Dayang Chem (Hangzhou) Co.,Ltd.
Dayangchem's R&D center can offer custom synthesis according to the contract research and development services for the fine chemicals, pharmaceutical, biotechnique and some of the other chemicals. DayangChem can provide different quantities
Cas:594-09-2
Min.Order:1 Kilogram
FOB Price: $3.0
Type:Lab/Research institutions
inquiryChemwill Asia Co., Ltd.
Our main production base is located in Xuzhou industry park. We are certified both to the ISO 9001 and ISO 14001 Standards, have a safety management system in place.Our R&D team masters core technology for process-design of target building block
Cas:594-09-2
Min.Order:5 Kiloliter
FOB Price: $1.2 / 5.0
Type:Manufacturers
inquiryZhuozhou Wenxi import and Export Co., Ltd
WITH US,YOUR MONEY IN SAFE,YOUR BUSINESS IN SAFE 1)Quick Response Within 12 hours; 2)Quality Guarantee: All products are strictly tested by our QC, confirmed by QA and approved by third party lab in China, USA, Canada, Germany, UK, Italy, France et
Cas:594-09-2
Min.Order:1 Kilogram
FOB Price: $139.0 / 210.0
Type:Trading Company
inquiryHebei yanxi chemical co.,LTD.
hebei yanxi chemical co., LTD who registered capital of 10 million yuan, nearly to $2 million, we have a pharmaceutical raw materials factory production of pharmaceutical raw materials, and a reagent r&d center, and we do research and developmen
Cas:594-09-2
Min.Order:1 Kilogram
FOB Price: $2.0 / 6.0
Type:Trading Company
inquiryHebei Sankai Chemical Technology Co., Ltd
1. Product advantages High purity, all above 98.5%, no impurities after dissolution We will test each batch to ensure quality OEM and private brand services designed for free Various cap colors available We can also provide MT1 peptide powd
Cas:594-09-2
Min.Order:1 Kilogram
FOB Price: $2.5 / 5.0
Type:Trading Company
inquiryHenan Wentao Chemical Product Co., Ltd.
We are leading fine chemicals supplier in China with ISO certificate, Our main business covers the fields below: 1.Noble Metal Catalysts (Pt.Pd...) 2.Organic Phosphine Ligands (Tert-butyl-phosphine.Cyclohexyl-phosphine...) 3.OLED
Cas:594-09-2
Min.Order:1 Gram
FOB Price: $3.0
Type:Lab/Research institutions
inquiryHebei Nengqian Chemical Import and Export Co., LTD
Our advantages: 1. All inquiries will be replied within 12 hours. 2. Dedication to quality, supply & service. 3. Strictly on selecting raw materials. 4. Reasonable & competitive price, fast lead time. 5. Sample is available for your eva
Cas:594-09-2
Min.Order:1 Kilogram
FOB Price: $9.0 / 99.0
Type:Trading Company
inquiryShanghai Upbio Tech Co.,Ltd
1.In No Less 10 years exporting experience. you can 100% received goods 2.Lower Price with higher quality 3,Free sample 4,We are sincerely responsible for the "product quality" and "After Service" Upbio is Specializ
Cas:594-09-2
Min.Order:1 Gram
Negotiable
Type:Lab/Research institutions
inquiryBaoji Guokang Healthchem co.,ltd
Our company has been in existence for 10 years since its establishment. We have our own unique team. The company integrates independent research and development, production and sales. We have established famous brands at home and abroad. At prese
Cas:594-09-2
Min.Order:1 Kilogram
FOB Price: $90.5 / 94.6
Type:Trading Company
inquiryQingdao Beluga Import and Export Co., LTD
Trimethylphosphine CAS:594-09-2 Qingdao Belugas Import and Export Co., Ltd. is a scientific and technological company integrating research and development, production and trade of chemical intermediates, specializing in high quality organic intermed
Cas:594-09-2
Min.Order:1 Gram
Negotiable
Type:Lab/Research institutions
inquiryShandong Hanjiang Chemical Co., Ltd.
Hello, dear friend! I'm Hansen and Allen from China. Welcome to my lookchem mall! The following is a brief introduction of our company's products and services. If you are interested in our products, please contact us by emai
Cas:594-09-2
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryHangzhou J&H Chemical Co., Ltd.
J&H CHEM R&D center can offer custom synthesis according to the contract research and development services for the fine chemicals, pharmaceutical, biotechnique and some of the other chemicals. J&H CHEM has some Manufacturing base in Jia
Ality Chemical Corporation
The above product is Ality Chemical's strong item with best price, good quality and fast supply. Ality Chemical has been focusing on the research and production of this field for over 14 years. At the same time, we are always committed to providi
