-
Name
(1R,2R)-(-)-2-Amino-1-phenyl-1,3-propanediol
- EINECS 256-250-8
- CAS No. 46032-98-8
- Article Data45
- CAS DataBase
- Density 1.206 g/cm3
- Solubility
- Melting Point 112 - 115 C
- Formula C9H13 N O2
- Boiling Point 360.6 °C at 760 mmHg
- Molecular Weight 167.208
- Flash Point 171.9 °C
- Transport Information UN 3259
- Appearance light yellow powder
- Safety 26-36-37/39
- Risk Codes R36/37/38
-
Molecular Structure
-
Hazard Symbols

- Synonyms 1,3-Propanediol,2-amino-1-phenyl-, [R-(R*,R*)]-; (1R,2R)-(-)-2-Amino-1-phenyl-1,3-propanediol;(1R,2R)-2-Amino-1-phenylpropane-1,3-diol;D-threo-1-Phenyl-2-amino-1,3-propanediol;D-threo-3-Phenyl-2-amino-1,3-propanediol
- PSA 66.48000
- LogP 0.73990
Synthetic route
-
-
912296-87-8
syn-(2R,3R)-[2-azido-3-hydroxy-3-phenyl-1-propanol]

-
-
46032-98-8
(1R,2R)-2-amino-1-phenylpropane-1,3-diol

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal In methanol at 20℃; under 760 Torr; for 12h; | 99% |
-
-
849357-84-2
(2S,3R)-2-Azido-3-nitrosooxy-3-phenyl-propionic acid methyl ester

-
-
46032-98-8
(1R,2R)-2-amino-1-phenylpropane-1,3-diol

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal In methanol at 20℃; under 760 Torr; for 12h; | 97% |
-
-
3306-06-7, 5285-86-9, 28143-91-1, 36391-67-0, 36391-68-1, 46032-98-8, 55057-81-3, 105452-39-9, 119364-52-2
(+/-)-threo-2-amino-1-phenyl-1,3-propanediol

-
-
46032-98-8
(1R,2R)-2-amino-1-phenylpropane-1,3-diol

| Conditions | Yield |
|---|---|
| With L-Tartaric acid | |
| With L-(-)-O,O'-dibenzoyltartaric acid | |
| With (1S)-(+)-3-bromocamphor-10-sulfonic acid | |
| With D-Glutamic acid |
-
-
114530-99-3
(2S,3R)-2-amino-3-hydroxy-3-phenyl-propionic acid cyclohexyl ester

-
-
46032-98-8
(1R,2R)-2-amino-1-phenylpropane-1,3-diol

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride; diethyl ether |
-
-
91247-54-0
1-Phenyl-2-acetamido-1,3-propandiol

-
A
-
28143-91-1
(1S,2S)-2-amino-1-phenyl-1,3-diol

-
B
-
46032-98-8
(1R,2R)-2-amino-1-phenylpropane-1,3-diol

-
C
-
105452-39-9
(1R,2S)-(+)-2-amino-1-phenyl-1,3-propanediol

-
D
-
119364-52-2
(1S,2R)-2-amino-1-phenylpropane-1,3-diol

| Conditions | Yield |
|---|---|
| hydrolysis; |


-
-
46032-98-8
(1R,2R)-2-amino-1-phenylpropane-1,3-diol

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride In tetrahydrofuran for 4h; Heating; |
-
-
285567-86-4
(1R,2R)-2-amino-(N-benzyloxycarbonyl)-1-phenylpropane-1,3-diol

-
-
46032-98-8
(1R,2R)-2-amino-1-phenylpropane-1,3-diol

| Conditions | Yield |
|---|---|
| With hydrogen |

-
A
-
28143-91-1
(1S,2S)-2-amino-1-phenyl-1,3-diol

-
B
-
46032-98-8
(1R,2R)-2-amino-1-phenylpropane-1,3-diol

| Conditions | Yield |
|---|---|
| With (1S)-(+)-3-bromocamphor-10-sulfonic acid; ethyl acetate |

-
-
46032-98-8
(1R,2R)-2-amino-1-phenylpropane-1,3-diol

| Conditions | Yield |
|---|---|
| With (1S)-(+)-3-bromocamphor-10-sulfonic acid; ethyl acetate |

