitrile / 5 h / 20 °C 2: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 17 steps 1.1: acetic acid; bromine / 20.25 h / 20 °C / Cooling with ice 2.1: potassium carbonate / acetone / 15 h / 30 °C 3.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 6 h / 20 °C 3.2: 2 h / Reflux 4.1: potassium carbonate / acetone / 12 h / 20 °C 5.1: n-butyllithium / tetrahydrofuran / 0.5 h / -78 °C / Inert atmosphere 5.2: 10 h / -78 - -30 °C / Inert atmosphere 6.1: potassium tert-butylate / tetrahydrofuran / 3 h / 20 °C 7.1: acetic anhydride; sodium acetate / 2 h / Reflux 8.1: sodium hydroxide / methanol; water / Reflux 9.1: potassium carbonate / acetone / 12 h / 20 °C 10.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 10.2: 10 h / 20 °C 11.1: silver(l) oxide / methanol / 3 h / 20 °C 12.1: potassium carbonate / acetone / 5 h / 20 °C 13.1: dichloromethane / 0.5 h / 20 °C 14.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 15.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 16.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 17.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 17 steps 1.1: acetic acid; bromine / 20.25 h / 20 °C / Cooling with ice 2.1: potassium carbonate / acetone / 15 h / 30 °C 3.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 6 h / 20 °C 3.2: 2 h / Reflux 4.1: potassium carbonate / acetone / 12 h / 20 °C 5.1: n-butyllithium / tetrahydrofuran / 0.5 h / -78 °C / Inert atmosphere 5.2: 10 h / -78 - -30 °C / Inert atmosphere 6.1: potassium tert-butylate / tetrahydrofuran / 3 h / 20 °C 7.1: acetic anhydride; sodium acetate / 2 h / Reflux 8.1: sodium hydroxide / methanol; water / Reflux 9.1: potassium carbonate / acetone / 12 h / 20 °C 10.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 10.2: 10 h / 20 °C 11.1: silver(l) oxide / 2 h / Reflux 12.1: potassium carbonate / acetone / 5 h / 20 °C 13.1: dichloromethane / 0.5 h / 20 °C 14.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 15.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 16.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 17.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 18 steps 1.1: acetic acid; bromine / 20.25 h / 20 °C / Cooling with ice 2.1: potassium carbonate / acetone / 15 h / 30 °C 3.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 6 h / 20 °C 3.2: 2 h / Reflux 4.1: potassium carbonate / acetone / 12 h / 20 °C 5.1: n-butyllithium / tetrahydrofuran / 0.5 h / -78 °C / Inert atmosphere 5.2: 10 h / -78 - -30 °C / Inert atmosphere 6.1: potassium tert-butylate / tetrahydrofuran / 3 h / 20 °C 7.1: acetic anhydride; sodium acetate / 2 h / Reflux 8.1: sodium hydroxide / methanol; water / Reflux 9.1: potassium carbonate / acetone / 12 h / 20 °C 10.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 10.2: 10 h / 20 °C 11.1: silver(l) oxide / methanol / 3 h / 20 °C 12.1: potassium carbonate / acetone / 5 h / 20 °C 13.1: dichloromethane / 0.5 h / 20 °C 14.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 15.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 16.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 17.1: hydrogenchloride / water; acetonitrile / 5 h / 20 °C 18.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 18 steps 1.1: acetic acid; bromine / 20.25 h / 20 °C / Cooling with ice 2.1: potassium carbonate / acetone / 15 h / 30 °C 3.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 6 h / 20 °C 3.2: 2 h / Reflux 4.1: potassium carbonate / acetone / 12 h / 20 °C 5.1: n-butyllithium / tetrahydrofuran / 0.5 h / -78 °C / Inert atmosphere 5.2: 10 h / -78 - -30 °C / Inert atmosphere 6.1: potassium tert-butylate / tetrahydrofuran / 3 h / 20 °C 7.1: acetic anhydride; sodium acetate / 2 h / Reflux 8.1: sodium hydroxide / methanol; water / Reflux 9.1: potassium carbonate / acetone / 12 h / 20 °C 10.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 10.2: 10 h / 20 °C 11.1: silver(l) oxide / 2 h / Reflux 12.1: potassium carbonate / acetone / 5 h / 20 °C 13.1: dichloromethane / 0.5 h / 20 °C 14.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 15.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 16.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 17.1: hydrogenchloride / water; acetonitrile / 5 h / 20 °C 18.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 18 steps 1.1: tin(IV) chloride; triethylamine / toluene / 0.33 h / 20 °C / Inert atmosphere 1.2: 8 h / 100 °C / Inert atmosphere 2.1: acetic acid; bromine / 20.25 h / 20 °C / Cooling with ice 3.1: potassium carbonate / acetone / 15 h / 30 °C 4.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 6 h / 20 °C 4.2: 2 h / Reflux 5.1: potassium carbonate / acetone / 12 h / 20 °C 6.1: n-butyllithium / tetrahydrofuran / 0.5 h / -78 °C / Inert atmosphere 6.2: 10 h / -78 - -30 °C / Inert atmosphere 7.1: potassium tert-butylate / tetrahydrofuran / 3 h / 20 °C 8.1: acetic anhydride; sodium acetate / 2 h / Reflux 9.1: sodium hydroxide / methanol; water / Reflux 10.1: potassium carbonate / acetone / 12 h / 20 °C 11.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 11.2: 10 h / 20 °C 12.1: silver(l) oxide / methanol / 3 h / 20 °C 13.1: potassium carbonate / acetone / 5 h / 20 °C 14.1: dichloromethane / 0.5 h / 20 °C 15.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 16.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 17.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 18.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 18 steps 1.1: tin(IV) chloride; triethylamine / toluene / 0.33 h / 20 °C / Inert atmosphere 1.2: 8 h / 100 °C / Inert atmosphere 2.1: acetic acid; bromine / 20.25 h / 20 °C / Cooling with ice 3.1: potassium carbonate / acetone / 15 h / 30 °C 4.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 6 h / 20 °C 4.2: 2 h / Reflux 5.1: potassium carbonate / acetone / 12 h / 20 °C 6.1: n-butyllithium / tetrahydrofuran / 0.5 h / -78 °C / Inert atmosphere 6.2: 10 h / -78 - -30 °C / Inert atmosphere 7.1: potassium tert-butylate / tetrahydrofuran / 3 h / 20 °C 8.1: acetic anhydride; sodium acetate / 2 h / Reflux 9.1: sodium hydroxide / methanol; water / Reflux 10.1: potassium carbonate / acetone / 12 h / 20 °C 11.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 11.2: 10 h / 20 °C 12.1: silver(l) oxide / 2 h / Reflux 13.1: potassium carbonate / acetone / 5 h / 20 °C 14.1: dichloromethane / 0.5 h / 20 °C 15.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 16.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 17.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 18.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 19 steps 1.1: tin(IV) chloride; triethylamine / toluene / 0.33 h / 20 °C / Inert atmosphere 1.2: 8 h / 100 °C / Inert atmosphere 2.1: acetic acid; bromine / 20.25 h / 20 °C / Cooling with ice 3.1: potassium carbonate / acetone / 15 h / 30 °C 4.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 6 h / 20 °C 4.2: 2 h / Reflux 5.1: potassium carbonate / acetone / 12 h / 20 °C 6.1: n-butyllithium / tetrahydrofuran / 0.5 h / -78 °C / Inert atmosphere 6.2: 10 h / -78 - -30 °C / Inert atmosphere 7.1: potassium tert-butylate / tetrahydrofuran / 3 h / 20 °C 8.1: acetic anhydride; sodium acetate / 2 h / Reflux 9.1: sodium hydroxide / methanol; water / Reflux 10.1: potassium carbonate / acetone / 12 h / 20 °C 11.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 11.2: 10 h / 20 °C 12.1: silver(l) oxide / methanol / 3 h / 20 °C 13.1: potassium carbonate / acetone / 5 h / 20 °C 14.1: dichloromethane / 0.5 h / 20 °C 15.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 16.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 17.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 18.1: hydrogenchloride / water; acetonitrile / 5 h / 20 °C 19.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 19 steps 1.1: tin(IV) chloride; triethylamine / toluene / 0.33 h / 20 °C / Inert atmosphere 1.2: 8 h / 100 °C / Inert atmosphere 2.1: acetic acid; bromine / 20.25 h / 20 °C / Cooling with ice 3.1: potassium carbonate / acetone / 15 h / 30 °C 4.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 6 h / 20 °C 4.2: 2 h / Reflux 5.1: potassium carbonate / acetone / 12 h / 20 °C 6.1: n-butyllithium / tetrahydrofuran / 0.5 h / -78 °C / Inert atmosphere 6.2: 10 h / -78 - -30 °C / Inert atmosphere 7.1: potassium tert-butylate / tetrahydrofuran / 3 h / 20 °C 8.1: acetic anhydride; sodium acetate / 2 h / Reflux 9.1: sodium hydroxide / methanol; water / Reflux 10.1: potassium carbonate / acetone / 12 h / 20 °C 11.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 11.2: 10 h / 20 °C 12.1: silver(l) oxide / 2 h / Reflux 13.1: potassium carbonate / acetone / 5 h / 20 °C 14.1: dichloromethane / 0.5 h / 20 °C 15.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 16.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 17.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 18.1: hydrogenchloride / water; acetonitrile / 5 h / 20 °C 19.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme |

