Hubei CuiRan Biotechnology Co., Ltd
Hubei CuiRan Biotechnology Co., Ltd is a leading company in the research, development, manufacture and marketing of High Quality Phytochemicals and Extracts(especially Active Ingredients from Traditional Chinese Medicine,Traditional Chinese Medicine)
Dayang Chem (Hangzhou) Co.,Ltd.
As a leading manufacturer and supplier of chemicals in China, DayangChem not only supply popular chemicals, but also DayangChem's R&D center offer custom synthesis services. DayangChem can provide different quantities of custom synthesis ch
Cas:83-79-4
Min.Order:1 Kilogram
FOB Price: $3.0
Type:Lab/Research institutions
inquiryHangzhou Dingyan Chem Co., Ltd
Items Standard Result Appearance -- White solid Purity -- 99.09%
Cas:83-79-4
Min.Order:1 Gram
FOB Price: $100.0 / 500.0
Type:Trading Company
inquirySimagchem Corporation
Welcome to Simagchem, your partner in China as a premier supply of bulk specialty chemicals for industry and life science. We introduce experienced quality product and exceptional JIT service with instant market intelligence in China to benefit our
Cas:83-79-4
Min.Order:0 Metric Ton
Negotiable
Type:Manufacturers
inquiryKono Chem Co.,Ltd
Superiority kono chem is a leading manufacturer and supplier of chemicals in China. We develop ,produce and distribute high quality pharmaceuticals, intermediates, special chemicals and other fine chemicals. We could give you: 1.Best
Cas:83-79-4
Min.Order:1 Kilogram
FOB Price: $800.0 / 1000.0
Type:Other
inquiryXi'an Quanao Biotech Co., Ltd.
Top quality insecticide Rotenone 98% Rotenone powder Derris Root Extract Professional Factory We has a complete production and operating system, with an annual output capacity of 2000 tons. It can produce variety of content and proportion of extrac
Cas:83-79-4
Min.Order:100 Kilogram
FOB Price: $255.0 / 259.0
Type:Manufacturers
inquiryAlity Chemical Corporation
The above product is Ality Chemical's strong item with best price, good quality and fast supply. Ality Chemical has been focusing on the research and production of this field for over 14 years. At the same time, we are always committed to providi
Cas:83-79-4
Min.Order:1
Negotiable
Type:Other
inquiryXi'an Xszo Chem Co., Ltd.
1. Factory price and high quality must be guaranteed, base on 8 years of production and R&D experience2. Free samples will be provided,ensure specifications and quality are right for customer3. Customers will receive the most professional technical s
Cas:83-79-4
Min.Order:1 Gram
FOB Price: $0.1
Type:Manufacturers
inquiryCOLORCOM LTD.
Colorcom is a global leader in industrial chemical manufacturing and is continuously innovating and transforming to exceed client expectations and industry standards. Colorcom prides itself on superior customer and technical focus, while focusing on
Chemwill Asia Co., Ltd.
Our main production base is located in Xuzhou industry park. We are certified both to the ISO 9001 and ISO 14001 Standards, have a safety management system in place.Our R&D team masters core technology for process-design of target building block
Hebei Nengqian Chemical Import and Export Co., LTD
Our Advantage Rich Experience Our products are sold all over Europe,North&South America, Sino-East, Asia and pacific area as well as Africa,we establish long term. Quality service Company cooperates with research institutes. We strictly con
Cas:83-79-4
Min.Order:1 Kilogram
FOB Price: $15.0 / 50.0
Type:Trading Company
inquiryZhuozhou Wenxi import and Export Co., Ltd
WITH US,YOUR MONEY IN SAFE,YOUR BUSINESS IN SAFE 1)Quick Response Within 12 hours; 2)Quality Guarantee: All products are strictly tested by our QC, confirmed by QA and approved by third party lab in China, USA, Canada, Germany, UK, Italy, France et
Cas:83-79-4
Min.Order:1 Kilogram
FOB Price: $139.0 / 210.0
Type:Trading Company
inquiryHebei Sankai Chemical Technology Co., Ltd
1. Product advantages ♦ High purity, all above 98.5%, no impurities after the dissolution ♦ We will test each batch to ensure quality ♦ OEM and private brand services designed for free ♦ Various cap colors available &diam
Cas:83-79-4
Min.Order:1 Kilogram
FOB Price: $7.0 / 10.0
Type:Trading Company
inquiryHenan Sinotech Import&Export Corporation
AS No 83-79-4 Appearance White powder Specifications (COA) Purity: 98% minLoss on Drying: 5.0% max Formulations 98% TC, 4% EC, 2.5% EC
Shanghai Upbio Tech Co.,Ltd
1.No Less 8 years exporting experience. Clients can 100% received goods 2.Lower Price with higher quality 3,Free sample 4,We are sincerely responsible for the "product quality" and "After Service" Upbio is Specialized
Cas:83-79-4
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryQingdao Beluga Import and Export Co., LTD
Rotenone CAS:83-79-4 Qingdao Belugas Import and Export Co., Ltd. is a scientific and technological company integrating research and development, production and trade of chemical intermediates, specializing in high quality organic intermediates, ster
Shandong Hanjiang Chemical Co., Ltd.
Hello, dear friend! I'm Hansen and Allen from China. Welcome to my lookchem mall! The following is a brief introduction of our company's products and services. If you are interested in our products, please contact us by emai
Henan Wentao Chemical Product Co., Ltd.
Henan Wentao Chemical Product Co.,Ltd is Located in Zhengzhou High-tech Development Zone with import and export license, We passed ISO 9001:2008 as well, Henan Wentao has developed more than 1000 compounds, which are widely used in the fields of prod
Cas:83-79-4
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryAfine Chemicals Limited
Our Services 1. New Molecules R&D 2. Own test center HPLC NMR GC LC-MS 3. API and Intermediates from China reputed manufacturers 4. Documents support COA MOA MSDS DMF open part Our advantages 1. Government awarded company. Top 100 enter
Rely Chemicals Ltd.
Best quality/price/service Professional manufacturing FAO Standards Appearance:white or grey white powder Package:Solid:50g,100g,200g,250g,400g,500g,1kg,10kg bag ,20kg, 25kg,40kg bag,50kg,600kg, 900kg; Aluminum Foil Bag,plastic bag, fiber bag,
Cas:83-79-4
Min.Order:0 Metric Ton
Negotiable
Type:Trading Company
inquiryHangzhou J&H Chemical Co., Ltd.
J&H CHEM R&D center can offer custom synthesis according to the contract research and development services for the fine chemicals, pharmaceutical, biotechnique and some of the other chemicals. J&H CHEM has some Manufacturing base in Jia
Zibo Hangyu Biotechnology Development Co., Ltd
Zibo Hangyu Biotechnology Development Co., Ltd is a leading manufacturer and supplier of chemicals in China. We develop produce and distribute high quality pharmaceuticals, intermediates, special chemicals and OLED intermediates and other fine chemi
Cas:83-79-4
Min.Order:10 Gram
FOB Price: $100.0
Type:Lab/Research institutions
inquiryShandong Zhimao New Materials Co., Ltd.
Rotenone is widely present in the root bark of plants. It is a highly specific substance in toxicology. It has strong touch killing and stomach poisoning effects on insects, especially the larva of butterfly butterfly, Diamondback moth and aphids. Ea
HANGZHOU YUNUO CHEMICAL CO.,LTD
Superior quality, moderate price & quick delivery. Appearance:white powder Storage:Stored in cool, dry and ventilation place; Away from fire and heat Package:25kg/drum, or as per your request. Application:Used as Pharmaceutical Intermediates Tr
Cas:83-79-4
Min.Order:1 Kilogram
Negotiable
Type:Trading Company
inquiryXiamen Jenny Chemical Technology Co., Ltd.
GMP standard, high purity, competitive price, in stock 1. Quick Response: within 6 hours after receiving your email. 2. Quality Guarantee: All products are strictly tested by our QC, confirmed by QA, and approved by a third-party lab in China, USA,
Suzhou Health Chemicals Co., Ltd.
High quality,stable supply chain.Appearance:white/off-white or light yellow Storage:Store in cool and dry place, keep away from strong light and heat. Package:aluminum bottle,glass bottle,PTFE bottle,cardboard drum Application:This product can be use
Hebei yanxi chemical co.,LTD.
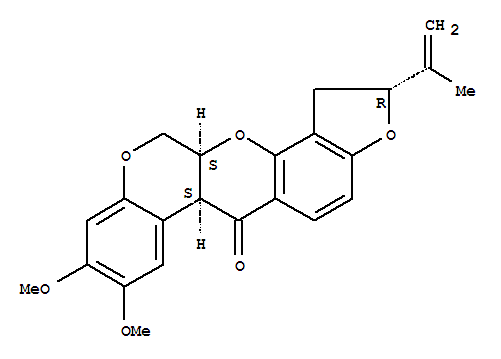
Content: 20%,40%, 90% Chemical name: (1,2,12,12a-tetrahidro-2 -iso-propeny-8, 9-dimethoxy-[1]-benzopyrane-[3,4-b] -furo-[2,3-h]-[1]-benzopyran-6-OH)
shanghai Tauto Biotech Co., Ltd
The quality is guaranteed. If you find the product is wrong compared with COA, we promise 100% refund or change product. COA and HPLC will be shipped out with goods. You can also inform your analysis method and we will follow your analysis me
Hubei Vanz Pharm Co.,Ltd
ISO/factory/goodqualityAppearance:off white Storage:Dry,cool place Package:drum Application:active pharmaceutical ingredients Transportation:by air/sea/express Port:shenzhen/shanghai
Wuxi TAA Chemical Industry Co.,LTD.
1.A strong technical force and advanced processing equipments. The quality of the products has been strictly inspected and all kinds of index have reached or exceeded domestic and international standards.2. Now we have established long-term stable re
Synthetic route
-
-
23355-70-6
(6aS,12aS,5'R)-rotenone enol acetate