-
-
46032-98-8
(1R,2R)-2-amino-1-phenylpropane-1,3-diol

| Conditions | Yield |
|---|---|
| With 1,4-dioxane; sodium tetrahydroborate und Erwaermen des Reaktionsprodukts mit wss.HCl; |

-
-
46032-98-8
(1R,2R)-2-amino-1-phenylpropane-1,3-diol

| Conditions | Yield |
|---|---|
| With diethyl ether; nitric acid; acetic acid |
-
-
127181-72-0
anti-(4S,2'S,3'S)-3-(3'-Hydroxy-3'-phenyl-2'-bromo-1'-oxopropyl)-4-(1-methylethyl)-2-oxazolidinone

-
-
46032-98-8
(1R,2R)-2-amino-1-phenylpropane-1,3-diol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 89 percent / NaN3 / dimethylformamide / 6 h / 25 °C 2: 90 percent / LiBH4 / tetrahydrofuran; methanol / 1 h / 0 °C 3: 99 percent / H2 / Pd/C / methanol / 12 h / 20 °C / 760 Torr View Scheme |
-
-
112459-60-6
(4S)-4-isopropyl-3-[(2E)-3-phenylprop-2-enoyl]-1,3-oxazolidin-2-one

-
-
46032-98-8
(1R,2R)-2-amino-1-phenylpropane-1,3-diol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: Ag2O; Br2 / acetone / -10 °C 2: 89 percent / NaN3 / dimethylformamide / 6 h / 25 °C 3: 90 percent / LiBH4 / tetrahydrofuran; methanol / 1 h / 0 °C 4: 99 percent / H2 / Pd/C / methanol / 12 h / 20 °C / 760 Torr View Scheme |
-
-
151003-65-5
syn-(4S,2'S,3'R)-3-(2'-azido-3'-hydroxy-3'-phenyl-propionyl)-4-(1-methylethyl)-2-oxazolidinone

-
-
46032-98-8
(1R,2R)-2-amino-1-phenylpropane-1,3-diol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 90 percent / LiBH4 / tetrahydrofuran; methanol / 1 h / 0 °C 2: 99 percent / H2 / Pd/C / methanol / 12 h / 20 °C / 760 Torr View Scheme |
-
-
115794-67-7
methyl (2R,3S)-3-phenylglycidate

-
-
46032-98-8
(1R,2R)-2-amino-1-phenylpropane-1,3-diol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 89 percent / NaNO2; AcOH / H2O / 2 h / 0 - 20 °C 2: 82 percent / diphenyl phosphoryl azide; DEAD; PPh3 / tetrahydrofuran / 12 h / 0 - 20 °C 3: 97 percent / H2 / Pd/C / methanol / 12 h / 20 °C / 760 Torr View Scheme |
-
-
849357-83-1
(2R,3R)-2-Hydroxy-3-nitrosooxy-3-phenyl-propionic acid methyl ester

-
-
46032-98-8
(1R,2R)-2-amino-1-phenylpropane-1,3-diol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 82 percent / diphenyl phosphoryl azide; DEAD; PPh3 / tetrahydrofuran / 12 h / 0 - 20 °C 2: 97 percent / H2 / Pd/C / methanol / 12 h / 20 °C / 760 Torr View Scheme |
-
-
104320-68-5
methyl (4S,5R)-5-phenyl-4,5-dihydro-1,3-oxazole-4-carboxylate

-
-
46032-98-8
(1R,2R)-2-amino-1-phenylpropane-1,3-diol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: conc. HCl / methanol / 3 h / 50 °C 2: LiAlH4 / tetrahydrofuran / 4 h / Heating View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: chiral ferrocenylphosphine-gold(I) complex / CH2Cl2 / 20 h / 25 °C 2: conc. HCl / methanol / 3 h / 50 °C 3: LiAlH4 / tetrahydrofuran / 4 h / Heating View Scheme |

-
-
46032-98-8
(1R,2R)-2-amino-1-phenylpropane-1,3-diol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: HCl 2: (S)-5-oxo-pyrrolidine-2-carboxylic acid 3: LiAlH4; diethyl ether View Scheme |
-
-
64153-18-0, 102207-94-3, 114530-99-3
(2RS,3SR)-2-amino-3-hydroxy-3-phenyl-propionic acid cyclohexyl ester