Shanghai Massive Chemical Technology Co., Ltd.
Massive Chemical is certified with ISO9001 and ISO14001 manufacturer for this product. We will offer all documents as requirement for the materials which includes, Certificate of Analysis, Material Safety Data Sheet, and Method of Analysis and
Cas:594-09-2
Min.Order:1 Gram
FOB Price: $1.0
Type:Lab/Research institutions
inquiryZibo Hangyu Biotechnology Development Co., Ltd
Zibo Hangyu Biotechnology Development Co., Ltd is a leading manufacturer and supplier of chemicals in China. We develop produce and distribute high quality pharmaceuticals, intermediates, special chemicals and OLED intermediates and other fine chemi
Cas:594-09-2
Min.Order:10 Gram
FOB Price: $100.0
Type:Lab/Research institutions
inquiryShanghai Minstar Chemical Co., Ltd
Trimethylphosphine Chemical Properties Melting point -86 °C (lit.) Boiling point 38-40 °C (lit.) density 0.738 g/mL at 20 °C (lit.) vapor pressure 7.24 psi ( 20 °C) refractive index n20/D 1.428(lit.) Fp −
Cas:594-09-2
Min.Order:1 Gram
FOB Price: $66.0
Type:Lab/Research institutions
inquiryHenan Tianfu Chemical Co., Ltd.
594-09-2 Application:594-09-2
Cas:594-09-2
Min.Order:0 Metric Ton
Negotiable
Type:Lab/Research institutions
inquiryHunan chemfish Pharmaceutical co.,Ltd
Appearance:95%+ Package:R&D,Pilot run Transportation:per client require Port:Express ,Air, Sea
Suzhou Health Chemicals Co., Ltd.
High quality,stable supply chain.Appearance:white/off-white or light yellow Storage:Store in cool and dry place, keep away from strong light and heat. Package:aluminum bottle,glass bottle,PTFE bottle,cardboard drum Application:This product can be use
GIHI CHEMICALS CO.,LIMITED
Lower price, sample is available,SDS test documents are available,large stock in warehouseAppearance:White powder Storage:Sealed and preserved Package:200/Kilograms Application:Fine chemical intermediates, used as the main raw material for the synthe
JINHUA HUAYI CHEMICAL CO., LTD.
Jinhua huayi chemical co., ltd. is dedicated to the development, production and marketing of chemicals. On the basis of equality and mutual benefit, and under the principle of customer first, credit first, quality first, we are ready to join hands
Cas:594-09-2
Min.Order:100 Gram
Negotiable
Type:Lab/Research institutions
inquiryHenan Allgreen Chemical Co.,Ltd
high quality Storage:Sealed, dry, microtherm , avoid light and smell. Package:According to the demand of customer Application:Organic synthesis Transportation:by air or by sea
Antimex Chemical Limied
Ansciep Chemical is a professional enterprise manufacturing and distributing fine chemicals and speciality chemicals. We have been dedicated to heterocycle compounds and phenyl rings for tens of years. This is our mature product for export. Our quali
Cas:594-09-2
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryXiamen AmoyChem Co.,Ltd
Amoychem is committed to providing the top-quality chemical products and services Internationally. We offer our customers with friendly, professional service and reliable, high performance products that have been manufactured according to the accredi
Guangdong Juda Chemical Industrial Co.,Limited
Appearance:solid or liquid Storage:sealed in cool and dry place Package:As customer's requested Application:Pharma Intermediate Transportation:by courier/air/sea Port:Any port in China
Hangzhou Fandachem Co.,Ltd
Trimethylphosphine cas 594-09-2Appearance:white crystalline powder Storage:Store in dry, dark and ventilated place Package:25KG drum Application:intermediate Transportation:by air, by sea, by express
Henan Kanbei Chemical Co.,LTD
factory?direct?saleAppearance:White powder Storage:Sealed and preserved Package:200/Kilograms Application:healing drugs Transportation:By sea Port:Shanghai/tianjin
Chemlyte Solutions
Stock products, own laboratory Package:Grams, Kilograms Application:For R&D Transportation:According to customer request Port:Shanghai
Shanghai Chinqesen Biotechnology Co., Ltd.
Good Quality Package:1kg/bag Application:Medical or chemical Transportation:Air/Train/Sea Port:Shenzhen
Hunan Russell Chemicals Technology Co.,Ltd
high purity lowest priceAppearance:solid or liquid Storage:in sealed air resistant place Package:Foil bag; Drum; Plastic bottle Application:Pharma;Industry;Agricultural Transportation:by sea or air Port:any port in China
Synthetic route