-
-
83-79-4
rotenone

| Conditions | Yield |
|---|---|
| With hydrogenchloride In methanol for 2h; Heating; | 83% |
-
-
186581-53-3, 908094-01-9
diazomethane

-
-
34487-52-0
(2R,6aS,12aS)-8-hydroxy-9-methoxy-2-(prop-1-en-2-yl)-1,2,12,12a- tetrahydrochromeno[3,4-b]furo[2,3-h]chromen-6(6aH)-one

-
-
83-79-4
rotenone

| Conditions | Yield |
|---|---|
| In diethyl ether | 59% |
-
-
30462-22-7
2-((2R,6aS,12aS)-8,9-dimethoxy-1,2,6,6a,12,12a-hexahydrochromeno[3,4-b]furo[2,3-h]chromen-2-yl)propan-2-ol

-
A
-
83-79-4
rotenone

-
B
-
549-22-4
Isorotenone

| Conditions | Yield |
|---|---|
| With Burgess Reagent In toluene for 0.5h; Reflux; | A 50% B 13% |
| With Burgess Reagent In toluene for 0.5h; Inert atmosphere; Reflux; | A 50% B 13% |
-
-
30462-22-7
2-((2R,6aS,12aS)-8,9-dimethoxy-1,2,6,6a,12,12a-hexahydrochromeno[3,4-b]furo[2,3-h]chromen-2-yl)propan-2-ol

-
-
83-79-4
rotenone

| Conditions | Yield |
|---|---|
| With pyridine; thionyl chloride at 0℃; for 0.5h; | 40% |
-
-
123000-20-4
(6aS,12aR,5'R)-(trans)-(+)-rotenone

-
-
83-79-4
rotenone

| Conditions | Yield |
|---|---|
| With hydrogenchloride In methanol; chloroform for 2h; |
-
-
123000-19-1
(6aR,12aS,5'R)-rotenone

-
-
83-79-4
rotenone

| Conditions | Yield |
|---|---|
| With hydrogenchloride In methanol; chloroform | |
| With hydrogenchloride In methanol; chloroform epimerisation; |

| Conditions | Yield |
|---|---|
| With acetic acid; zinc(II) chloride; zinc In water at 100℃; for 2.5h; | 20 mg |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 31 mg / diisobutyaluminium hydride / toluene; tetrahydrofuran / 1 h / -78 °C 2: aq. HCl / methanol; CHCl3 / 2 h View Scheme | |
| Multi-step reaction with 2 steps 1: di-isobutylaluminium hydride 2: hydrogen chloride / methanol; CHCl3 / epimerisation View Scheme |
-
-
15130-81-1
(6aS,12aS,5′R)-rotenone-6′-norketone

-
A
-
83-79-4
rotenone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 50 percent / conc. H2SO4 / 3 h / Heating 2: 1.) n-BuLi / 1.) THF, 35 deg C, 5 min, 2.) THF, 1 h 3: 83 percent / conc. HCl / methanol / 2 h / Heating View Scheme |
-
-
23295-59-2, 23295-65-0
rotenone 6'-norketone enol acetate

-
A
-
83-79-4
rotenone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 1.) n-BuLi / 1.) THF, 35 deg C, 5 min, 2.) THF, 1 h 2: 83 percent / conc. HCl / methanol / 2 h / Heating View Scheme |