-
-
303-45-7
(rac)-gossypol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 16 steps 1.1: potassium carbonate / acetone / 15 h / 30 °C 2.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 6 h / 20 °C 2.2: 2 h / Reflux 3.1: potassium carbonate / acetone / 12 h / 20 °C 4.1: n-butyllithium / tetrahydrofuran / 0.5 h / -78 °C / Inert atmosphere 4.2: 10 h / -78 - -30 °C / Inert atmosphere 5.1: potassium tert-butylate / tetrahydrofuran / 3 h / 20 °C 6.1: acetic anhydride; sodium acetate / 2 h / Reflux 7.1: sodium hydroxide / methanol; water / Reflux 8.1: potassium carbonate / acetone / 12 h / 20 °C 9.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 9.2: 10 h / 20 °C 10.1: silver(l) oxide / methanol / 3 h / 20 °C 11.1: potassium carbonate / acetone / 5 h / 20 °C 12.1: dichloromethane / 0.5 h / 20 °C 13.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 14.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 15.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 16.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 16 steps 1.1: potassium carbonate / acetone / 15 h / 30 °C 2.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 6 h / 20 °C 2.2: 2 h / Reflux 3.1: potassium carbonate / acetone / 12 h / 20 °C 4.1: n-butyllithium / tetrahydrofuran / 0.5 h / -78 °C / Inert atmosphere 4.2: 10 h / -78 - -30 °C / Inert atmosphere 5.1: potassium tert-butylate / tetrahydrofuran / 3 h / 20 °C 6.1: acetic anhydride; sodium acetate / 2 h / Reflux 7.1: sodium hydroxide / methanol; water / Reflux 8.1: potassium carbonate / acetone / 12 h / 20 °C 9.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 9.2: 10 h / 20 °C 10.1: silver(l) oxide / 2 h / Reflux 11.1: potassium carbonate / acetone / 5 h / 20 °C 12.1: dichloromethane / 0.5 h / 20 °C 13.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 14.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 15.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 16.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 17 steps 1.1: potassium carbonate / acetone / 15 h / 30 °C 2.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 6 h / 20 °C 2.2: 2 h / Reflux 3.1: potassium carbonate / acetone / 12 h / 20 °C 4.1: n-butyllithium / tetrahydrofuran / 0.5 h / -78 °C / Inert atmosphere 4.2: 10 h / -78 - -30 °C / Inert atmosphere 5.1: potassium tert-butylate / tetrahydrofuran / 3 h / 20 °C 6.1: acetic anhydride; sodium acetate / 2 h / Reflux 7.1: sodium hydroxide / methanol; water / Reflux 8.1: potassium carbonate / acetone / 12 h / 20 °C 9.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 9.2: 10 h / 20 °C 10.1: silver(l) oxide / methanol / 3 h / 20 °C 11.1: potassium carbonate / acetone / 5 h / 20 °C 12.1: dichloromethane / 0.5 h / 20 °C 13.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 14.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 15.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 16.1: hydrogenchloride / water; acetonitrile / 5 h / 20 °C 17.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 17 steps 1.1: potassium carbonate / acetone / 15 h / 30 °C 2.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 6 h / 20 °C 2.2: 2 h / Reflux 3.1: potassium carbonate / acetone / 12 h / 20 °C 4.1: n-butyllithium / tetrahydrofuran / 0.5 h / -78 °C / Inert atmosphere 4.2: 10 h / -78 - -30 °C / Inert atmosphere 5.1: potassium tert-butylate / tetrahydrofuran / 3 h / 20 °C 6.1: acetic anhydride; sodium acetate / 2 h / Reflux 7.1: sodium hydroxide / methanol; water / Reflux 8.1: potassium carbonate / acetone / 12 h / 20 °C 9.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 9.2: 10 h / 20 °C 10.1: silver(l) oxide / 2 h / Reflux 11.1: potassium carbonate / acetone / 5 h / 20 °C 12.1: dichloromethane / 0.5 h / 20 °C 13.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 14.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 15.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 16.1: hydrogenchloride / water; acetonitrile / 5 h / 20 °C 17.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme |

-
-
303-45-7
(rac)-gossypol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 10 steps 1.1: potassium carbonate / acetone / 12 h / 20 °C 2.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 2.2: 10 h / 20 °C 3.1: silver(l) oxide / methanol / 3 h / 20 °C 4.1: potassium carbonate / acetone / 5 h / 20 °C 5.1: dichloromethane / 0.5 h / 20 °C 6.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 7.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 8.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 9.1: hydrogenchloride / water; acetonitrile / 5 h / 20 °C 10.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 10 steps 1.1: potassium carbonate / acetone / 12 h / 20 °C 2.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 2.2: 10 h / 20 °C 3.1: silver(l) oxide / 2 h / Reflux 4.1: potassium carbonate / acetone / 5 h / 20 °C 5.1: dichloromethane / 0.5 h / 20 °C 6.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 7.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 8.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 9.1: hydrogenchloride / water; acetonitrile / 5 h / 20 °C 10.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 9 steps 1.1: potassium carbonate / acetone / 12 h / 20 °C 2.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 2.2: 10 h / 20 °C 3.1: silver(l) oxide / 2 h / Reflux 4.1: potassium carbonate / acetone / 5 h / 20 °C 5.1: dichloromethane / 0.5 h / 20 °C 6.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 7.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 8.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 9.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 9 steps 1.1: potassium carbonate / acetone / 12 h / 20 °C 2.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 2.2: 10 h / 20 °C 3.1: silver(l) oxide / methanol / 3 h / 20 °C 4.1: potassium carbonate / acetone / 5 h / 20 °C 5.1: dichloromethane / 0.5 h / 20 °C 6.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 7.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 8.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 9.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme |

-
-
303-45-7
(rac)-gossypol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 15 steps 1.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 6 h / 20 °C 1.2: 2 h / Reflux 2.1: potassium carbonate / acetone / 12 h / 20 °C 3.1: n-butyllithium / tetrahydrofuran / 0.5 h / -78 °C / Inert atmosphere 3.2: 10 h / -78 - -30 °C / Inert atmosphere 4.1: potassium tert-butylate / tetrahydrofuran / 3 h / 20 °C 5.1: acetic anhydride; sodium acetate / 2 h / Reflux 6.1: sodium hydroxide / methanol; water / Reflux 7.1: potassium carbonate / acetone / 12 h / 20 °C 8.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 8.2: 10 h / 20 °C 9.1: silver(l) oxide / methanol / 3 h / 20 °C 10.1: potassium carbonate / acetone / 5 h / 20 °C 11.1: dichloromethane / 0.5 h / 20 °C 12.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 13.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 14.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 15.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 15 steps 1.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 6 h / 20 °C 1.2: 2 h / Reflux 2.1: potassium carbonate / acetone / 12 h / 20 °C 3.1: n-butyllithium / tetrahydrofuran / 0.5 h / -78 °C / Inert atmosphere 3.2: 10 h / -78 - -30 °C / Inert atmosphere 4.1: potassium tert-butylate / tetrahydrofuran / 3 h / 20 °C 5.1: acetic anhydride; sodium acetate / 2 h / Reflux 6.1: sodium hydroxide / methanol; water / Reflux 7.1: potassium carbonate / acetone / 12 h / 20 °C 8.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 8.2: 10 h / 20 °C 9.1: silver(l) oxide / 2 h / Reflux 10.1: potassium carbonate / acetone / 5 h / 20 °C 11.1: dichloromethane / 0.5 h / 20 °C 12.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 13.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 14.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 15.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 16 steps 1.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 6 h / 20 °C 1.2: 2 h / Reflux 2.1: potassium carbonate / acetone / 12 h / 20 °C 3.1: n-butyllithium / tetrahydrofuran / 0.5 h / -78 °C / Inert atmosphere 3.2: 10 h / -78 - -30 °C / Inert atmosphere 4.1: potassium tert-butylate / tetrahydrofuran / 3 h / 20 °C 5.1: acetic anhydride; sodium acetate / 2 h / Reflux 6.1: sodium hydroxide / methanol; water / Reflux 7.1: potassium carbonate / acetone / 12 h / 20 °C 8.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 8.2: 10 h / 20 °C 9.1: silver(l) oxide / methanol / 3 h / 20 °C 10.1: potassium carbonate / acetone / 5 h / 20 °C 11.1: dichloromethane / 0.5 h / 20 °C 12.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 13.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 14.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 15.1: hydrogenchloride / water; acetonitrile / 5 h / 20 °C 16.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 16 steps 1.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 6 h / 20 °C 1.2: 2 h / Reflux 2.1: potassium carbonate / acetone / 12 h / 20 °C 3.1: n-butyllithium / tetrahydrofuran / 0.5 h / -78 °C / Inert atmosphere 3.2: 10 h / -78 - -30 °C / Inert atmosphere 4.1: potassium tert-butylate / tetrahydrofuran / 3 h / 20 °C 5.1: acetic anhydride; sodium acetate / 2 h / Reflux 6.1: sodium hydroxide / methanol; water / Reflux 7.1: potassium carbonate / acetone / 12 h / 20 °C 8.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 8.2: 10 h / 20 °C 9.1: silver(l) oxide / 2 h / Reflux 10.1: potassium carbonate / acetone / 5 h / 20 °C 11.1: dichloromethane / 0.5 h / 20 °C 12.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 13.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 14.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 15.1: hydrogenchloride / water; acetonitrile / 5 h / 20 °C 16.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme |

-
-
303-45-7
(rac)-gossypol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 2: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 3: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 4: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 5 steps 1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 2: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 3: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 4: hydrogenchloride / water; acetonitrile / 5 h / 20 °C 5: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme |

-
-
303-45-7
(rac)-gossypol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 14 steps 1.1: potassium carbonate / acetone / 12 h / 20 °C 2.1: n-butyllithium / tetrahydrofuran / 0.5 h / -78 °C / Inert atmosphere 2.2: 10 h / -78 - -30 °C / Inert atmosphere 3.1: potassium tert-butylate / tetrahydrofuran / 3 h / 20 °C 4.1: acetic anhydride; sodium acetate / 2 h / Reflux 5.1: sodium hydroxide / methanol; water / Reflux 6.1: potassium carbonate / acetone / 12 h / 20 °C 7.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 7.2: 10 h / 20 °C 8.1: silver(l) oxide / methanol / 3 h / 20 °C 9.1: potassium carbonate / acetone / 5 h / 20 °C 10.1: dichloromethane / 0.5 h / 20 °C 11.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 12.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 13.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 14.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 14 steps 1.1: potassium carbonate / acetone / 12 h / 20 °C 2.1: n-butyllithium / tetrahydrofuran / 0.5 h / -78 °C / Inert atmosphere 2.2: 10 h / -78 - -30 °C / Inert atmosphere 3.1: potassium tert-butylate / tetrahydrofuran / 3 h / 20 °C 4.1: acetic anhydride; sodium acetate / 2 h / Reflux 5.1: sodium hydroxide / methanol; water / Reflux 6.1: potassium carbonate / acetone / 12 h / 20 °C 7.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 7.2: 10 h / 20 °C 8.1: silver(l) oxide / 2 h / Reflux 9.1: potassium carbonate / acetone / 5 h / 20 °C 10.1: dichloromethane / 0.5 h / 20 °C 11.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 12.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 13.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 14.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 15 steps 1.1: potassium carbonate / acetone / 12 h / 20 °C 2.1: n-butyllithium / tetrahydrofuran / 0.5 h / -78 °C / Inert atmosphere 2.2: 10 h / -78 - -30 °C / Inert atmosphere 3.1: potassium tert-butylate / tetrahydrofuran / 3 h / 20 °C 4.1: acetic anhydride; sodium acetate / 2 h / Reflux 5.1: sodium hydroxide / methanol; water / Reflux 6.1: potassium carbonate / acetone / 12 h / 20 °C 7.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 7.2: 10 h / 20 °C 8.1: silver(l) oxide / methanol / 3 h / 20 °C 9.1: potassium carbonate / acetone / 5 h / 20 °C 10.1: dichloromethane / 0.5 h / 20 °C 11.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 12.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 13.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 14.1: hydrogenchloride / water; acetonitrile / 5 h / 20 °C 15.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 15 steps 1.1: potassium carbonate / acetone / 12 h / 20 °C 2.1: n-butyllithium / tetrahydrofuran / 0.5 h / -78 °C / Inert atmosphere 2.2: 10 h / -78 - -30 °C / Inert atmosphere 3.1: potassium tert-butylate / tetrahydrofuran / 3 h / 20 °C 4.1: acetic anhydride; sodium acetate / 2 h / Reflux 5.1: sodium hydroxide / methanol; water / Reflux 6.1: potassium carbonate / acetone / 12 h / 20 °C 7.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 7.2: 10 h / 20 °C 8.1: silver(l) oxide / 2 h / Reflux 9.1: potassium carbonate / acetone / 5 h / 20 °C 10.1: dichloromethane / 0.5 h / 20 °C 11.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 12.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 13.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 14.1: hydrogenchloride / water; acetonitrile / 5 h / 20 °C 15.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme |

-
-
303-45-7
(rac)-gossypol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 13 steps 1.1: n-butyllithium / tetrahydrofuran / 0.5 h / -78 °C / Inert atmosphere 1.2: 10 h / -78 - -30 °C / Inert atmosphere 2.1: potassium tert-butylate / tetrahydrofuran / 3 h / 20 °C 3.1: acetic anhydride; sodium acetate / 2 h / Reflux 4.1: sodium hydroxide / methanol; water / Reflux 5.1: potassium carbonate / acetone / 12 h / 20 °C 6.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 6.2: 10 h / 20 °C 7.1: silver(l) oxide / methanol / 3 h / 20 °C 8.1: potassium carbonate / acetone / 5 h / 20 °C 9.1: dichloromethane / 0.5 h / 20 °C 10.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 11.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 12.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 13.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 13 steps 1.1: n-butyllithium / tetrahydrofuran / 0.5 h / -78 °C / Inert atmosphere 1.2: 10 h / -78 - -30 °C / Inert atmosphere 2.1: potassium tert-butylate / tetrahydrofuran / 3 h / 20 °C 3.1: acetic anhydride; sodium acetate / 2 h / Reflux 4.1: sodium hydroxide / methanol; water / Reflux 5.1: potassium carbonate / acetone / 12 h / 20 °C 6.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 6.2: 10 h / 20 °C 7.1: silver(l) oxide / 2 h / Reflux 8.1: potassium carbonate / acetone / 5 h / 20 °C 9.1: dichloromethane / 0.5 h / 20 °C 10.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 11.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 12.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 13.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 14 steps 1.1: n-butyllithium / tetrahydrofuran / 0.5 h / -78 °C / Inert atmosphere 1.2: 10 h / -78 - -30 °C / Inert atmosphere 2.1: potassium tert-butylate / tetrahydrofuran / 3 h / 20 °C 3.1: acetic anhydride; sodium acetate / 2 h / Reflux 4.1: sodium hydroxide / methanol; water / Reflux 5.1: potassium carbonate / acetone / 12 h / 20 °C 6.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 6.2: 10 h / 20 °C 7.1: silver(l) oxide / methanol / 3 h / 20 °C 8.1: potassium carbonate / acetone / 5 h / 20 °C 9.1: dichloromethane / 0.5 h / 20 °C 10.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 11.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 12.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 13.1: hydrogenchloride / water; acetonitrile / 5 h / 20 °C 14.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 14 steps 1.1: n-butyllithium / tetrahydrofuran / 0.5 h / -78 °C / Inert atmosphere 1.2: 10 h / -78 - -30 °C / Inert atmosphere 2.1: potassium tert-butylate / tetrahydrofuran / 3 h / 20 °C 3.1: acetic anhydride; sodium acetate / 2 h / Reflux 4.1: sodium hydroxide / methanol; water / Reflux 5.1: potassium carbonate / acetone / 12 h / 20 °C 6.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 6.2: 10 h / 20 °C 7.1: silver(l) oxide / 2 h / Reflux 8.1: potassium carbonate / acetone / 5 h / 20 °C 9.1: dichloromethane / 0.5 h / 20 °C 10.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 11.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 12.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 13.1: hydrogenchloride / water; acetonitrile / 5 h / 20 °C 14.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme |

-
-
303-45-7
(rac)-gossypol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 12 steps 1.1: potassium tert-butylate / tetrahydrofuran / 3 h / 20 °C 2.1: acetic anhydride; sodium acetate / 2 h / Reflux 3.1: sodium hydroxide / methanol; water / Reflux 4.1: potassium carbonate / acetone / 12 h / 20 °C 5.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 5.2: 10 h / 20 °C 6.1: silver(l) oxide / methanol / 3 h / 20 °C 7.1: potassium carbonate / acetone / 5 h / 20 °C 8.1: dichloromethane / 0.5 h / 20 °C 9.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 10.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 11.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 12.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 12 steps 1.1: potassium tert-butylate / tetrahydrofuran / 3 h / 20 °C 2.1: acetic anhydride; sodium acetate / 2 h / Reflux 3.1: sodium hydroxide / methanol; water / Reflux 4.1: potassium carbonate / acetone / 12 h / 20 °C 5.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 5.2: 10 h / 20 °C 6.1: silver(l) oxide / 2 h / Reflux 7.1: potassium carbonate / acetone / 5 h / 20 °C 8.1: dichloromethane / 0.5 h / 20 °C 9.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 10.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 11.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 12.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 13 steps 1.1: potassium tert-butylate / tetrahydrofuran / 3 h / 20 °C 2.1: acetic anhydride; sodium acetate / 2 h / Reflux 3.1: sodium hydroxide / methanol; water / Reflux 4.1: potassium carbonate / acetone / 12 h / 20 °C 5.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 5.2: 10 h / 20 °C 6.1: silver(l) oxide / methanol / 3 h / 20 °C 7.1: potassium carbonate / acetone / 5 h / 20 °C 8.1: dichloromethane / 0.5 h / 20 °C 9.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 10.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 11.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 12.1: hydrogenchloride / water; acetonitrile / 5 h / 20 °C 13.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 13 steps 1.1: potassium tert-butylate / tetrahydrofuran / 3 h / 20 °C 2.1: acetic anhydride; sodium acetate / 2 h / Reflux 3.1: sodium hydroxide / methanol; water / Reflux 4.1: potassium carbonate / acetone / 12 h / 20 °C 5.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 5.2: 10 h / 20 °C 6.1: silver(l) oxide / 2 h / Reflux 7.1: potassium carbonate / acetone / 5 h / 20 °C 8.1: dichloromethane / 0.5 h / 20 °C 9.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 10.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 11.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 12.1: hydrogenchloride / water; acetonitrile / 5 h / 20 °C 13.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme |

-
-
303-45-7
(rac)-gossypol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 11 steps 1.1: acetic anhydride; sodium acetate / 2 h / Reflux 2.1: sodium hydroxide / methanol; water / Reflux 3.1: potassium carbonate / acetone / 12 h / 20 °C 4.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 4.2: 10 h / 20 °C 5.1: silver(l) oxide / methanol / 3 h / 20 °C 6.1: potassium carbonate / acetone / 5 h / 20 °C 7.1: dichloromethane / 0.5 h / 20 °C 8.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 9.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 10.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 11.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 11 steps 1.1: acetic anhydride; sodium acetate / 2 h / Reflux 2.1: sodium hydroxide / methanol; water / Reflux 3.1: potassium carbonate / acetone / 12 h / 20 °C 4.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 4.2: 10 h / 20 °C 5.1: silver(l) oxide / 2 h / Reflux 6.1: potassium carbonate / acetone / 5 h / 20 °C 7.1: dichloromethane / 0.5 h / 20 °C 8.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 9.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 10.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 11.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 12 steps 1.1: acetic anhydride; sodium acetate / 2 h / Reflux 2.1: sodium hydroxide / methanol; water / Reflux 3.1: potassium carbonate / acetone / 12 h / 20 °C 4.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 4.2: 10 h / 20 °C 5.1: silver(l) oxide / methanol / 3 h / 20 °C 6.1: potassium carbonate / acetone / 5 h / 20 °C 7.1: dichloromethane / 0.5 h / 20 °C 8.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 9.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 10.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 11.1: hydrogenchloride / water; acetonitrile / 5 h / 20 °C 12.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 12 steps 1.1: acetic anhydride; sodium acetate / 2 h / Reflux 2.1: sodium hydroxide / methanol; water / Reflux 3.1: potassium carbonate / acetone / 12 h / 20 °C 4.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 4.2: 10 h / 20 °C 5.1: silver(l) oxide / 2 h / Reflux 6.1: potassium carbonate / acetone / 5 h / 20 °C 7.1: dichloromethane / 0.5 h / 20 °C 8.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 9.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 10.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 11.1: hydrogenchloride / water; acetonitrile / 5 h / 20 °C 12.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme |

-
-
303-45-7
(rac)-gossypol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 10 steps 1.1: sodium hydroxide / methanol; water / Reflux 2.1: potassium carbonate / acetone / 12 h / 20 °C 3.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 3.2: 10 h / 20 °C 4.1: silver(l) oxide / methanol / 3 h / 20 °C 5.1: potassium carbonate / acetone / 5 h / 20 °C 6.1: dichloromethane / 0.5 h / 20 °C 7.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 8.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 9.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 10.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 10 steps 1.1: sodium hydroxide / methanol; water / Reflux 2.1: potassium carbonate / acetone / 12 h / 20 °C 3.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 3.2: 10 h / 20 °C 4.1: silver(l) oxide / 2 h / Reflux 5.1: potassium carbonate / acetone / 5 h / 20 °C 6.1: dichloromethane / 0.5 h / 20 °C 7.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 8.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 9.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 10.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 11 steps 1.1: sodium hydroxide / methanol; water / Reflux 2.1: potassium carbonate / acetone / 12 h / 20 °C 3.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 3.2: 10 h / 20 °C 4.1: silver(l) oxide / methanol / 3 h / 20 °C 5.1: potassium carbonate / acetone / 5 h / 20 °C 6.1: dichloromethane / 0.5 h / 20 °C 7.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 8.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 9.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 10.1: hydrogenchloride / water; acetonitrile / 5 h / 20 °C 11.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 11 steps 1.1: sodium hydroxide / methanol; water / Reflux 2.1: potassium carbonate / acetone / 12 h / 20 °C 3.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 3.2: 10 h / 20 °C 4.1: silver(l) oxide / 2 h / Reflux 5.1: potassium carbonate / acetone / 5 h / 20 °C 6.1: dichloromethane / 0.5 h / 20 °C 7.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 8.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 9.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 10.1: hydrogenchloride / water; acetonitrile / 5 h / 20 °C 11.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme |

-
-
303-45-7
(rac)-gossypol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 1.2: 10 h / 20 °C 2.1: silver(l) oxide / 2 h / Reflux 3.1: potassium carbonate / acetone / 5 h / 20 °C 4.1: dichloromethane / 0.5 h / 20 °C 5.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 6.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 7.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 8.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 8 steps 1.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 1.2: 10 h / 20 °C 2.1: silver(l) oxide / methanol / 3 h / 20 °C 3.1: potassium carbonate / acetone / 5 h / 20 °C 4.1: dichloromethane / 0.5 h / 20 °C 5.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 6.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 7.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 8.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 9 steps 1.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 1.2: 10 h / 20 °C 2.1: silver(l) oxide / 2 h / Reflux 3.1: potassium carbonate / acetone / 5 h / 20 °C 4.1: dichloromethane / 0.5 h / 20 °C 5.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 6.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 7.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 8.1: hydrogenchloride / water; acetonitrile / 5 h / 20 °C 9.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 9 steps 1.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 20 °C 1.2: 10 h / 20 °C 2.1: silver(l) oxide / methanol / 3 h / 20 °C 3.1: potassium carbonate / acetone / 5 h / 20 °C 4.1: dichloromethane / 0.5 h / 20 °C 5.1: lithium hydroxide / water; tetrahydrofuran / 1 h / 20 °C 6.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C 7.1: boron tribromide / dichloromethane / 6 h / -78 - -10 °C / Inert atmosphere 8.1: hydrogenchloride / water; acetonitrile / 5 h / 20 °C 9.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 4 h / 80 °C / Inert atmosphere View Scheme |

-
-
303-45-7
(rac)-gossypol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 13 steps 1.1: sodium tetrahydroborate; methanol / 0.5 h / 0 °C / Inert atmosphere; Schlenk technique 2.1: sodium hydride / tetrahydrofuran; mineral oil / 0.75 h / 0 °C / Inert atmosphere; Schlenk technique 2.2: 14 h / 0 - 20 °C / Inert atmosphere; Schlenk technique 3.1: palladium dichloride; copper(l) chloride; oxygen / water; N,N-dimethyl-formamide / 32 h / 20 °C / Schlenk technique 4.1: potassium tert-butylate / tetrahydrofuran / 0.75 h / 0 - 20 °C / Inert atmosphere; Schlenk technique 4.2: 2 h / 20 °C / Inert atmosphere; Schlenk technique 5.1: 2,3-dicyano-5,6-dichloro-p-benzoquinone / dichloromethane; water / 4 h / 20 °C / Inert atmosphere; Schlenk technique 6.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C / Inert atmosphere; Schlenk technique 7.1: 2,6-di-tert-butyl-4-methyl-phenol / chlorobenzene / 13 h / 160 °C / Schlenk technique; Inert atmosphere 8.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C / Inert atmosphere; Schlenk technique 9.1: potassium carbonate / acetonitrile / 20 h / 80 °C / Inert atmosphere; Schlenk technique 10.1: trifluoroacetic acid / dichloromethane / 1 h / 20 °C / Inert atmosphere; Schlenk technique 10.2: 20 °C / Inert atmosphere; Schlenk technique 11.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C / Inert atmosphere; Schlenk technique 12.1: boron tribromide / dichloromethane / 10 h / -78 - -10 °C / Inert atmosphere; Schlenk technique 13.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane; mineral oil / 2.5 h / 80 °C / Inert atmosphere; Schlenk technique View Scheme |

-
-
303-45-7
(rac)-gossypol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: potassium carbonate / acetonitrile / 20 h / 80 °C / Inert atmosphere; Schlenk technique 2.1: trifluoroacetic acid / dichloromethane / 1 h / 20 °C / Inert atmosphere; Schlenk technique 2.2: 20 °C / Inert atmosphere; Schlenk technique 3.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 12 h / 20 °C / Inert atmosphere; Schlenk technique 4.1: boron tribromide / dichloromethane / 10 h / -78 - -10 °C / Inert atmosphere; Schlenk technique 5.1: tert-butyl peroxyacetate / 1,2-dichloro-ethane; mineral oil / 2.5 h / 80 °C / Inert atmosphere; Schlenk technique View Scheme | |
| Multi-step reaction with 5 steps 1: potassium carbonate / acetonitrile / 4 h / 80 °C 2: trifluoroacetic acid / dichloromethane / 1 h / 20 °C 3: 2-Iodobenzoic acid / dimethyl sulfoxide / 12 h 4: boron tribromide / dichloromethane / 10 h / -78 - -10 °C 5: tert-butyl peroxyacetate / 1,2-dichloro-ethane / 2.5 h / 80 °C / Inert atmosphere View Scheme |

| Conditions | Yield |
|---|---|
| In methanol for 5h; Ambient temperature; | 100% |
-
-
303-45-7
(rac)-gossypol

-
-
5619-09-0, 7524-52-9, 14907-27-8, 26988-71-6, 41222-70-2, 67557-19-1, 109492-62-8
methyl tryptophanate hydrochloride