-
-
46032-98-8
(1R,2R)-2-amino-1-phenylpropane-1,3-diol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: (S)-5-oxo-pyrrolidine-2-carboxylic acid 2: LiAlH4; diethyl ether View Scheme |
-
-
46032-98-8
(1R,2R)-2-amino-1-phenylpropane-1,3-diol

-
-
108-24-7
acetic anhydride

-
-
111136-67-5
(1R,2R)-2-acetamido-1-phenylpropane-1,3-diyl diacetate

| Conditions | Yield |
|---|---|
| With pyridine; dmap | 100% |
| With pyridine |
-
-
46032-98-8
(1R,2R)-2-amino-1-phenylpropane-1,3-diol

-
-
501-53-1
benzyl chloroformate

-
-
285567-86-4
(1R,2R)-2-amino-(N-benzyloxycarbonyl)-1-phenylpropane-1,3-diol

| Conditions | Yield |
|---|---|
| With sodium carbonate In 1,4-dioxane; water at 30℃; for 2h; | 99% |
-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
46032-98-8
(1R,2R)-2-amino-1-phenylpropane-1,3-diol

-
-
67-64-1
acetone

-
-
1009092-91-4
tert-butyl (4R,5R)-4-(hydroxymethyl)-2,2-dimethyl-5-phenyl-1,3-oxazolidine-3-carboxylate

| Conditions | Yield |
|---|---|
| Stage #1: (1R,2R)-2-amino-1-phenylpropane-1,3-diol; acetone In toluene for 5h; Reflux; Stage #2: di-tert-butyl dicarbonate In tetrahydrofuran at 20℃; for 5h; | 98% |
| Stage #1: (1R,2R)-2-amino-1-phenylpropane-1,3-diol; acetone In toluene Reflux; Stage #2: di-tert-butyl dicarbonate In tetrahydrofuran at 20℃; | 91% |
| Stage #1: (1R,2R)-2-amino-1-phenylpropane-1,3-diol; acetone In toluene for 22h; Dean-Stark; Reflux; Stage #2: di-tert-butyl dicarbonate In toluene at 20℃; | 78.5% |
| Stage #1: (1R,2R)-2-amino-1-phenylpropane-1,3-diol; acetone In dichloromethane at 50℃; Stage #2: di-tert-butyl dicarbonate at 50℃; |

-
-
46032-98-8
(1R,2R)-2-amino-1-phenylpropane-1,3-diol

-
-
100-39-0
benzyl bromide

| Conditions | Yield |
|---|---|
| With tetra-(n-butyl)ammonium iodide; potassium carbonate In acetonitrile Heating; | 96% |
| With tetra-(n-butyl)ammonium iodide; potassium carbonate In acetonitrile Heating; | 96% |
| With tetra-(n-butyl)ammonium iodide; potassium carbonate In acetonitrile Heating; | |
| With potassium carbonate In acetonitrile for 8h; Heating; |
-
-
46032-98-8
(1R,2R)-2-amino-1-phenylpropane-1,3-diol

-
-
98-88-4
benzoyl chloride

-
-
1005196-97-3
benzoic acid (2R,3R)-2-benzoylamino-3-hydroxy-3-phenylpropyl ester

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran at 20℃; for 24h; | 96% |
-
-
46032-98-8
(1R,2R)-2-amino-1-phenylpropane-1,3-diol

-
-
70090-26-5
cholic acid N-succinimidyl ester

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 25℃; for 1.5h; | 92% |
-
-
46032-98-8
(1R,2R)-2-amino-1-phenylpropane-1,3-diol

-
-
174069-00-2
3α,12α-dihydroxy-5β-cholan-24-oic acid 2,5-dioxopyrrolidin-1-yl ester

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 25℃; for 1.5h; | 88% |
-
-
60280-45-7
N-Boc-L-methionine sulfone

