 This product is a nationally controlled contraband, and the Lookchem platform doesn't provide relevant sales information.
This product is a nationally controlled contraband, and the Lookchem platform doesn't provide relevant sales information.
Synthetic route

| Conditions | Yield |
|---|---|
| With solution aqueuse d'hydroxide de sodium; tetrabutylammomium bromide In dichloromethane 1)room temp. 12h 2)reflux 3h; | 100% |

-
-
58-08-2
3,7-dihydro-1,3,7-trimethyl-1H-purine-2,6-dione

| Conditions | Yield |
|---|---|
| In xylene at 140℃; for 0.75h; Rearrangement; | 100% |
-
-
3485-82-3
theophylline sodium salt

-
-
616-38-6
carbonic acid dimethyl ester

-
-
58-08-2
3,7-dihydro-1,3,7-trimethyl-1H-purine-2,6-dione

| Conditions | Yield |
|---|---|
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In water; ethylene glycol at 135℃; for 4h; Autoclave; Green chemistry; | 98.4% |
| With PEG 400 In 5,5-dimethyl-1,3-cyclohexadiene at 140℃; for 12h; Solvent; Concentration; Temperature; Reagent/catalyst; | 94.9% |
| With NaY (Y-type sodium zeolite catalyst); turkey red oil In water for 5h; Reflux; |
-
-
74-83-9
methyl bromide

-
-
58-55-9
theophylline

-
A
-
58-08-2
3,7-dihydro-1,3,7-trimethyl-1H-purine-2,6-dione

-
B
-
7280-81-1
3-benzyladenine

| Conditions | Yield |
|---|---|
| With solution aqueuse d'hydroxide de sodium; tetrabutylammomium bromide In dichloromethane 1)20 deg C, 12h 2)40 deg C, 3h; | A 98% B n/a |

-
-
107-31-3
Methyl formate

-
-
57533-87-6
1,3-Dimethylxanthine potassium salt

-
A
-
58-55-9
theophylline

-
B
-
58-08-2
3,7-dihydro-1,3,7-trimethyl-1H-purine-2,6-dione

| Conditions | Yield |
|---|---|
| In methanol | A 97.5% B n/a |

-
-
67-56-1
methanol

-
-
57533-87-6
1,3-Dimethylxanthine potassium salt

-
A
-
107-31-3
Methyl formate

-
B
-
58-08-2
3,7-dihydro-1,3,7-trimethyl-1H-purine-2,6-dione

| Conditions | Yield |
|---|---|
| With carbon monoxide at 140℃; under 37503 Torr; for 20h; | A n/a B 97% |


| Conditions | Yield |
|---|---|
| With sodium hydride In dimethyl sulfoxide for 0.75h; | 95% |
| With sodium hydride In dimethyl sulfoxide at 20℃; for 24h; Reagent/catalyst; | 90% |
-
-
74-83-9
methyl bromide

-
-
83-67-0
theobromine /

-
A
-
58-08-2
3,7-dihydro-1,3,7-trimethyl-1H-purine-2,6-dione

-
B
-
7280-81-1
3-benzyladenine

| Conditions | Yield |
|---|---|
| With solution aqueuse d'hydroxide de sodium; tetrabutylammomium bromide In dichloromethane 1)20 deg C, 12h 2)40 deg C, 3h; | A 94% B n/a |

-
-
1076-22-8
3-methylxanthine

-
-
77-78-1
dimethyl sulfate

-
-
58-08-2
3,7-dihydro-1,3,7-trimethyl-1H-purine-2,6-dione

| Conditions | Yield |
|---|---|
| With tetramethyl ammoniumhydroxide; potassium carbonate In methanol; water at 65 - 70℃; | 92.5% |
-
-
83-67-0
theobromine /

-
-
77-78-1
dimethyl sulfate

-
-
58-08-2
3,7-dihydro-1,3,7-trimethyl-1H-purine-2,6-dione

| Conditions | Yield |
|---|---|
| potassium fluoride on basic alumina In acetonitrile for 24h; Ambient temperature; | 92% |
| With sodium hydroxide |
-
-
58-55-9
theophylline