| Conditions | Yield |
|---|---|
| In acetone for 1 h; evapd. slowly under vacuo; | A 98% B n/a |
| In [(2)H6]acetone at room temp. for 1 day; | |
| In tetrahydrofuran Kinetics; monitored by UV; |
-
-
593-75-9, 685498-28-6
methyl isocyanate

-
B
-
594-09-2
trimethylphosphane

| Conditions | Yield |
|---|---|
| In diethyl ether under Ar, reaction temp.: -78°C, warmed to room temp.; removal of solvent, extn. (pentane), brought to dryness in vac., stored at -78°C for 2 days; | A 95% B n/a |

-
-
74-87-3
methylene chloride

-
-
7803-51-2
phosphan

-
A
-
593-54-4
methylphosphine

-
B
-
676-59-5
dimethylphosphane

-
C
-
880343-42-0
chloro-tetramethyl-phosphorane

-
D
-
594-09-2
trimethylphosphane

| Conditions | Yield |
|---|---|
| With potassium hydroxide; PTK In toluene at 15℃; for 18h; | A 4% B 92% C 4 g D 2% |

-
-
114130-53-9
methylenetriphenylphosphorane-(4,5-diethyl-2,5-dihydro-2,2,3-trimethyl-1-phenyl-1,2,5-phosphasilaborole)

-
A
-
114182-21-7
dimeric (P-B)(2)-4,5-diethyl-1,2,5,6-tetrahydro-2,2,3-trimethyl-1-phenyl-1,2,5-phosphasilaborine * toluene

-
B
-
594-09-2
trimethylphosphane

| Conditions | Yield |
|---|---|
| With toluene In toluene Ar atmosphere, refluxing (3 h); sublimation of PMe3, recrystn. of B-complex (hot toluene), washing (toluene), drying (0.001 Torr); elem. anal.; | A 84% B 92% |
-
-
87640-52-6
(η5-C5Me5)(PMe3)2Ru(CH2SiMe3)

-
-
998-30-1
Triethoxysilane

-
A
-
75-76-3
tetramethylsilane

-
C
-
594-09-2
trimethylphosphane

| Conditions | Yield |
|---|---|
| In inert atmosphere, heating of Ru-complex with HSi(OEt)3 (80°C, 12 h, closed vessel), briefly pumping to remove PMe3, continued heating (80°C, 6 h).; Evapn. in vac. gives a waxy solid, recrystn. (acetone), elem. anal.; | A n/a B 92% C n/a |

-
-
113533-26-9
(bis(diisopropylamino)phosphanyl)diazomethane, lithium salt

-
-
70525-09-6, 92670-96-7
rhodium(trimethylphosphine)4Cl

-
-
116405-38-0
5-bis(trimethylphosphine)rhoda-4-bis(diisopropylamino)phospha-1-n-butyl-Δ2-pyrazoline

-
B
-
594-09-2
trimethylphosphane

| Conditions | Yield |
|---|---|
| With n-C4H9Li byproducts: LiCl; Excess of n-Buli.; | A 90% B n/a |

-
-
82555-25-7
cis-HRh(COCH3)(P(CH3)3)3Cl

-
A
-
36713-95-8, 133577-23-8, 22710-50-5
RhCl(CO)(PMe3)2

-
-
36103-64-7
[Rh(trimethylphosphine)3]Cl

-
C
-
34557-54-5
methane

-
D
-
75-07-0
acetaldehyde

-
E
-
594-09-2
trimethylphosphane

| Conditions | Yield |
|---|---|
| Rh complex heated at 90°C for 2 h; collection of the volatile products under high vaccum; | A 13% B 80% C 13% D 87% E 19% |
| In benzene-d6 soln. of Rh complex in benzene-d6 heated at 70°C for 2 h; | A 54% B 15% C 51% D 14% E 50% |


| Conditions | Yield |
|---|---|
| In diethyl ether addn. of fumaric acid dimethylester to the Co compd. in ether under Ar and stirring at room temp. for 30 min; evapn. (vac.), chromy. on Al2O3, elution with ether-pentane, concn. (vac.), filtn., washing with pentane and drying (vac.); elem. anal.; | A 85% B n/a |