-
-
46032-98-8
(1R,2R)-2-amino-1-phenylpropane-1,3-diol

-
-
1258542-74-3
(1R,2R)-1-phenyl-2-(Nα-Boc-L-methionylsulfone-amido)-1,3-propadiol

| Conditions | Yield |
|---|---|
| With 1-hydroxy-pyrrolidine-2,5-dione; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 20℃; for 24h; | 81% |

| Conditions | Yield |
|---|---|
| With O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In dichloromethane at 20℃; for 4h; | 79% |
| With O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In dichloromethane at 20℃; for 4h; | 79% |
-
-
46032-98-8
(1R,2R)-2-amino-1-phenylpropane-1,3-diol

-
-
123-54-6
acetylacetone

| Conditions | Yield |
|---|---|
| With niobium oxide phosphate In neat (no solvent) at 50℃; for 0.5h; | 78% |
-
-
46032-98-8
(1R,2R)-2-amino-1-phenylpropane-1,3-diol

-
-
106-93-4
ethylene dibromide

| Conditions | Yield |
|---|---|
| Stage #1: (1R,2R)-2-amino-1-phenylpropane-1,3-diol; ethylene dibromide at 100℃; for 10h; Stage #2: With sodium hydroxide In water | 75% |
-
-
625-38-7
but-3-enoic acid

-
-
201230-82-2
carbon monoxide

-
-
46032-98-8
(1R,2R)-2-amino-1-phenylpropane-1,3-diol

-
A
-
1231260-20-0
(2R,3R,8aS)-3-(hydroxymethyl)-2-phenylhexahydrooxazolo[3,2-a]pyridin-5-one

| Conditions | Yield |
|---|---|
| With acetylacetonatodicarbonylrhodium(l); hydrogen; pyridinium p-toluenesulfonate; 6,6′-[(3,3′-di-tert-butyl-5,5′-dimethoxy-1,1′-biphenyl-2,2′-diyl)bis(oxy)]bis(di-benzo[d,f][1,3,2]dioxaphosphepin) In tetrahydrofuran at 75℃; under 5168.02 Torr; for 1.5h; Inert atmosphere; Microwave irradiation; optical yield given as %de; regioselective reaction; | A 70% B n/a |


| Conditions | Yield |
|---|---|
| With O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In dichloromethane at 20℃; for 4h; | 70% |
| With O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In dichloromethane at 20℃; for 4h; | 70% |

| Conditions | Yield |
|---|---|
| With O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In dichloromethane at 20℃; for 4h; | 65% |
| With O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In dichloromethane at 20℃; for 4h; | 65% |

-
-
46032-98-8
(1R,2R)-2-amino-1-phenylpropane-1,3-diol

| Conditions | Yield |
|---|---|
| With O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In dichloromethane at 20℃; for 16h; | 62% |
| With O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In dichloromethane at 20℃; for 16h; | 62% |
-
-
26384-76-9
Ethyl 2-hydroxybenzimidate

-
-
46032-98-8
(1R,2R)-2-amino-1-phenylpropane-1,3-diol

| Conditions | Yield |
|---|---|
| In chlorobenzene at 80 - 110℃; for 25h; | 61% |
-
-
609-67-6
2-Iodobenzoyl chloride

-
-
46032-98-8
(1R,2R)-2-amino-1-phenylpropane-1,3-diol

-
-
872052-36-3
(R,R)-[2-(2-iodophenyl)-4,5-dihydrooxazol-4-yl]phenylmethanol

| Conditions | Yield |
|---|---|
| Stage #1: 2-Iodobenzoyl chloride; (1R,2R)-2-amino-1-phenylpropane-1,3-diol With triethylamine In dichloromethane at 0℃; for 0.5h; Stage #2: With dmap; p-toluenesulfonyl chloride In dichloromethane for 16h; Heating; | 59% |
-
-
90055-55-3
2-(2-chlorophenyl)-2-(6,7-dihydrothieno[3,2-c]pyridine-5(4H)-yl)acetic acid