-
-
616-38-6
carbonic acid dimethyl ester

-
-
58-08-2
3,7-dihydro-1,3,7-trimethyl-1H-purine-2,6-dione

| Conditions | Yield |
|---|---|
| With cetyltrimethylammonium bromide; N-ethyl-N,N-diisopropylamine In toluene at 140℃; for 9h; Solvent; Concentration; Reagent/catalyst; Temperature; | 90.7% |
| With 18-crown-6 ether In N,N-dimethyl-formamide at 90℃; for 12h; | 67% |
| With DABCO-NaY (1:1 DABCO Y-type sodium zeolite catalyst); turkey red oil In water for 5h; Reagent/catalyst; Reflux; |
-
-
58-55-9
theophylline

-
-
74-88-4
methyl iodide

-
A
-
58-08-2
3,7-dihydro-1,3,7-trimethyl-1H-purine-2,6-dione

-
B
-
7280-81-1
3-benzyladenine

| Conditions | Yield |
|---|---|
| With solution aqueuse d'hydroxide de sodium; tetrabutylammomium bromide In dichloromethane 1)20 deg C, 12h 2)40 deg C, 3h; | A 90% B n/a |

-
-
58-55-9
theophylline

-
-
77-78-1
dimethyl sulfate

-
-
58-08-2
3,7-dihydro-1,3,7-trimethyl-1H-purine-2,6-dione

| Conditions | Yield |
|---|---|
| potassium fluoride on basic alumina In acetonitrile for 24h; Ambient temperature; | 88% |
| With sodium hydroxide | |
| With sodium hydroxide | |
| With potassium hydroxide; Aliquat 336 1.) 20 deg C, 2 h, 2.) 1 h; Yield given. Multistep reaction; |
-
-
58-55-9
theophylline

-
-
10504-60-6
diphenylmethylsulfonium tetrafluoroborate

-
-
58-08-2
3,7-dihydro-1,3,7-trimethyl-1H-purine-2,6-dione

| Conditions | Yield |
|---|---|
| With potassium hydroxide In water; N,N-dimethyl-formamide at 20℃; for 3h; | 88% |
-
-
353-39-9
Trimethylsulfonium Fluoride

-
-
58-55-9
Theophylline

-
-
58-08-2
3,7-dihydro-1,3,7-trimethyl-1H-purine-2,6-dione

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 100℃; for 1h; | 86% |
-
-
69-89-6
xanthin

-
-
6257-10-9
N,N'-dicyclohexyl-O-methyl isourea

-
-
58-08-2
3,7-dihydro-1,3,7-trimethyl-1H-purine-2,6-dione

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 100℃; for 48h; | 85% |

| Conditions | Yield |
|---|---|
| With copper(I) thiophene-2-carboxylate; (4,4'-di-tert-butyl-2,2'-dipyridyl)-bis-(5-methyl-2-(4-fluorophenyl)pyridine(-1H))-iridium(III) hexafluorophosphate; N,N,N′,N′-tetramethyl-N″-tert-butylguanidine; bathophenanthroline; iodomesitylene diacetate In 1,4-dioxane at 20℃; for 1h; Inert atmosphere; Irradiation; regioselective reaction; | 82% |
-
-
107605-83-4
4--1-methyl-5-methylaminocarbonylimidazole

-
-
58-08-2
3,7-dihydro-1,3,7-trimethyl-1H-purine-2,6-dione

| Conditions | Yield |
|---|---|
| With sodium hydride In ethanol for 1.5h; Heating; | 81% |
-
-
83-67-0
theobromine /

-
-
616-38-6
carbonic acid dimethyl ester

-
-
58-08-2
3,7-dihydro-1,3,7-trimethyl-1H-purine-2,6-dione

| Conditions | Yield |
|---|---|
| With 18-crown-6 ether In N,N-dimethyl-formamide at 90℃; for 12h; | 78% |
-
-
113613-79-9
1-methyl-9-aminoxanthine potassium salt

-
-
74-88-4
methyl iodide

-
-
58-08-2
3,7-dihydro-1,3,7-trimethyl-1H-purine-2,6-dione

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 100℃; for 3h; | 76% |
-
-
616-38-6
carbonic acid dimethyl ester