-
-
83-79-4
rotenone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 13 steps 1.1: sodium hydride / hexane; mineral oil; N,N-dimethyl-formamide / 0 - 20 °C 2.1: N-ethyl-N,N-diisopropylamine; tetra-(n-butyl)ammonium iodide / dichloromethane / 24 h / 20 °C 3.1: N,N,N,N,-tetramethylethylenediamine; sec.-butyllithium / hexane; toluene; diethyl ether; cyclohexane / 1 h / -78 °C 3.2: 1.5 h / -78 - -30 °C 4.1: boron trifluoride diethyl etherate / dichloromethane / 0.25 h / 0 °C 5.1: sodium tetrahydroborate / ethanol / 0.5 h / 20 °C 6.1: pyridinium p-toluenesulfonate / dichloromethane / 4 h / 20 °C 7.1: tetrabutyl ammonium fluoride / tetrahydrofuran / 1 h / 20 °C 8.1: bis(1,5-cyclooctadiene)nickel (0); tricyclohexylphosphine; potassium tert-butylate / toluene / 2 h / Reflux 9.1: aluminum (III) chloride; lithium aluminium tetrahydride / dichloromethane; toluene; diethyl ether / 0.33 h / 0 °C 10.1: sodium hydride; 1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone; 15-crown-5 / toluene / 2 h / 80 °C 11.1: palladium 10% on activated carbon; hydrogen / tert-butyl alcohol; water / 15 h / 20 °C 12.1: Dess-Martin periodane / dichloromethane / 0.25 h / 20 °C 12.2: 1.5 h / 50 °C 13.1: pyridine; thionyl chloride / 0.5 h / 0 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 13 steps 1.1: sodium hydride / hexane; mineral oil; N,N-dimethyl-formamide / 0 - 20 °C 2.1: N-ethyl-N,N-diisopropylamine; tetra-(n-butyl)ammonium iodide / dichloromethane / 24 h / 20 °C 3.1: N,N,N,N,-tetramethylethylenediamine; sec.-butyllithium / hexane; toluene; diethyl ether; cyclohexane / 1 h / -78 °C 3.2: 1.5 h / -78 - -30 °C 4.1: boron trifluoride diethyl etherate / dichloromethane / 0.25 h / 0 °C 5.1: sodium tetrahydroborate / ethanol / 0.5 h / 20 °C 6.1: pyridinium p-toluenesulfonate / dichloromethane / 4 h / 20 °C 7.1: tetrabutyl ammonium fluoride / tetrahydrofuran / 1 h / 20 °C 8.1: bis(1,5-cyclooctadiene)nickel (0); tricyclohexylphosphine; potassium tert-butylate / toluene / 2 h / Reflux 9.1: aluminum (III) chloride; lithium aluminium tetrahydride / dichloromethane; toluene; diethyl ether / 0.33 h / 0 °C 10.1: sodium hydride; 1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone; 15-crown-5 / toluene / 2 h / 80 °C 11.1: palladium 10% on activated carbon; hydrogen / tert-butyl alcohol; water / 15 h / 20 °C 12.1: Dess-Martin periodane / dichloromethane / 0.25 h / 20 °C 12.2: 1.5 h / 50 °C 13.1: Burgess Reagent / toluene / 0.5 h / Reflux View Scheme |

-
-
83-79-4
rotenone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 12 steps 1.1: N-ethyl-N,N-diisopropylamine; tetra-(n-butyl)ammonium iodide / dichloromethane / 24 h / 20 °C 2.1: N,N,N,N,-tetramethylethylenediamine; sec.-butyllithium / hexane; toluene; diethyl ether; cyclohexane / 1 h / -78 °C 2.2: 1.5 h / -78 - -30 °C 3.1: boron trifluoride diethyl etherate / dichloromethane / 0.25 h / 0 °C 4.1: sodium tetrahydroborate / ethanol / 0.5 h / 20 °C 5.1: pyridinium p-toluenesulfonate / dichloromethane / 4 h / 20 °C 6.1: tetrabutyl ammonium fluoride / tetrahydrofuran / 1 h / 20 °C 7.1: bis(1,5-cyclooctadiene)nickel (0); tricyclohexylphosphine; potassium tert-butylate / toluene / 2 h / Reflux 8.1: aluminum (III) chloride; lithium aluminium tetrahydride / dichloromethane; toluene; diethyl ether / 0.33 h / 0 °C 9.1: sodium hydride; 1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone; 15-crown-5 / toluene / 2 h / 80 °C 10.1: palladium 10% on activated carbon; hydrogen / tert-butyl alcohol; water / 15 h / 20 °C 11.1: Dess-Martin periodane / dichloromethane / 0.25 h / 20 °C 11.2: 1.5 h / 50 °C 12.1: pyridine; thionyl chloride / 0.5 h / 0 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 12 steps 1.1: N-ethyl-N,N-diisopropylamine; tetra-(n-butyl)ammonium iodide / dichloromethane / 24 h / 20 °C 2.1: N,N,N,N,-tetramethylethylenediamine; sec.-butyllithium / hexane; toluene; diethyl ether; cyclohexane / 1 h / -78 °C 2.2: 1.5 h / -78 - -30 °C 3.1: boron trifluoride diethyl etherate / dichloromethane / 0.25 h / 0 °C 4.1: sodium tetrahydroborate / ethanol / 0.5 h / 20 °C 5.1: pyridinium p-toluenesulfonate / dichloromethane / 4 h / 20 °C 6.1: tetrabutyl ammonium fluoride / tetrahydrofuran / 1 h / 20 °C 7.1: bis(1,5-cyclooctadiene)nickel (0); tricyclohexylphosphine; potassium tert-butylate / toluene / 2 h / Reflux 8.1: aluminum (III) chloride; lithium aluminium tetrahydride / dichloromethane; toluene; diethyl ether / 0.33 h / 0 °C 9.1: sodium hydride; 1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone; 15-crown-5 / toluene / 2 h / 80 °C 10.1: palladium 10% on activated carbon; hydrogen / tert-butyl alcohol; water / 15 h / 20 °C 11.1: Dess-Martin periodane / dichloromethane / 0.25 h / 20 °C 11.2: 1.5 h / 50 °C 12.1: Burgess Reagent / toluene / 0.5 h / Reflux View Scheme |