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol at 45℃; for 2h; Resolution of racemate; | A 99.4% B 95% |


| Conditions | Yield |
|---|---|
| With acid In ethanol for 3h; Heating; | 98.7% |

| Conditions | Yield |
|---|---|
| at 20℃; | 98% |
-
-
303-45-7
(rac)-gossypol

-
-
14907-27-8, 5619-09-0, 7524-52-9, 26988-71-6, 41222-70-2, 67557-19-1, 109492-62-8
(R)-(+)-tryptophan methyl ester hydrochloride

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol Reflux; | 98% |
-
-
303-45-7
(rac)-gossypol

-
-
7524-52-9, 26988-71-6, 5619-09-0, 14907-27-8, 41222-70-2, 67557-19-1, 109492-62-8
(S)-tryptophan methyl ester hydrochloride

-
-
132303-09-4
C54H54N4O10

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol Reflux; | 98% |
-
-
303-45-7
(rac)-gossypol

-
-
7524-52-9, 26988-71-6, 5619-09-0, 14907-27-8, 41222-70-2, 67557-19-1, 109492-62-8
(S)-tryptophan methyl ester hydrochloride

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol at 20℃; for 0.5h; | A 97% B 94% |

-
-
303-45-7
(rac)-gossypol

-
-
75-04-7
ethylamine

-
-
307976-02-9
1,6,7,1',6',7'-hexahydroxy-5,5'-diisopropyl-3,3'-dimethyl-[2,2']binaphthyl-8,8'-dicarbaldehyde bis-ethylimine

| Conditions | Yield |
|---|---|
| In diethyl ether; isopropyl alcohol at 20 - 45℃; for 2.08333h; Condensation; | 96% |
| With isopropyl alcohol |

| Conditions | Yield |
|---|---|
| In diethyl ether; isopropyl alcohol at 20 - 45℃; for 2.08333h; Condensation; | 96% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol for 3h; Heating; | 95.8% |

| Conditions | Yield |
|---|---|
| In ethanol for 3h; Heating; | 95.31% |
-
-
303-45-7
(rac)-gossypol

-
-
784206-41-3
apogossypol

| Conditions | Yield |
|---|---|
| Stage #1: (rac)-gossypol With sodium hydroxide; acetic acid In water at 90℃; for 3.5h; darkness; Stage #2: With sulfuric acid In water at 0℃; | 95% |
| Stage #1: (rac)-gossypol With water; sodium hydroxide at 90℃; for 3.5h; Inert atmosphere; Darkness; Stage #2: With sulfuric acid In water Cooling with ice; | 95% |
| Stage #1: (rac)-gossypol With sodium hydroxide In water at 90℃; for 3.5h; Inert atmosphere; Darkness; Stage #2: With sulfuric acid In water | 95% |

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide Condensation; | 95% |
-
-
303-45-7
(rac)-gossypol

-
-
850480-50-1
4-hydroxynon-2-enal

| Conditions | Yield |
|---|---|
| With zinc(II) chloride In acetonitrile at 60℃; for 5h; Reagent/catalyst; Solvent; Temperature; | 93.5% |

| Conditions | Yield |
|---|---|
| Stage #1: 1.3-propanedithiol; (rac)-gossypol In chloroform at 20℃; for 1h; Stage #2: With boron trifluoride diethyl etherate In chloroform at 20℃; for 15h; | 93% |

| Conditions | Yield |
|---|---|
| In methanol at 20℃; for 3h; Inert atmosphere; | 92.9% |
-
-
303-45-7
(rac)-gossypol

-
-
17460-45-6, 23707-23-5, 23739-93-7, 23743-51-3, 26108-75-8, 59042-16-9, 124516-07-0, 124580-56-9
tetra-acetyl glucosamine

| Conditions | Yield |
|---|---|
| In methanol at 20℃; for 3h; Inert atmosphere; | 92.6% |

| Conditions | Yield |
|---|---|
| With acid In ethanol for 3h; Heating; | 92.3% |

| Conditions | Yield |
|---|---|
| In diethyl ether; isopropyl alcohol at 20 - 45℃; for 2.08333h; Condensation; | 92% |

| Conditions | Yield |
|---|---|
| In diethyl ether; isopropyl alcohol at 20 - 45℃; for 2.08333h; Condensation; | 92% |

| Conditions | Yield |
|---|---|
| In ethanol for 3h; Heating; | 91.9% |
-
-
303-45-7
(rac)-gossypol

-
-
86133-36-0
N-Aminoanabasine

-
-
86133-35-9
5,5'-Diisopropyl-3,3'-dimethyl-8,8'-bis-{[(Z)-3,4,5,6-tetrahydro-2H-[2,3']bipyridinyl-1-ylimino]-methyl}-[2,2']binaphthalenyl-1,6,7,1',6',7'-hexaol

| Conditions | Yield |
|---|---|
| In ethanol Heating; | 91% |
-
-
124-22-1
n-Dodecylamine

-
-
303-45-7
(rac)-gossypol

-
-
5463-57-0
1,6,7,1',6',7'-hexahydroxy-5,5'-diisopropyl-3,3'-dimethyl-[2,2']binaphthyl-8,8'-dicarbaldehyde bis-dodecylimine

| Conditions | Yield |
|---|---|
| In diethyl ether; isopropyl alcohol at 20 - 45℃; for 2.08333h; Condensation; | 91% |

| Conditions | Yield |
|---|---|
| Stage #1: (rac)-gossypol With iron(III) chloride; acetic acid In water; acetone at 100℃; for 2h; Stage #2: With sulfuric acid In diethyl ether; water | 91% |
| With iron(III) chloride In acetic acid; acetone at 60 - 70℃; Oxidation; | 49% |
| With iron(III) chloride; acetic acid In acetone at 70℃; for 0.75h; |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In methanol at 20℃; for 3h; pH=7.4; Inert atmosphere; | 90.8% |

| Conditions | Yield |
|---|---|
| In ethanol for 3h; Heating; | 90.17% |
-
-
60835-71-4
4'-aminobenzo-15-crown-5-ether

-
-
303-45-7
(rac)-gossypol

| Conditions | Yield |
|---|---|
| In ethanol for 6h; Heating; | 90.1% |
-
-
123-00-2
4-(3-Aminopropyl)morpholine