-
-
69-89-6
xanthin

-
-
58-08-2
3,7-dihydro-1,3,7-trimethyl-1H-purine-2,6-dione

| Conditions | Yield |
|---|---|
| With 18-crown-6 ether In N,N-dimethyl-formamide at 90℃; for 12h; | 74% |
-
-
10381-82-5
8-bromocaffeine

-
-
58-08-2
3,7-dihydro-1,3,7-trimethyl-1H-purine-2,6-dione

| Conditions | Yield |
|---|---|
| With copper In N,N-dimethyl-formamide for 2h; Heating; | 72% |
-
-
113613-76-6
1-methyl-9-aminoxanthine

-
-
74-88-4
methyl iodide

-
-
58-08-2
3,7-dihydro-1,3,7-trimethyl-1H-purine-2,6-dione

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 100℃; for 1h; | 71% |
-
-
107605-87-8
4-(N-trichloroacetyl-N-methylamino)-1-methyl-5-methylaminocarbonylimidazole

-
-
58-08-2
3,7-dihydro-1,3,7-trimethyl-1H-purine-2,6-dione

| Conditions | Yield |
|---|---|
| With sodium hydride In ethanol for 2h; Heating; | 66% |

| Conditions | Yield |
|---|---|
| With cerium(III) chloride; 9,10-diphenylanthracene; 1,2-bis(2,4,6-triisopropylphenyl)disulfane; tetrabutyl-ammonium chloride In acetonitrile for 168h; Irradiation; Inert atmosphere; | 63% |

| Conditions | Yield |
|---|---|
| potassium fluoride on basic alumina In acetonitrile for 24h; Ambient temperature; | 61% |
| With alkaline solution | |
| With sodium hydroxide |
-
-
83-67-0
theobromine /

-
-
74-88-4
methyl iodide

-
A
-
58-08-2
3,7-dihydro-1,3,7-trimethyl-1H-purine-2,6-dione

-
B
-
7280-81-1
3-benzyladenine

| Conditions | Yield |
|---|---|
| With solution aqueuse d'hydroxide de sodium; tetrabutylammomium bromide In dichloromethane 1)20 deg C, 12h 2)40 deg C, 3h; | A 56% B n/a |

-
-
18623-34-2
1,3,7,9-tetramethylxanthinium methyl sulfate

-
-
58-08-2
3,7-dihydro-1,3,7-trimethyl-1H-purine-2,6-dione

| Conditions | Yield |
|---|---|
| at 260℃; for 15h; | 25% |
-
-
58-55-9
theophylline

-
-
80-48-8
methyl p-toluene sulfonate

-
-
58-08-2
3,7-dihydro-1,3,7-trimethyl-1H-purine-2,6-dione

| Conditions | Yield |
|---|---|
| With sodium hydroxide |

| Conditions | Yield |
|---|---|
| With chlorine In chloroform | 100% |
| With chlorine In chloroform at 50℃; for 2h; | 100% |
| With trichloroisocyanuric acid; brilliant green carbocation In acetonitrile at 20℃; for 2h; Irradiation; regioselective reaction; | 78% |
-
-
58-08-2
3,7-dihydro-1,3,7-trimethyl-1H-purine-2,6-dione

-
-
10381-82-5
8-bromocaffeine

| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide In dichloromethane; water for 120h; | 100% |
| With N-Bromosuccinimide In dichloromethane; water for 120h; | 99% |
| With N-Bromosuccinimide In dichloromethane; water for 120h; | 99% |
-
-
58-08-2
3,7-dihydro-1,3,7-trimethyl-1H-purine-2,6-dione

| Conditions | Yield |
|---|---|
| With [(N,N’-bis(1R,2R,3R,5S)-(−)-isopinocampheyl-1,2-ethanediimine-radical)NiI(μ2-H)]2; deuterium In tetrahydrofuran at -196 - 45℃; under 760.051 Torr; for 24h; Sealed tube; | 100% |
| With deuteromethanol; silver carbonate; johnphos In dichloromethane at 50 - 80℃; | 98% |
| With (1,5-cyclooctadiene)(methoxy)iridium(I) dimer; deuterium In tetrahydrofuran at 55℃; under 750.075 Torr; for 22h; Inert atmosphere; | 92% |
| With palladium 10% on activated carbon; hydrogen; water-d2 at 90℃; for 1.5h; | 86.9% |
| With water-d2 at 150℃; for 0.25h; Microwave irradiation; |

| Conditions | Yield |
|---|---|
| With palladium diacetate; caesium carbonate; tricyclohexylphosphine tetrafluoroborate In toluene at 20 - 130℃; for 24h; Inert atmosphere; | 99% |
| With potassium phosphate; palladium diacetate In 1-methyl-pyrrolidin-2-one at 125℃; for 24h; | 86% |
-
-
104-92-7
1-bromo-4-methoxy-benzene