-
-
292638-85-8
acrylic acid methyl ester

-
B
-
594-09-2
trimethylphosphane

| Conditions | Yield |
|---|---|
| In hexane addn. of acrylic acid methylester to the Co compd. in hexane under Ar and stirring at room temp. for 30 min; evapn. (vac.), dissolving in ether, chromy. on Al2O3, elution with ether-hexane, concn., cooling to -78°C, filtn., washing with cold pentane and drying (vac.); elem. anal.; | A 85% B n/a |
| In diethyl ether addn. of acrylic acid methylester to the Co compd. in ether under Ar and stirring at room temp. for 30 min; evapn. (vac.), dissolving in ether, chromy. on Al2O3, elution with ether-hexane, concn., cooling to -78°C, filtn., washing with cold pentane and drying (vac.); elem. anal.; | A 85% B n/a |


-
-
88333-03-3, 10340-91-7
Benzyl isocyanide

-
B
-
594-09-2
trimethylphosphane

| Conditions | Yield |
|---|---|
| In benzene under Ar, stirred for a few minutes at room temp.; removal of solvent in vac., extn. (pentane), cooling to -78°C; elem. anal.; | A 85% B n/a |

-
-
133911-64-5
(η2-1,2-diphenyl-1-cyclopropene)(trimethylphosphane)zirconocene

-
A
-
133911-65-6
1,1-bis(η5-cyclopentadienyl)-2,3-diphenyl-1-(trimethylphosphane)-1-zirconacyclobut-2-ene

-
B
-
133911-66-7
1,1-bis(η5-cyclopentadienyl)-2,3-diphenyl-1-zirconacyclobut-2-ene

-
C
-
594-09-2
trimethylphosphane

| Conditions | Yield |
|---|---|
| In tetrahydrofuran-d8 (Ar), -100°C; (31)P-NMR; | A 84% B 0% C 16% |


-
-
292638-85-8
acrylic acid methyl ester

-
B
-
594-09-2
trimethylphosphane

| Conditions | Yield |
|---|---|
| In hexane addn. of acrylic acid methylester to the Co compd. in hexane under Ar and stirring at room temp. for 30 min; evapn. (vac.), dissolving in ether, chromy. on Al2O3, elution with ether-hexane, concn., cooling to -78°C, filtn., washing with cold pentane and drying (vac.); elem. anal.; | A 83% B n/a |
| In diethyl ether addn. of acrylic acid methylester to the Co compd. in ether under Ar and stirring at room temp. for 30 min; evapn. (vac.), dissolving in ether, chromy. on Al2O3, elution with ether-hexane, concn., cooling to -78°C, filtn., washing with cold pentane and drying (vac.); elem. anal.; | A 83% B n/a |

-
-
33937-27-8
tetrakis(trimethylphosphine)platinum(0)

-
-
95-15-8
Benzo[b]thiophene

-
-
202662-81-5
[(CH3)3P]2PtC8SH6

-
B
-
594-09-2
trimethylphosphane

| Conditions | Yield |
|---|---|
| In toluene reflux; elem. anal.; | A 83% B n/a |

-
-
75-15-0
carbon disulfide

-
-
28069-69-4
tetrakis(trimethylphosphine)nickel(0)

-
B
-
594-09-2
trimethylphosphane

| Conditions | Yield |
|---|---|
| In diethyl ether at 0 - 20℃; for 24h; Inert atmosphere; Schlenk technique; | A 81% B n/a |


-
B
-
594-09-2
trimethylphosphane

| Conditions | Yield |
|---|---|
| In not given byproducts: NaCl; N2 atmosphere; react. of Ni-complex with excess of NaCp (1.5 equiv) at 20°C for 12 h; elem. anal.; | A 80% B n/a |