-
-
303-45-7
(rac)-gossypol

-
-
104757-46-2
5,5'-Diisopropyl-3,3'-dimethyl-8,8'-bis-{[(Z)-3-morpholin-4-yl-propylimino]-methyl}-[2,2']binaphthalenyl-1,6,7,1',6',7'-hexaol

| Conditions | Yield |
|---|---|
| In isopropyl alcohol Heating; | 90% |

| Conditions | Yield |
|---|---|
| In diethyl ether; isopropyl alcohol at 20 - 45℃; for 2.08333h; Condensation; | 90% |

| Conditions | Yield |
|---|---|
| In diethyl ether; isopropyl alcohol at 20 - 45℃; for 2.08333h; Condensation; | 90% |
Gossypol Consensus Reports
EPA Genetic Toxicology Program.
Gossypol Specification
The Gossypol, with the CAS registry number 303-45-7, is also known as 2,2'-Bis(8-Formyl-1,6,7-trihydroxy-5-isopropyl-3-methylnaphthalene). It belongs to the product categories of Aromatic Phenols; Antitumour. This chemical's molecular formula is C30H30O8 and molecular weight is 518.55. What's more, its IUPAC name is called 7-(8-Formyl-1,6,7-trihydroxy-3-methyl-5-propan-2-ylnaphthalen-2-yl)-2,3,8-trihydroxy-6-methyl-4-propan-2-ylnaphthalene-1-carbaldehyde. Gossypol is a natural phenol derived from the cotton plant (genus Gossypium). Gossypol is a phenolic aldehyde that permeates cells and acts as an inhibitor for several dehydrogenase enzymes. It is a yellow pigment. And this chemical should be kept in a cold, dry place.
Physical properties about Gossypol are: (1)ACD/LogP: 5.42; (2)# of Rule of 5 Violations: 3; (3)ACD/LogD (pH 5.5): 5.405; (4)ACD/LogD (pH 7.4): 4.795; (5)ACD/BCF (pH 5.5): 7488.263; (6)ACD/BCF (pH 7.4): 1838.539; (7)ACD/KOC (pH 5.5): 20462.531; (8)ACD/KOC (pH 7.4): 5024.019; (9)#H bond acceptors: 8; (10)#H bond donors: 6; (11)#Freely Rotating Bonds: 11; (12)Polar Surface Area: 155.52 Å2; (13)Index of Refraction: 1.742; (14)Molar Refractivity: 149.345 cm3; (15)Molar Volume: 369.555 cm3; (16)Surface Tension: 71.297 dyne/cm; (17)Density: 1.403 g/cm3; (18)Flash Point: 395.872 °C; (19)Enthalpy of Vaporization: 107.239 kJ/mol; (20)Boiling Point: 707.888 °C at 760 mmHg; (21)Vapour Pressure: 0 mmHg at 25 °C.
Preparation of Gossypol: it is a polyphenol derived from the cotton plant (genus Gossypium, family Malvaceae). It is formed metabolically through acetate via the isopernoid pathway.
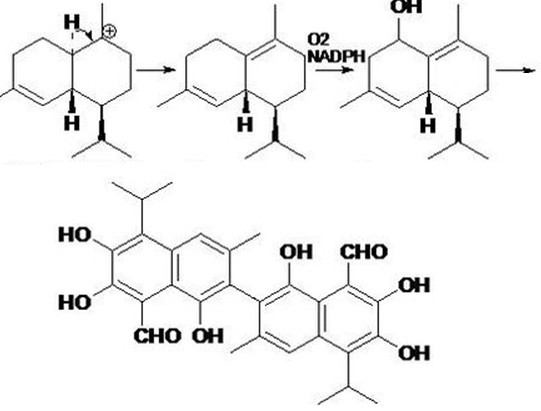
Uses of Gossypol: it is used to produce other chemicals. For example, it can react with Ethylamine to get 1,6,7,1',6',7'-Hexahydroxy-5,5'-diisopropyl-3,3'-dimethyl-[2,2']binaphthyl-8,8'-dicarbaldehyde bis-ethylimine. The reaction occurs with solvents diethyl ether, propan-2-ol at temperature of 20-45 °C. The yield is 96 %.
![Gossypol can react with Ethylamine to get 1,6,7,1',6',7'-Hexahydroxy-5,5'-diisopropyl-3,3'-dimethyl-[2,2']binaphthyl-8,8'-dicarbaldehyde bis-ethylimine.](/UserFilesUpload/Uses of Gossypol.jpg)
When you are dealing with this chemical, you should be very careful. This chemical is inflammation to the skin, eyes and respiratory system or other mucous membranes. If swallowed, it's harmful to health. It may cause damage to health. Therefore, you should wear suitable protective clothing, gloves and eye/face protection. And you must take off immediately all contaminated clothing. In case of contacting with eyes, you should rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
(1) SMILES: Cc1cc2c(c(c(c(c2C(C)C)O)O)C=O)c(c1c3c(cc4c(c3O)c(c(c(c4C(C)C)O)O)C=O)C)O
(2) InChI: InChI=1S/C30H30O8/c1-11(2)19-15-7-13(5)21(27(35)23(15)17(9-31)25(33)29(19)37)22-14(6)8-16-20(12(3)4)30(38)26(34)18(10-32)24(16)28(22)36/h7-12,33-38H,1-6H3
(3) InChIKey: QBKSWRVVCFFDOT-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| pig | LD50 | oral | 550mg/kg (550mg/kg) | BEHAVIORAL: MUSCLE WEAKNESS GASTROINTESTINAL: "HYPERMOTILITY, DIARRHEA" | Journal of the American Oil Chemists' Society. Vol. 40, Pg. 571, 1963. |
| rat | LD50 | oral | 2315mg/kg (2315mg/kg) | Journal of the American Oil Chemists' Society. Vol. 37, Pg. 40, 1960. |
Related Products
- Gossypol
- Gossypol acetate
- Gossypol-acetic acid
- 3034-57-9
- 3034-62-6
- 30346-88-4
- 303-47-9
- 3034-86-4
- 303-49-1
- 3034-94-4
- 303-53-7
- 30355-60-3
- 30357-42-7
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View