-
-
83-79-4
rotenone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 11 steps 1.1: N,N,N,N,-tetramethylethylenediamine; sec.-butyllithium / hexane; toluene; diethyl ether; cyclohexane / 1 h / -78 °C 1.2: 1.5 h / -78 - -30 °C 2.1: boron trifluoride diethyl etherate / dichloromethane / 0.25 h / 0 °C 3.1: sodium tetrahydroborate / ethanol / 0.5 h / 20 °C 4.1: pyridinium p-toluenesulfonate / dichloromethane / 4 h / 20 °C 5.1: tetrabutyl ammonium fluoride / tetrahydrofuran / 1 h / 20 °C 6.1: bis(1,5-cyclooctadiene)nickel (0); tricyclohexylphosphine; potassium tert-butylate / toluene / 2 h / Reflux 7.1: aluminum (III) chloride; lithium aluminium tetrahydride / dichloromethane; toluene; diethyl ether / 0.33 h / 0 °C 8.1: sodium hydride; 1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone; 15-crown-5 / toluene / 2 h / 80 °C 9.1: palladium 10% on activated carbon; hydrogen / tert-butyl alcohol; water / 15 h / 20 °C 10.1: Dess-Martin periodane / dichloromethane / 0.25 h / 20 °C 10.2: 1.5 h / 50 °C 11.1: pyridine; thionyl chloride / 0.5 h / 0 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 11 steps 1.1: N,N,N,N,-tetramethylethylenediamine; sec.-butyllithium / hexane; toluene; diethyl ether; cyclohexane / 1 h / -78 °C 1.2: 1.5 h / -78 - -30 °C 2.1: boron trifluoride diethyl etherate / dichloromethane / 0.25 h / 0 °C 3.1: sodium tetrahydroborate / ethanol / 0.5 h / 20 °C 4.1: pyridinium p-toluenesulfonate / dichloromethane / 4 h / 20 °C 5.1: tetrabutyl ammonium fluoride / tetrahydrofuran / 1 h / 20 °C 6.1: bis(1,5-cyclooctadiene)nickel (0); tricyclohexylphosphine; potassium tert-butylate / toluene / 2 h / Reflux 7.1: aluminum (III) chloride; lithium aluminium tetrahydride / dichloromethane; toluene; diethyl ether / 0.33 h / 0 °C 8.1: sodium hydride; 1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone; 15-crown-5 / toluene / 2 h / 80 °C 9.1: palladium 10% on activated carbon; hydrogen / tert-butyl alcohol; water / 15 h / 20 °C 10.1: Dess-Martin periodane / dichloromethane / 0.25 h / 20 °C 10.2: 1.5 h / 50 °C 11.1: Burgess Reagent / toluene / 0.5 h / Reflux View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 15 steps 1.1: N,N,N,N,N,N-hexamethylphosphoric triamide; n-butyllithium / tetrahydrofuran; hexane / 1.42 h / -78 °C 1.2: 2 h / 20 °C 2.1: potassium osmate; potassium hexacyanoferrate(III); potassium carbonate; methanesulfonamide; (9S,9"S)-9,9"-[phthalazine-1,4-diylbis-(oxy)]bis[10,11-dihydro-6'-methoxycinchonane] / tert-butyl alcohol; water / 120 h / 0 - 20 °C 3.1: sodium hydride / hexane; mineral oil; N,N-dimethyl-formamide / 0 - 20 °C 4.1: N-ethyl-N,N-diisopropylamine; tetra-(n-butyl)ammonium iodide / dichloromethane / 24 h / 20 °C 5.1: N,N,N,N,-tetramethylethylenediamine; sec.-butyllithium / hexane; toluene; diethyl ether; cyclohexane / 1 h / -78 °C 5.2: 1.5 h / -78 - -30 °C 6.1: boron trifluoride diethyl etherate / dichloromethane / 0.25 h / 0 °C 7.1: sodium tetrahydroborate / ethanol / 0.5 h / 20 °C 8.1: pyridinium p-toluenesulfonate / dichloromethane / 4 h / 20 °C 9.1: tetrabutyl ammonium fluoride / tetrahydrofuran / 1 h / 20 °C 10.1: bis(1,5-cyclooctadiene)nickel (0); tricyclohexylphosphine; potassium tert-butylate / toluene / 2 h / Reflux 11.1: aluminum (III) chloride; lithium aluminium tetrahydride / dichloromethane; toluene; diethyl ether / 0.33 h / 0 °C 12.1: sodium hydride; 1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone; 15-crown-5 / toluene / 2 h / 80 °C 13.1: palladium 10% on activated carbon; hydrogen / tert-butyl alcohol; water / 15 h / 20 °C 14.1: Dess-Martin periodane / dichloromethane / 0.25 h / 20 °C 14.2: 1.5 h / 50 °C 15.1: pyridine; thionyl chloride / 0.5 h / 0 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 15 steps 1.1: N,N,N,N,N,N-hexamethylphosphoric triamide; n-butyllithium / tetrahydrofuran; hexane / 1.42 h / -78 °C 1.2: 2 h / 20 °C 2.1: potassium osmate; potassium hexacyanoferrate(III); potassium carbonate; methanesulfonamide; (9S,9"S)-9,9"-[phthalazine-1,4-diylbis-(oxy)]bis[10,11-dihydro-6'-methoxycinchonane] / tert-butyl alcohol; water / 120 h / 0 - 20 °C 3.1: sodium hydride / hexane; mineral oil; N,N-dimethyl-formamide / 0 - 20 °C 4.1: N-ethyl-N,N-diisopropylamine; tetra-(n-butyl)ammonium iodide / dichloromethane / 24 h / 20 °C 5.1: N,N,N,N,-tetramethylethylenediamine; sec.-butyllithium / hexane; toluene; diethyl ether; cyclohexane / 1 h / -78 °C 5.2: 1.5 h / -78 - -30 °C 6.1: boron trifluoride diethyl etherate / dichloromethane / 0.25 h / 0 °C 7.1: sodium tetrahydroborate / ethanol / 0.5 h / 20 °C 8.1: pyridinium p-toluenesulfonate / dichloromethane / 4 h / 20 °C 9.1: tetrabutyl ammonium fluoride / tetrahydrofuran / 1 h / 20 °C 10.1: bis(1,5-cyclooctadiene)nickel (0); tricyclohexylphosphine; potassium tert-butylate / toluene / 2 h / Reflux 11.1: aluminum (III) chloride; lithium aluminium tetrahydride / dichloromethane; toluene; diethyl ether / 0.33 h / 0 °C 12.1: sodium hydride; 1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone; 15-crown-5 / toluene / 2 h / 80 °C 13.1: palladium 10% on activated carbon; hydrogen / tert-butyl alcohol; water / 15 h / 20 °C 14.1: Dess-Martin periodane / dichloromethane / 0.25 h / 20 °C 14.2: 1.5 h / 50 °C 15.1: Burgess Reagent / toluene / 0.5 h / Reflux View Scheme |