-
B
-
594-09-2
trimethylphosphane

| Conditions | Yield |
|---|---|
| In not given byproducts: NaBr; N2 atmosphere; react. of Ni-complex with excess of NaCp (1.5 equiv) at 20°C for 12 h; elem. anal.; | A 80% B n/a |


| Conditions | Yield |
|---|---|
| In diethyl ether addn. of fumaric acid dimethylester to the Co compd. in ether under Ar and stirring at room temp. for 30 min; evapn. (vac.), chromy. on Al2O3, elution with ether-pentane, concn. (vac.), filtn., washing with pentane and drying (vac.); elem. anal.; | A 80% B n/a |


| Conditions | Yield |
|---|---|
| In diethyl ether addn. of fumaric acid dimethylester to the Co compd. in ether under Ar and stirring at room temp. for 30 min; evapn. (vac.), chromy. on Al2O3, elution with ether-pentane, concn. (vac.), filtn., washing with pentane and drying (vac.); elem. anal.; | A 78% B n/a |

-
-
87640-52-6
(η5-C5Me5)(PMe3)2Ru(CH2SiMe3)

-
-
1631-83-0
diphenylsilyl chloride

-
A
-
75-76-3
tetramethylsilane

-
C
-
594-09-2
trimethylphosphane

| Conditions | Yield |
|---|---|
| In inert atmosphere, heating of Ru-complex with HSi(Ph2Cl)3 (110°C, 16 h, closed vessel, stirring), periodic pumping to remove PMe3.; Cooling to room temp., addn. of pentane, cooling (-78°C, 6 h), filtn. of pale yellow crystals, washing (pentane, -78°C), drying in vac, elem. anal.; | A n/a B 78% C n/a |


-
-
23936-60-9
1,2-bis(dimethylphosphanyl)ethane

-
B
-
594-09-2
trimethylphosphane

| Conditions | Yield |
|---|---|
| In toluene N2 or Ar atmosphere of vac.; stirring (1 h); filtn., concn. (vac.), cooling (-20°C); elem. anal.; | A 76% B n/a |


| Conditions | Yield |
|---|---|
| In tetrahydrofuran; diethyl ether inert atmosphere; -78°C, then room temp., stirring 15 min.; evapn. (vac.), drying, extn. (petroleum ether), partial evapn. of filtrate, crystn. (-78°C), collection (filtration), drying (vac.); elem. anal.; | A 76% B n/a |
-
-
119998-04-8
trans-MoCl(NO)(PMe3)4

-
-
119998-14-0
MoCl(η3-S2CPMe3)(NO)(PMe3)2

-
B
-
594-09-2
trimethylphosphane

| Conditions | Yield |
|---|---|
| With CS2 In tetrahydrofuran Ar or N2 atmosphere; addn. of 1.4 equiv of CS2 to a soln. of Mo-complex, stirring (24 h at room temp.), pptn.; collection (filtration), washing (Et2O), crystn. (CHCl3), -30°C; | A 75% B n/a |

| Conditions | Yield |
|---|---|
| In water heating an aq. soln. of the Ag compound with a concd. soln. of thiourea with N2 bubbling through the soln.; | 75% |

-
-
292638-85-8
acrylic acid methyl ester

-
B
-
594-09-2
trimethylphosphane

| Conditions | Yield |
|---|---|
| In hexane addn. of acrylic acid methylester to the Co compd. in hexane under Ar and stirring at room temp. for 30 min; evapn. (vac.), dissolving in ether, chromy. on Al2O3, elution with ether-hexane, concn., cooling to -78°C, filtn., washing with cold pentane and drying (vac.); elem. anal.; | A 73% B n/a |
| In diethyl ether addn. of acrylic acid methylester to the Co compd. in ether under Ar and stirring at room temp. for 30 min; evapn. (vac.), dissolving in ether, chromy. on Al2O3, elution with ether-hexane, concn., cooling to -78°C, filtn., washing with cold pentane and drying (vac.); elem. anal.; | A 73% B n/a |


| Conditions | Yield |
|---|---|
| In diethyl ether addn. of acrylonitrile to the Co compd. in ether under Ar and stirring at room temp. for 1 h; evapn. (vac.), dissolving in ether-hexane, chromy. on Al2O3, elution with ether-hexane, concn., cooling to -78°C, filtn., washing with pentane and drying (vac.); elem. anal.; exo-endo-mixture, identified by (1)H-NMR; | A 73% B n/a |


| Conditions | Yield |
|---|---|
| In diethyl ether addn. of acrylonitrile to the Co compd. in ether under Ar and stirring at room temp. for 1 h; evapn. (vac.), dissolving in ether-hexane, chromy. on Al2O3, elution with ether-hexane, concn., cooling to -78°C, filtn., washing with pentane and drying (vac.); elem. anal.; exo-endo-mixture, identified by (1)H- and (13)C-NMR; | A 72% B n/a |