-
-
58-08-2
3,7-dihydro-1,3,7-trimethyl-1H-purine-2,6-dione

-
-
439927-43-2
1,3,7-trimethyl-8-(p-methoxyphenyl)-xanthine

| Conditions | Yield |
|---|---|
| With potassium phosphate; palladium diacetate; Trimethylacetic acid In N,N-dimethyl-formamide at 20 - 120℃; for 7.16667h; Inert atmosphere; | 99% |
| With potassium phosphate; copper(l) iodide; 1,10-Phenanthroline In 5,5-dimethyl-1,3-cyclohexadiene; N,N-dimethyl-formamide at 20 - 140℃; for 36.1667h; Inert atmosphere; regioselective reaction; | 98% |
-
-
58-08-2
3,7-dihydro-1,3,7-trimethyl-1H-purine-2,6-dione

-
-
501-65-5
diphenyl acetylene

-
-
99765-12-5
(E)-8-(1,2-Diphenylethenyl)-3,7-dihydro-1,3,7-trimethyl-1H-purin-2,6-dion

| Conditions | Yield |
|---|---|
| With chloro(1,5-cyclooctadiene)rhodium(I) dimer; cesium acetate; 1,2-bis-(diphenylphosphino)ethane In toluene at 120℃; for 24h; Sealed vial; optical yield given as %de; stereoselective reaction; | 99% |
| With bis(dibenzylideneacetone)-palladium(0); tricyclohexylphosphine; Trimethylacetic acid In N,N-dimethyl acetamide at 130℃; for 14h; Inert atmosphere; Glovebox; stereoselective reaction; | 69% |
-
-
401485-19-6
tert-butyl N-(thiophen-2-ylmethyl)carbamate

-
-
58-08-2
3,7-dihydro-1,3,7-trimethyl-1H-purine-2,6-dione

-
-
1403938-43-1
tert-butyl N-((5-(1,3,7-trimethyl-2,6-dioxo-2,3,6,7-tetrahydro-1H-purin-8-yl) thiophen-2-yl)methyl)carbamate

| Conditions | Yield |
|---|---|
| With pyridine; palladium diacetate; copper(II) acetate monohydrate; copper(l) chloride In 1,4-dioxane at 120℃; for 20h; Schlenk technique; Inert atmosphere; | 99% |
-
-
58-08-2
3,7-dihydro-1,3,7-trimethyl-1H-purine-2,6-dione

-
-
612-55-5
2-iodonaphthalene

-
-
1257390-78-5
1,3,7-trimethyl-8-(naphthalen-2-yl)-xanthine

| Conditions | Yield |
|---|---|
| With (1-(2-(tert-butylamino)ethyl)-3-mesityl-2,3-dihydro-1H-imidazol-2-yl)copper(I) iodide; lithium tert-butoxide In N,N-dimethyl-formamide at 140℃; for 8h; Glovebox; | 99% |

| Conditions | Yield |
|---|---|
| With tetrabutylammonium tetrafluoroborate In acetonitrile at 45℃; for 10h; Temperature; Inert atmosphere; Electrochemical reaction; Green chemistry; diastereoselective reaction; | 99% |

| Conditions | Yield |
|---|---|
| With tetrabutylammonium tetrafluoroborate In acetonitrile at 45℃; for 10h; Inert atmosphere; Electrochemical reaction; Green chemistry; diastereoselective reaction; | 99% |
-
-
58-08-2
3,7-dihydro-1,3,7-trimethyl-1H-purine-2,6-dione