-
-
83-79-4
rotenone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 10 steps 1.1: boron trifluoride diethyl etherate / dichloromethane / 0.25 h / 0 °C 2.1: sodium tetrahydroborate / ethanol / 0.5 h / 20 °C 3.1: pyridinium p-toluenesulfonate / dichloromethane / 4 h / 20 °C 4.1: tetrabutyl ammonium fluoride / tetrahydrofuran / 1 h / 20 °C 5.1: bis(1,5-cyclooctadiene)nickel (0); tricyclohexylphosphine; potassium tert-butylate / toluene / 2 h / Reflux 6.1: aluminum (III) chloride; lithium aluminium tetrahydride / dichloromethane; toluene; diethyl ether / 0.33 h / 0 °C 7.1: sodium hydride; 1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone; 15-crown-5 / toluene / 2 h / 80 °C 8.1: palladium 10% on activated carbon; hydrogen / tert-butyl alcohol; water / 15 h / 20 °C 9.1: Dess-Martin periodane / dichloromethane / 0.25 h / 20 °C 9.2: 1.5 h / 50 °C 10.1: pyridine; thionyl chloride / 0.5 h / 0 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 10 steps 1.1: boron trifluoride diethyl etherate / dichloromethane / 0.25 h / 0 °C 2.1: sodium tetrahydroborate / ethanol / 0.5 h / 20 °C 3.1: pyridinium p-toluenesulfonate / dichloromethane / 4 h / 20 °C 4.1: tetrabutyl ammonium fluoride / tetrahydrofuran / 1 h / 20 °C 5.1: bis(1,5-cyclooctadiene)nickel (0); tricyclohexylphosphine; potassium tert-butylate / toluene / 2 h / Reflux 6.1: aluminum (III) chloride; lithium aluminium tetrahydride / dichloromethane; toluene; diethyl ether / 0.33 h / 0 °C 7.1: sodium hydride; 1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone; 15-crown-5 / toluene / 2 h / 80 °C 8.1: palladium 10% on activated carbon; hydrogen / tert-butyl alcohol; water / 15 h / 20 °C 9.1: Dess-Martin periodane / dichloromethane / 0.25 h / 20 °C 9.2: 1.5 h / 50 °C 10.1: Burgess Reagent / toluene / 0.5 h / Reflux View Scheme | |
| Multi-step reaction with 10 steps 1.1: boron trifluoride diethyl etherate / dichloromethane / 0.25 h / 0 °C / Inert atmosphere 1.2: 0.5 h / 20 °C / Inert atmosphere 2.1: pyridinium p-toluenesulfonate / dichloromethane / 3 h / 20 °C / Inert atmosphere 3.1: tetrabutyl ammonium fluoride / tetrahydrofuran / 1 h / 20 °C / Inert atmosphere 4.1: potassium tert-butylate; bis(1,5-cyclooctadiene)nickel (0); tricyclohexylphosphine / toluene / 2 h / Inert atmosphere; Reflux 5.1: aluminum (III) chloride; lithium aluminium tetrahydride / diethyl ether; dichloromethane / 0.33 h / 0 °C / Inert atmosphere 6.1: sodium hydride; 15-crown-5; 1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone / toluene / 2 h / 80 °C / Inert atmosphere 7.1: 10 wt% Pd(OH)2 on carbon; hydrogen / tetrahydrofuran; tert-butyl alcohol; water / 3 h / 20 °C 8.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 20 °C / Inert atmosphere 9.1: hydrogenchloride / methanol / 1.5 h / 50 °C / Inert atmosphere 10.1: Burgess Reagent / toluene / 0.5 h / Inert atmosphere; Reflux View Scheme |

-
-
83-79-4
rotenone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1.1: pyridinium p-toluenesulfonate / dichloromethane / 4 h / 20 °C 2.1: tetrabutyl ammonium fluoride / tetrahydrofuran / 1 h / 20 °C 3.1: bis(1,5-cyclooctadiene)nickel (0); tricyclohexylphosphine; potassium tert-butylate / toluene / 2 h / Reflux 4.1: aluminum (III) chloride; lithium aluminium tetrahydride / dichloromethane; toluene; diethyl ether / 0.33 h / 0 °C 5.1: sodium hydride; 1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone; 15-crown-5 / toluene / 2 h / 80 °C 6.1: palladium 10% on activated carbon; hydrogen / tert-butyl alcohol; water / 15 h / 20 °C 7.1: Dess-Martin periodane / dichloromethane / 0.25 h / 20 °C 7.2: 1.5 h / 50 °C 8.1: pyridine; thionyl chloride / 0.5 h / 0 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1.1: pyridinium p-toluenesulfonate / dichloromethane / 4 h / 20 °C 2.1: tetrabutyl ammonium fluoride / tetrahydrofuran / 1 h / 20 °C 3.1: bis(1,5-cyclooctadiene)nickel (0); tricyclohexylphosphine; potassium tert-butylate / toluene / 2 h / Reflux 4.1: aluminum (III) chloride; lithium aluminium tetrahydride / dichloromethane; toluene; diethyl ether / 0.33 h / 0 °C 5.1: sodium hydride; 1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone; 15-crown-5 / toluene / 2 h / 80 °C 6.1: palladium 10% on activated carbon; hydrogen / tert-butyl alcohol; water / 15 h / 20 °C 7.1: Dess-Martin periodane / dichloromethane / 0.25 h / 20 °C 7.2: 1.5 h / 50 °C 8.1: Burgess Reagent / toluene / 0.5 h / Reflux View Scheme | |
| Multi-step reaction with 9 steps 1: pyridinium p-toluenesulfonate / dichloromethane / 3 h / 20 °C / Inert atmosphere 2: tetrabutyl ammonium fluoride / tetrahydrofuran / 1 h / 20 °C / Inert atmosphere 3: potassium tert-butylate; bis(1,5-cyclooctadiene)nickel (0); tricyclohexylphosphine / toluene / 2 h / Inert atmosphere; Reflux 4: aluminum (III) chloride; lithium aluminium tetrahydride / diethyl ether; dichloromethane / 0.33 h / 0 °C / Inert atmosphere 5: sodium hydride; 15-crown-5; 1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone / toluene / 2 h / 80 °C / Inert atmosphere 6: 10 wt% Pd(OH)2 on carbon; hydrogen / tetrahydrofuran; tert-butyl alcohol; water / 3 h / 20 °C 7: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 20 °C / Inert atmosphere 8: hydrogenchloride / methanol / 1.5 h / 50 °C / Inert atmosphere 9: Burgess Reagent / toluene / 0.5 h / Inert atmosphere; Reflux View Scheme |