-
-
28069-69-4
tetrakis(trimethylphosphine)nickel(0)

-
-
103-72-0
phenyl isothiocyanate

-
B
-
594-09-2
trimethylphosphane

| Conditions | Yield |
|---|---|
| In diethyl ether at 0 - 20℃; for 24h; Inert atmosphere; Schlenk technique; | A 72% B n/a |

-
-
83828-53-9
MoCl2(CO)2{P(CH3)3}3

-
-
140-92-1
potassium isopropylxanthate

-
A
-
125841-34-1
Mo(η3-(S,S',C)S2CO-i-Pr)2(CO)(trimethylphosphine)2

-
B
-
594-09-2
trimethylphosphane

| Conditions | Yield |
|---|---|
| In tetrahydrofuran byproducts: CO, KCl; N2 or Ar atmosphere; stirring (2 h, room temp.); evapn. (vac.), extn. (petroleum ether/Et2O 1:1), centrifugation and cooling (-35°C); elem. anal.; | A 70% B n/a |


| Conditions | Yield |
|---|---|
| In benzene addn. of maleic anhydride to the Co compd. in benzene under Ar and stirring at room temp. for 30 min; evapn. (vac.), washing with hexane, recrystn. from acetone-hexane, filtn., washing with ether and pentane and drying (vac.); elem. anal.; | A 70% B n/a |

-
-
84879-23-2
trans-{W(ethylene)2(P(CH3)3)4}

-
A
-
122214-24-8
{WH(OOCCHCH2)(C2H4)(P(CH3)3)2}2*0.5(C2H5)2O

-
B
-
594-09-2
trimethylphosphane

| Conditions | Yield |
|---|---|
| With CO2 In diethyl ether under N2; reacting ethene complex with CO2, 20°C, 1 atm, 30 min; filtering off ppt., 2nd crop from mother liquor by cooling to -20°C overnight; elem. anal.; | A 65% B n/a |
-
-
629-03-8
1 ,6-dibromohexane

-
-
594-09-2
trimethylphosphane

-
-
72385-53-6
(6-Bromhexyl)trimethylphosphoniumbromid

| Conditions | Yield |
|---|---|
| In toluene for 72h; Ambient temperature; | 100% |
-
-
106-95-6
allyl bromide

-
-
594-09-2
trimethylphosphane

-
-
41301-43-3
trimethyl-2-propen-1-ylphosphonium bromide

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; dichloromethane at 0 - 20℃; for 2.5h; Inert atmosphere; | 100% |
| In diethyl ether Ambient temperature; | |
| In toluene at 20℃; Inert atmosphere; | |
| In dichloromethane for 12h; Inert atmosphere; |
-
-
1765-40-8
(bromomethyl)pentafluorobenzene

-
-
594-09-2
trimethylphosphane

-
-
80431-31-8
Trimethyl<(pentafluorphenyl)methyl>phosphoniumbromid

| Conditions | Yield |
|---|---|
| In diethyl ether at 0℃; for 1h; | 100% |
-
-
16355-92-3
1,10-diiododecane

-
-
594-09-2
trimethylphosphane

-
-
138730-46-8
(10-Iodo-decyl)-trimethyl-phosphonium; iodide

| Conditions | Yield |
|---|---|
| In toluene for 72h; Ambient temperature; | 100% |
-
-
5055-11-8
(2-chloroethyl)diphenylphosphane

-
-
594-09-2
trimethylphosphane

-
-
138710-61-9
(2-Diphenylphosphanyl-ethyl)-trimethyl-phosphonium; chloride

| Conditions | Yield |
|---|---|
| In toluene for 72h; Heating; | 100% |
-
-
57137-55-0
3-(diphenylphosphino)propyl chloride