-
-
13808-64-5
4-bromo-3-methyl-1H-pyrazole

| Conditions | Yield |
|---|---|
| With tetrabutylammonium tetrafluoroborate In acetonitrile at 45℃; for 10h; Inert atmosphere; Electrochemical reaction; Green chemistry; diastereoselective reaction; | 99% |

| Conditions | Yield |
|---|---|
| In methanol; cyclohexane at 17 - 22℃; under 724.572 - 782.178 Torr; for 0.5h; | 98.3% |
-
-
58-08-2
3,7-dihydro-1,3,7-trimethyl-1H-purine-2,6-dione

-
-
32061-73-7
2,6-dithiocaffeine

| Conditions | Yield |
|---|---|
| With Lawessons reagent; aluminum oxide for 0.1h; microwave irradiation; | 98% |
| With Lawessons reagent In toluene for 25h; Reflux; | 75% |
| With bis(1,5-cyclooctadiylboryl)sulfide In 1,3,5-trimethyl-benzene for 336h; Heating; | 62% |
| With kerosine |
-
-
15199-43-6
d6-dimethyl sulfate

-
-
58-08-2
3,7-dihydro-1,3,7-trimethyl-1H-purine-2,6-dione

| Conditions | Yield |
|---|---|
| In nitrobenzene at 90 - 100℃; for 25h; | 98% |
-
-
64-17-5
ethanol

-
-
58-08-2
3,7-dihydro-1,3,7-trimethyl-1H-purine-2,6-dione

-
-
32086-89-8
8-(1-hydroxyethyl)-1,3,7-trimethyl-3,7-dihydro-1H-purine-2,6-dione

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide In decane at 120℃; for 0.333333h; Reagent/catalyst; Solvent; Temperature; Time; Microwave irradiation; Green chemistry; | 98% |
| With di-tert-butyl peroxide In water at 20℃; Inert atmosphere; UV-irradiation; | 96% |
| With benzophenone Ambient temperature; Irradiation; |
-
-
108-86-1
bromobenzene

-
-
58-08-2
3,7-dihydro-1,3,7-trimethyl-1H-purine-2,6-dione

-
-
6439-88-9
1,3,7-trimethyl-8-phenylxanthine

| Conditions | Yield |
|---|---|
| With potassium phosphate; palladium diacetate; Trimethylacetic acid In N,N-dimethyl-formamide at 20 - 120℃; for 7.16667h; Inert atmosphere; | 98% |
| With potassium phosphate; copper(l) iodide; 1,10-Phenanthroline In 5,5-dimethyl-1,3-cyclohexadiene; N,N-dimethyl-formamide at 20 - 140℃; for 36.1667h; Inert atmosphere; regioselective reaction; | 93% |
-
-
58-08-2
3,7-dihydro-1,3,7-trimethyl-1H-purine-2,6-dione

-
-
573-17-1
9-bromophenanthrene

-
-
99765-06-7
3,7-Dihydro-1,3,7-trimethyl-8-(9-phenanthryl)-1H-purin-2,6-dion

| Conditions | Yield |
|---|---|
| With potassium phosphate; palladium diacetate; Trimethylacetic acid In N,N-dimethyl-formamide at 20 - 120℃; for 7.16667h; Inert atmosphere; | 98% |
| With potassium phosphate; copper(l) iodide; 1,10-Phenanthroline In 5,5-dimethyl-1,3-cyclohexadiene; N,N-dimethyl-formamide at 20 - 140℃; for 36.1667h; Inert atmosphere; regioselective reaction; | 95% |
-
-
58-08-2
3,7-dihydro-1,3,7-trimethyl-1H-purine-2,6-dione

-
-
1220356-33-1
8-deuterio-1,3,7-tris(trideuteriomethyl)xanthine

| Conditions | Yield |
|---|---|
| With palladium 10% on activated carbon; hydrogen; water-d2 at 160℃; for 24h; | 98% |

| Conditions | Yield |
|---|---|
| Stage #1: 3,7-dihydro-1,3,7-trimethyl-1H-purine-2,6-dione; trimethoxonium tetrafluoroborate In acetonitrile at 20℃; for 0.0833333h; Inert atmosphere; Schlenk technique; Stage #2: With sodium carbonate In acetonitrile at 20℃; for 0.916667h; | 97% |
| In 1,2-dichloro-ethane at 100℃; for 1h; | 66% |
| In 1,2-dichloro-ethane Inert atmosphere; Schlenk technique; Reflux; |
-
-
58-08-2
3,7-dihydro-1,3,7-trimethyl-1H-purine-2,6-dione