-
-
83-79-4
rotenone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 9 steps 1.1: sodium tetrahydroborate / ethanol / 0.5 h / 20 °C 2.1: pyridinium p-toluenesulfonate / dichloromethane / 4 h / 20 °C 3.1: tetrabutyl ammonium fluoride / tetrahydrofuran / 1 h / 20 °C 4.1: bis(1,5-cyclooctadiene)nickel (0); tricyclohexylphosphine; potassium tert-butylate / toluene / 2 h / Reflux 5.1: aluminum (III) chloride; lithium aluminium tetrahydride / dichloromethane; toluene; diethyl ether / 0.33 h / 0 °C 6.1: sodium hydride; 1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone; 15-crown-5 / toluene / 2 h / 80 °C 7.1: palladium 10% on activated carbon; hydrogen / tert-butyl alcohol; water / 15 h / 20 °C 8.1: Dess-Martin periodane / dichloromethane / 0.25 h / 20 °C 8.2: 1.5 h / 50 °C 9.1: pyridine; thionyl chloride / 0.5 h / 0 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 9 steps 1.1: sodium tetrahydroborate / ethanol / 0.5 h / 20 °C 2.1: pyridinium p-toluenesulfonate / dichloromethane / 4 h / 20 °C 3.1: tetrabutyl ammonium fluoride / tetrahydrofuran / 1 h / 20 °C 4.1: bis(1,5-cyclooctadiene)nickel (0); tricyclohexylphosphine; potassium tert-butylate / toluene / 2 h / Reflux 5.1: aluminum (III) chloride; lithium aluminium tetrahydride / dichloromethane; toluene; diethyl ether / 0.33 h / 0 °C 6.1: sodium hydride; 1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone; 15-crown-5 / toluene / 2 h / 80 °C 7.1: palladium 10% on activated carbon; hydrogen / tert-butyl alcohol; water / 15 h / 20 °C 8.1: Dess-Martin periodane / dichloromethane / 0.25 h / 20 °C 8.2: 1.5 h / 50 °C 9.1: Burgess Reagent / toluene / 0.5 h / Reflux View Scheme |

-
-
83-79-4
rotenone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1.1: tetrabutyl ammonium fluoride / tetrahydrofuran / 1 h / 20 °C 2.1: bis(1,5-cyclooctadiene)nickel (0); tricyclohexylphosphine; potassium tert-butylate / toluene / 2 h / Reflux 3.1: aluminum (III) chloride; lithium aluminium tetrahydride / dichloromethane; toluene; diethyl ether / 0.33 h / 0 °C 4.1: sodium hydride; 1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone; 15-crown-5 / toluene / 2 h / 80 °C 5.1: palladium 10% on activated carbon; hydrogen / tert-butyl alcohol; water / 15 h / 20 °C 6.1: Dess-Martin periodane / dichloromethane / 0.25 h / 20 °C 6.2: 1.5 h / 50 °C 7.1: pyridine; thionyl chloride / 0.5 h / 0 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1.1: tetrabutyl ammonium fluoride / tetrahydrofuran / 1 h / 20 °C 2.1: bis(1,5-cyclooctadiene)nickel (0); tricyclohexylphosphine; potassium tert-butylate / toluene / 2 h / Reflux 3.1: aluminum (III) chloride; lithium aluminium tetrahydride / dichloromethane; toluene; diethyl ether / 0.33 h / 0 °C 4.1: sodium hydride; 1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone; 15-crown-5 / toluene / 2 h / 80 °C 5.1: palladium 10% on activated carbon; hydrogen / tert-butyl alcohol; water / 15 h / 20 °C 6.1: Dess-Martin periodane / dichloromethane / 0.25 h / 20 °C 6.2: 1.5 h / 50 °C 7.1: Burgess Reagent / toluene / 0.5 h / Reflux View Scheme | |
| Multi-step reaction with 8 steps 1: tetrabutyl ammonium fluoride / tetrahydrofuran / 1 h / 20 °C / Inert atmosphere 2: potassium tert-butylate; bis(1,5-cyclooctadiene)nickel (0); tricyclohexylphosphine / toluene / 2 h / Inert atmosphere; Reflux 3: aluminum (III) chloride; lithium aluminium tetrahydride / diethyl ether; dichloromethane / 0.33 h / 0 °C / Inert atmosphere 4: sodium hydride; 15-crown-5; 1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone / toluene / 2 h / 80 °C / Inert atmosphere 5: 10 wt% Pd(OH)2 on carbon; hydrogen / tetrahydrofuran; tert-butyl alcohol; water / 3 h / 20 °C 6: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 20 °C / Inert atmosphere 7: hydrogenchloride / methanol / 1.5 h / 50 °C / Inert atmosphere 8: Burgess Reagent / toluene / 0.5 h / Inert atmosphere; Reflux View Scheme |