-
-
594-09-2
trimethylphosphane

-
-
170125-48-1
(3-Diphenylphosphanyl-propyl)-trimethyl-phosphonium; chloride

| Conditions | Yield |
|---|---|
| In toluene for 72h; Heating; | 100% |
-
-
89094-99-5
Phosphoric acid (E)-2-chloro-1-chloromethyl-vinyl ester diethyl ester

-
-
594-09-2
trimethylphosphane

-
-
120115-73-3
O,O-diethyl α-(trimethylphosphoniummethyl)-β-chlorovinyl phosphate chloride

| Conditions | Yield |
|---|---|
| at 0℃; for 2h; | 100% |
| In diethyl ether for 12h; Ambient temperature; Yield given; |

| Conditions | Yield |
|---|---|
| In pentane at 0℃; Addition; | 100% |

| Conditions | Yield |
|---|---|
| In dichloromethane Irradiation (UV/VIS); | 100% |
| In chloroform Irradiation (UV/VIS); | 100% |
| In chloroform Irradiation (UV/VIS); addn. of PMe3 to the Mo compd. in CHCl3 and irradiation (Hanovia medium pressure mercury vapor lamp) for 30 min at room temp.; evapn. (vac.), dissolving in CH2Cl2 and pptn. with hexane; elem. anal.; | 60% |

-
-
594-09-2
trimethylphosphane

| Conditions | Yield |
|---|---|
| In acetone cooled to -76°C, stirred at 23°C for 3h; filtered; NMR, IR, elem. anal.; | 100% |
-
-
12146-37-1, 124717-04-0
(bicyclo[2.2.1]hepta-2,5-diene)tetracarbonylmolybdenum(0)

-
-
594-09-2
trimethylphosphane

-
-
16027-45-5
cis-bis(trimethylphosphine)tetracarbonylmolybdenum

-
B
-
121-46-0
bicyclo[2.2.1]hepta-2,5-diene

| Conditions | Yield |
|---|---|
| In tetrahydrofuran reaction in a calorimeter under argon; | A 100% B n/a |


| Conditions | Yield |
|---|---|
| With Mg In tetrahydrofuran addn. of Mg (177 mmol) and P(CH3)3 (200 mmol) to soln. of ((C5H5)2TiCl2) (40.2 mmol) in THF under Ar; stirring for 20 h;; evapg. solvent at 1E-2 bar; extg. residue with pentane; crystn. at -78°C; elem. anal.;; | 100% |
| With n-C4H9Li In tetrahydrofuran byproducts: LiCl, C4H8, C4H10; (Ar); to soln. of Ti complex was dropped hexane soln. of BuLi at -78°C within 0,5 h and stirred for 0,5 h, then was added PMe3, soln. was allowed to warm up to room temp. within 8 h and volatiles were removed at 0,1 Torr; pentane soln. of residue was filtered and filtrate cooled to -20°C, black crystals pptd., which were filtered off and dried at 0,01 Torr; elem. anal.; | 90% |
| With Na#Hg In diethyl ether byproducts: NaCl; (Ar); a suspn. of Ti complex in a soln. of ligand added slowly to Na amalgam, stirred for 12 h; filtered, evapd. (Ar); | 66% |
-
-
36223-69-5
cis-bis{(trimethylsilyl)methyl}(1,5-cyclooctadiene)platinum(II)

-
-
594-09-2
trimethylphosphane

| Conditions | Yield |
|---|---|
| In toluene under Ar, heated to 60°C for 14 days; removal of solvent in vacuo, addn. of n-hexane, cooled to -25°C; elem. anal.; | 100% |

-
-
594-09-2
trimethylphosphane

| Conditions | Yield |
|---|---|
| In benzene (under N2 or Ar) addn. of P-compd. to Pt-complex in benzene at room temp., standing overnight (ambient temp.); solvent removal (vac.); elem. anal.; | 100% |

| Conditions | Yield |
|---|---|
| In dichloromethane PMe3 was condensed into a flask with complex and CH2Cl2 at -196°C, the mixt. was warmed to 25°C and stirred for 10 min;; recrystd. from CH2Cl2-hexane;; | 100% |
-
-
128205-88-9
{(η4-2-methyl-1,3-pentadiene)cobalt(carbonyl)3} tetrafluoroborate