-
-
696-62-8
para-iodoanisole

-
-
439927-43-2
1,3,7-trimethyl-8-(p-methoxyphenyl)-xanthine

| Conditions | Yield |
|---|---|
| With (1-(2-(tert-butylamino)ethyl)-3-mesityl-2,3-dihydro-1H-imidazol-2-yl)copper(I) iodide; lithium tert-butoxide In N,N-dimethyl-formamide at 140℃; for 8h; Glovebox; | 97% |
| With potassium phosphate; palladium diacetate; Trimethylacetic acid In N,N-dimethyl-formamide at 20 - 120℃; for 7.16667h; Inert atmosphere; | 92% |
-
-
58-08-2
3,7-dihydro-1,3,7-trimethyl-1H-purine-2,6-dione

-
-
15802-75-2
4-iodo-3-methyl-1H-pyrazole

| Conditions | Yield |
|---|---|
| With tetrabutylammonium tetrafluoroborate In acetonitrile at 45℃; for 10h; Inert atmosphere; Electrochemical reaction; Green chemistry; diastereoselective reaction; | 97% |

| Conditions | Yield |
|---|---|
| With potassium phosphate; palladium diacetate; Trimethylacetic acid In N,N-dimethyl-formamide at 20 - 120℃; for 7.16667h; Inert atmosphere; | 96% |
| With potassium phosphate; copper(l) iodide; 1,10-Phenanthroline In 5,5-dimethyl-1,3-cyclohexadiene; N,N-dimethyl-formamide at 20 - 140℃; for 36.1667h; Inert atmosphere; regioselective reaction; | 96% |
| With 2-ethyl-[1,2,4] triazolo[4,3-a]pyridin-2-ium tetrafluoroborate; palladium diacetate; potassium carbonate In N,N-dimethyl-formamide at 115℃; for 22h; Inert atmosphere; | 94% |
-
-
58-08-2
3,7-dihydro-1,3,7-trimethyl-1H-purine-2,6-dione

-
-
95-46-5
2-methylphenyl bromide

-
-
1137486-72-6
1,3,7-trimethyl-8-(o-tolyl)-xanthine

| Conditions | Yield |
|---|---|
| With potassium phosphate; palladium diacetate; Trimethylacetic acid In N,N-dimethyl-formamide at 20 - 120℃; for 7.16667h; Inert atmosphere; | 96% |
| With potassium phosphate; copper(l) iodide; 1,10-Phenanthroline In 5,5-dimethyl-1,3-cyclohexadiene; N,N-dimethyl-formamide at 20 - 140℃; for 36.1667h; Inert atmosphere; regioselective reaction; | 88% |
-
-
554-14-3
2-Methylthiophene

-
-
58-08-2
3,7-dihydro-1,3,7-trimethyl-1H-purine-2,6-dione

-
-
1207995-92-3
1,3,7-trimethyl-8-(5-methylthiophen-2-yl)-2,3,6,7-tetrahydro-1H-purine-2,6-dione

| Conditions | Yield |
|---|---|
| Stage #1: 3,7-dihydro-1,3,7-trimethyl-1H-purine-2,6-dione With pyridine; palladium diacetate; copper(II) acetate monohydrate In 1,4-dioxane at 20℃; for 0.166667h; Inert atmosphere; Stage #2: 2-Methylthiophene In 1,4-dioxane at 120℃; for 20h; Inert atmosphere; | 96% |
| With pyridine; palladium diacetate; copper(II) acetate monohydrate In 1,4-dioxane at 120℃; for 24h; Schlenk technique; Inert atmosphere; | 92% |
| With pyridine; (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; oxygen; copper(II) acetate monohydrate In 1,4-dioxane at 140℃; under 760.051 Torr; for 30h; Schlenk technique; Green chemistry; | 89% |
Related products
Raw Materials
Downstream Products
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View
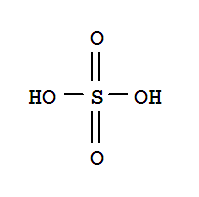






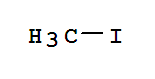








 Xn,
Xn,  T
T