-
-
83-79-4
rotenone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1.1: bis(1,5-cyclooctadiene)nickel (0); tricyclohexylphosphine; potassium tert-butylate / toluene / 2 h / Reflux 2.1: aluminum (III) chloride; lithium aluminium tetrahydride / dichloromethane; toluene; diethyl ether / 0.33 h / 0 °C 3.1: sodium hydride; 1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone; 15-crown-5 / toluene / 2 h / 80 °C 4.1: palladium 10% on activated carbon; hydrogen / tert-butyl alcohol; water / 15 h / 20 °C 5.1: Dess-Martin periodane / dichloromethane / 0.25 h / 20 °C 5.2: 1.5 h / 50 °C 6.1: pyridine; thionyl chloride / 0.5 h / 0 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1.1: bis(1,5-cyclooctadiene)nickel (0); tricyclohexylphosphine; potassium tert-butylate / toluene / 2 h / Reflux 2.1: aluminum (III) chloride; lithium aluminium tetrahydride / dichloromethane; toluene; diethyl ether / 0.33 h / 0 °C 3.1: sodium hydride; 1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone; 15-crown-5 / toluene / 2 h / 80 °C 4.1: palladium 10% on activated carbon; hydrogen / tert-butyl alcohol; water / 15 h / 20 °C 5.1: Dess-Martin periodane / dichloromethane / 0.25 h / 20 °C 5.2: 1.5 h / 50 °C 6.1: Burgess Reagent / toluene / 0.5 h / Reflux View Scheme | |
| Multi-step reaction with 7 steps 1: potassium tert-butylate; bis(1,5-cyclooctadiene)nickel (0); tricyclohexylphosphine / toluene / 2 h / Inert atmosphere; Reflux 2: aluminum (III) chloride; lithium aluminium tetrahydride / diethyl ether; dichloromethane / 0.33 h / 0 °C / Inert atmosphere 3: sodium hydride; 15-crown-5; 1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone / toluene / 2 h / 80 °C / Inert atmosphere 4: 10 wt% Pd(OH)2 on carbon; hydrogen / tetrahydrofuran; tert-butyl alcohol; water / 3 h / 20 °C 5: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 20 °C / Inert atmosphere 6: hydrogenchloride / methanol / 1.5 h / 50 °C / Inert atmosphere 7: Burgess Reagent / toluene / 0.5 h / Inert atmosphere; Reflux View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 15 steps 1.1: N,N,N,N,N,N-hexamethylphosphoric triamide; n-butyllithium / tetrahydrofuran; hexane / 1.42 h / -78 °C 1.2: 2 h / 20 °C 2.1: potassium osmate; potassium hexacyanoferrate(III); potassium carbonate; methanesulfonamide; (9S,9"S)-9,9"-[phthalazine-1,4-diylbis-(oxy)]bis[10,11-dihydro-6'-methoxycinchonane] / tert-butyl alcohol; water / 120 h / 0 - 20 °C 3.1: sodium hydride / hexane; mineral oil; N,N-dimethyl-formamide / 0 - 20 °C 4.1: N-ethyl-N,N-diisopropylamine; tetra-(n-butyl)ammonium iodide / dichloromethane / 24 h / 20 °C 5.1: N,N,N,N,-tetramethylethylenediamine; sec.-butyllithium / hexane; toluene; diethyl ether; cyclohexane / 1 h / -78 °C 5.2: 1.5 h / -78 - -30 °C 6.1: boron trifluoride diethyl etherate / dichloromethane / 0.25 h / 0 °C 7.1: sodium tetrahydroborate / ethanol / 0.5 h / 20 °C 8.1: pyridinium p-toluenesulfonate / dichloromethane / 4 h / 20 °C 9.1: tetrabutyl ammonium fluoride / tetrahydrofuran / 1 h / 20 °C 10.1: bis(1,5-cyclooctadiene)nickel (0); tricyclohexylphosphine; potassium tert-butylate / toluene / 2 h / Reflux 11.1: aluminum (III) chloride; lithium aluminium tetrahydride / dichloromethane; toluene; diethyl ether / 0.33 h / 0 °C 12.1: sodium hydride; 1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone; 15-crown-5 / toluene / 2 h / 80 °C 13.1: palladium 10% on activated carbon; hydrogen / tert-butyl alcohol; water / 15 h / 20 °C 14.1: Dess-Martin periodane / dichloromethane / 0.25 h / 20 °C 14.2: 1.5 h / 50 °C 15.1: pyridine; thionyl chloride / 0.5 h / 0 °C View Scheme |
-
-
83-79-4
rotenone

-
-
6659-45-6
2R,6aS,12aS-2-isopropyl-8,9-dimethoxy-1,2,12,12a-tetrahydrochromeno[3,4-b]furo[2,3-h]chromen-6(6aH)-one

| Conditions | Yield |
|---|---|
| With palladium 10% on activated carbon; hydrogen In dichloromethane at 20℃; | 98% |
| With hydrogen; palladium on activated charcoal In ethyl acetate Ambient temperature; | 94% |
| With hydrogen; palladium on activated charcoal In acetone | 90% |
-
-
83-79-4
rotenone

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In methanol at 50℃; for 2h; | 98% |
| With sodium tetrahydroborate In methanol at 0℃; for 3h; | 95% |
| Multi-step reaction with 2 steps 1: two-dimensional iron(II) coordination polymer based on a divergent 4'-(4-diphenylamino)phenyl-4,2';6',4''-terpyridine ligand; potassium tert-butylate / tetrahydrofuran / 4 h / 20 °C / Green chemistry 2: silica gel / ethyl acetate; hexane / 20 °C View Scheme | |
| With sodium tetrahydroborate In methanol at 0 - 20℃; for 2h; |

| Conditions | Yield |
|---|---|
| With caesium carbonate; Benzene-1,2-dithiol In 1,4-dioxane at 10℃; for 7h; Inert atmosphere; Glovebox; | 96% |
-
-
83-79-4
rotenone

-
-
88390-15-2
(6aS,12S,12aR,5′R)-12-deoxo-12-hydroxyrotenone

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In methanol at 20℃; for 2h; Inert atmosphere; | 92% |
| With sodium tetrahydroborate In methanol | 85% |
| With lithium aluminium tetrahydride Reduction; | |
| With lithium aluminium tetrahydride In tetrahydrofuran for 3h; Reduction; Heating; |

| Conditions | Yield |
|---|---|
| With dipotassium hydrogenphosphate; tris(1,10-phenanthroline)ruthenium(II) dichloride In dichloromethane at 25℃; for 15h; Inert atmosphere; Irradiation; regioselective reaction; | 92% |
| With dipotassium hydrogenphosphate; tris(1,10-phenanthroline)ruthenium(II) dichloride In dichloromethane at 25℃; for 15h; Photolysis; regioselective reaction; | 92% |
| With 2,4,6-trimethyl-pyridine; [Hf6(μ3-O)4(mu3-OH)4(formato)5.9(eosinato Y)0.1(4'-(4-carboxylatophenyl)[2,2':6',2''-terpyridine]-5,5''-dicarboxylato-Fe(OTf)2)2] In acetonitrile at 20℃; for 2h; Catalytic behavior; Inert atmosphere; Schlenk technique; Irradiation; | 70% |
-
-
83-79-4
rotenone

-
-
3276-14-0, 112838-10-5, 112838-11-6, 112838-12-7
(2R,6aR,12aS)-8,9-dimethoxy-2-(prop-1-en-2-yl)-1,2,12,12a-tetrahydrochromeno[3,4-b]furo[2,3-h]chromen-6(6aH)-one oxime

| Conditions | Yield |
|---|---|
| With hydroxylamine hydrochloride; sodium acetate In ethanol for 10h; Reflux; | 91% |
| With hydroxylamine hydrochloride; sodium acetate In ethanol for 18h; Reflux; | 74% |
| With pyridine; hydroxylamine hydrochloride | 70% |
| With hydroxylamine |
-
-
83-79-4
rotenone

| Conditions | Yield |
|---|---|
| With sodium acetate; hydrazine hydrate In ethanol at 80℃; for 5h; | 91% |

| Conditions | Yield |
|---|---|
| With Rh2(esp)2 In dichloromethane at 22℃; for 4h; Inert atmosphere; diastereoselective reaction; | 88% |
-
-
83-79-4
rotenone

| Conditions | Yield |
|---|---|
| With potassium hydroxide; hydrazine In ethanol Heating; | 87% |
| With hydrazine hydrate In ethanol |
-
-
83-79-4
rotenone