-
-
594-09-2
trimethylphosphane

| Conditions | Yield |
|---|---|
| In nitromethane addn. of the Co complex to CH3NO2 at 0°C, stirring to complete dissoln., dropwise addn. of PMe3 in CH3NO2 and react. for 10-15 min; addn. of Et2O, removing the solvent using cannula and drying (vac.); | 100% |

| Conditions | Yield |
|---|---|
| In dichloromethane byproducts: P(C6H5)3; mixing of the Mo compd. with the free phosphine (molar ratio 2.6:1) in CH2Cl2;; | 100% |

| Conditions | Yield |
|---|---|
| In pentane a solution of the alkyne-complex was cooled to -78°C; Me3P was added; standing over dry ice overnight; evaporation to dryness; dried in high vacuum; | 100% |

| Conditions | Yield |
|---|---|
| In dichloromethane mixing of the Mo compd. with the free phosphine (molar ratio 2.3:1) in CH2Cl2;; | A 100% B n/a |


| Conditions | Yield |
|---|---|
| In pentane a solution of the alkyne-complex was cooled to -78°C; Me3P was added; standing over dry ice overnight; evaporation to dryness; dried in high vacuum; | 100% |

| Conditions | Yield |
|---|---|
| In pentane a solution of the alkyne-complex was cooled to -78°C; Me3P was added; standing over dry ice overnight; evaporation to dryness; dried in high vacuum; | 100% |

| Conditions | Yield |
|---|---|
| In pentane a solution of the alkyne-complex was cooled to -78°C; Me3P was added; standing over dry ice overnight; evaporation to dryness; dried in high vacuum; | 100% |

| Conditions | Yield |
|---|---|
| In pentane a solution of the alkyne-complex was cooled to -78°C; Me3P was added; standing over dry ice overnight; evaporation to dryness; dried in high vacuum; | 100% |

| Conditions | Yield |
|---|---|
| In hexane evapd.; | 100% |
| In benzene-d6 under N2, soln. of educts in C6D6 heated at 51°C for 30 min; not isolated; detected by NMR; | |
| In [(2)H6]acetone in NMR tube at room temp.; |
-
-
67538-15-2
3-(NNN'N'-tetramethylethylenediamine)-1,2-dicarba-3-palladadodecaborane

-
-
594-09-2
trimethylphosphane

-
-
67538-14-1
3,3-bis(trimethylphosphine)-1,2-dicarba-3-palladadodecaborane

| Conditions | Yield |
|---|---|
| In dichloromethane PMe3 passed into soln. of Pd complex in CH2Cl2; added hexane, filtered, washed with Et2O/acetone, dried in vac.; elem. anal.; | 100% |

-
-
594-09-2
trimethylphosphane

| Conditions | Yield |
|---|---|
| In benzene-d6 under N2; to Ru compd. in C6D6 addn. of PMe3 at room temp.; monitored by NMR; evapn. of solvent; elem. anal.; | 100% |

| Conditions | Yield |
|---|---|
| In dichloromethane mixing of the Mo compd. with the free phosphine (molar ratio 4.5:1) in CH2Cl2;; | A 100% B n/a |
| In toluene mixing of the Mo compd. with the free phosphine (molar ratio 2.1:1) in toluene;; | A 100% B n/a |


| Conditions | Yield |
|---|---|
| In toluene mixing of the Mo compd. with the free phosphine (molar ratio 2.1:1) in toluene;; | A 100% B n/a |


| Conditions | Yield |
|---|---|
| In toluene mixing of the Mo compd. with the free phosphine (molar ratio 2.2:1) in toluene;; | A 100% B n/a |


-
-
594-09-2
trimethylphosphane

-
-
97225-94-0
tetra-iso-propoxy(phenylimido)trimethylphosphinetungsten(VI)

| Conditions | Yield |
|---|---|
| In petroleum ether PMe3 is added to soln. of W(NPh)(NH2CMe3)(OCHMe2)4 in petroleum ether and mixt. is stirred for 3 h; soln. is filtered, solvent removed; | 100% |
Related products
Raw Materials
Downstream Products
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View
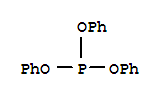







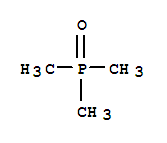







 F,
F, Xi
Xi