-
-
58277-58-0
(6aS,12aS,5’R)-rotenone hydrobromide

| Conditions | Yield |
|---|---|
| With hydrogen bromide; acetic acid at 20℃; for 0.5h; | 87% |

| Conditions | Yield |
|---|---|
| Stage #1: rotenone With copper diacetate; 2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl In tetrahydrofuran for 0.0833333h; Inert atmosphere; Stage #2: 1,2-propanediene With (dimethoxy)methylsilane In tetrahydrofuran at 20℃; for 12h; Inert atmosphere; diastereoselective reaction; | 86% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide In ethanol Heating; | 85% |
-
-
83-79-4
rotenone

-
-
30462-22-7
2-((2R,6aS,12aS)-8,9-dimethoxy-1,2,6,6a,12,12a-hexahydrochromeno[3,4-b]furo[2,3-h]chromen-2-yl)propan-2-ol

| Conditions | Yield |
|---|---|
| With iron(III)-acetylacetonate; methyl 4-nitrobenzenesulfonate; phenylsilane; sodium hydrogencarbonate In methanol at 0 - 20℃; for 12h; Schlenk technique; Inert atmosphere; regioselective reaction; | 85% |
| Stage #1: rotenone With mercury(II) diacetate In tetrahydrofuran; water at 20℃; for 18h; Stage #2: With sodium tetrahydroborate; sodium hydrogencarbonate In tetrahydrofuran; water | 48% |
| With sodium tetrahydroborate; mercury(II) diacetate 1) H2O, THF, 20 deg C, 10 h, 2) 30 s; Yield given. Multistep reaction; | |
| Multi-step reaction with 3 steps 1: 1) H2, pyridine / 1) 5percent Pd-BaSO4 2: 0.37 g / m-chloroperbenzoic acid, NaHCO3 / CH2Cl2; H2O / 0.75 h / 19 °C 3: 66 percent / activated Zn-dust / methanol / Ambient temperature View Scheme |
-
-
83-79-4
rotenone

-
-
811451-63-5
6aS,12aS-8,9-dimethoxy-2-(2-methyloxiran-2-yl)-1,2,12,12a-tetrahydrochromeno[3,4-b]furo[2,3-h]chromen-6(6aH)-one

| Conditions | Yield |
|---|---|
| With 3-chloro-benzenecarboperoxoic acid In dichloromethane at 20℃; for 2h; | 85% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide In ethanol Heating; | 84% |
-
-
83-79-4
rotenone

-
-
15130-81-1
(6aS,12aS,5′R)-rotenone-6′-norketone

| Conditions | Yield |
|---|---|
| With sodium periodate; osmium(VIII) oxide In tetrahydrofuran; water; tert-butyl alcohol | 82% |
| With sodium periodate; osmium(VIII) oxide | 46% |

| Conditions | Yield |
|---|---|
| With potassium dichromate; acetic acid In water at 20 - 60℃; for 18.5h; | 82% |
| With dichromate anion; acetic acid | 51% |
-
-
83-79-4
rotenone

-
-
82481-44-5
(2R,6S,6aS,12aS,2'R,6'S,6'aS,12'aS)-2,2'-Diisopropenyl-8,9,8',9'-tetramethoxy-1,2,12,12a,1',2',12',12'a-octahydro-6aH,6'aH-[6,6']bi[chromeno[3,4-b]furo[2,3-h]chromenyl]-6,6'-diol

| Conditions | Yield |
|---|---|
| In acetic acid; acetonitrile electrochemical reduction; | 80% |
| In acetic acid; acetonitrile Product distribution; Mechanism; electrochemical reduction; variation of solvent; | 80 % Chromat. |
-
-
83-79-4
rotenone

| Conditions | Yield |
|---|---|
| Stage #1: rotenone With dimethylsulfide borane complex In tetrahydrofuran at 0 - 20℃; for 1.5h; Inert atmosphere; Stage #2: With dihydrogen peroxide; sodium hydroxide In tetrahydrofuran; water at 0 - 20℃; for 18h; | 80% |
| Stage #1: rotenone With borane In tetrahydrofuran Reduction; hydroboration; Stage #2: With sodium hydroxide; dihydrogen peroxide Oxidation; |

| Conditions | Yield |
|---|---|
| In water; acetonitrile electrochemical reduction; | 75% |
| With sodium hydroxide In ethanol Mechanism; Electrochemical reduction; tetrabutylammonium perchlorate, aqueous or aprotic medium; | 70% |
| With sodium hydroxide In ethanol controlled potential electrolysis, tetrabutylammonium perchlorate; other solvent DMF; | 70% |

| Conditions | Yield |
|---|---|
| In ethanol Product distribution; Heating; other amines, also with nitrenonone; | 73% |
| In ethanol Heating; | 73% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide In ethanol Heating; | 71% |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran 1.) room temperature, 10 min, 2.) 45-55 deg C, 50 min; | 71% |
-
-
58310-28-4
(Difluoromethyl)triphenylphosphonium bromide

-
-
83-79-4
rotenone

| Conditions | Yield |
|---|---|
| With tris[2-phenylpyridinato-C2,N]iridium(III); copper(ll) bromide In N,N-dimethyl-formamide at 20℃; for 10h; Schlenk technique; Inert atmosphere; Irradiation; | 71% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide In ethanol Heating; | 70% |
-
-
74-88-4
methyl iodide

-
-
83-79-4
rotenone

-
-
59456-14-3, 143838-84-0, 143838-90-8, 149116-35-8
(6aS,12aR,5'R)-/(6aR,12aS,5'R)-12a-methylrotenone

| Conditions | Yield |
|---|---|
| With sodium hydride In N,N-dimethyl-formamide at 20℃; for 6h; | 66% |
-
-
83-79-4
rotenone

| Conditions | Yield |
|---|---|
| With potassium hydroxide; hydroxylamine hydrochloride In ethanol for 3.5h; Heating; | 66% |

| Conditions | Yield |
|---|---|
| Stage #1: dibromodifluoromethane; rotenone With tetrahydrofuran; eosin at 20℃; for 5h; Irradiation; Inert atmosphere; Schlenk technique; Stage #2: With potassium hydrogencarbonate for 5h; Inert atmosphere; Irradiation; Cooling with ice; Schlenk technique; | 65% |

| Conditions | Yield |
|---|---|
| With eosin y In tetrahydrofuran at 20℃; for 10h; Inert atmosphere; | 65% |
Related products
Raw Materials
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View



 N,
N, T
T