-
-
46032-98-8
(1R,2R)-2-amino-1-phenylpropane-1,3-diol

-
-
917613-69-5
(S)-(+)-α-(2-chlorophenyl)-6,7-dihydro-4H-thieno[3,2-c]pyridine-5-acetic acid, (1R,2R)-(-)-2-amino-1-phenyl-1,3-propanediol salt

| Conditions | Yield |
|---|---|
| In isopropyl alcohol at 5℃; for 2h; Heating / reflux; | 57% |
-
-
46032-98-8
(1R,2R)-2-amino-1-phenylpropane-1,3-diol


| Conditions | Yield |
|---|---|
| Stage #1: (1R,2R)-2-amino-1-phenylpropane-1,3-diol; 3,3'-bis(3-bromopropoxy)-2,2'-dimethyl-1,1'-biphenyl With N-ethyl-N,N-diisopropylamine In methanol at 65℃; for 24h; Inert atmosphere; Stage #2: (S)-3-amino-1,2-dihydroxypropane hydrochloride With N-ethyl-N,N-diisopropylamine In methanol at 65℃; Inert atmosphere; | A 54% B 17% |

-
-
46032-98-8
(1R,2R)-2-amino-1-phenylpropane-1,3-diol

| Conditions | Yield |
|---|---|
| In chlorobenzene at 80 - 110℃; for 25h; | 52% |
-
-
4387-36-4
2-iodobenzonitrile

-
-
46032-98-8
(1R,2R)-2-amino-1-phenylpropane-1,3-diol

-
-
872052-32-9
[(R,R)-2-(2-iodophenyl)-5-phenyl-4,5-dihydrooxazol-4-yl]methanol

| Conditions | Yield |
|---|---|
| With cadmium(II) acetate In chlorobenzene for 16h; Heating; | 40% |
-
-
46032-98-8
(1R,2R)-2-amino-1-phenylpropane-1,3-diol

-
-
74-88-4
methyl iodide

-
-
51594-35-5
(1R,2R)-2-amino-3-methoxy-1-phenylpropan-1-ol

| Conditions | Yield |
|---|---|
| Stage #1: (1R,2R)-2-amino-1-phenylpropane-1,3-diol With potassium hydride In tetrahydrofuran at 20℃; for 12h; Inert atmosphere; Stage #2: methyl iodide In tetrahydrofuran at 20℃; for 4h; Inert atmosphere; | 11% |
-
-
50-00-0
formaldehyd

-
-
46032-98-8
(1R,2R)-2-amino-1-phenylpropane-1,3-diol

-
-
906331-30-4
(7aR)-1c-phenyl-(7ar)-dihydro-oxazolo[3,4-c]oxazole

| Conditions | Yield |
|---|---|
| With benzene |
-
-
111-71-7
heptanal

-
-
46032-98-8
(1R,2R)-2-amino-1-phenylpropane-1,3-diol

-
-
114947-26-1
(7aR)-3ξ,5ξ-dihexyl-1c-phenyl-(7ar)-dihydro-oxazolo[3,4-c]oxazole

| Conditions | Yield |
|---|---|
| With benzene |
-
-
116-54-1
dichloroacetic acid methyl ester

-
-
46032-98-8
(1R,2R)-2-amino-1-phenylpropane-1,3-diol

-
-
25126-19-6
(1R,2R)-2-(2,2-dichloro-acetylamino)-1-phenyl-propane-1,3-diol

| Conditions | Yield |
|---|---|
| With methanol |
-
-
872302-03-9
dichloroacetyl-phosphorous acid diethyl ester

-
-
46032-98-8
(1R,2R)-2-amino-1-phenylpropane-1,3-diol

-
-
25126-19-6
(1R,2R)-2-(2,2-dichloro-acetylamino)-1-phenyl-propane-1,3-diol

| Conditions | Yield |
|---|---|
| With ethanol; triisoamylamine |
(1R,2R)-(-)-2-Amino-1-phenyl-1,3-propanediol Chemical Properties
The MF of (1R,2R)-(-)-2-Amino-1-phenyl-1,3-propanediol(46032-98-8): C9H13NO2
The MW of (1R,2R)-(-)-2-Amino-1-phenyl-1,3-propanediol(46032-98-8): 167.21
EINECS: 256-250-8
mp: 112-118 °C(lit.)
alpha: -39 °(c=1, 1N HCl)
refractive index: -26.5 ° (C=1, MeOH)
Molar Refractivity: 47 cm3
Molar Volume: 138.5 cm3
Polarizability: 18.63 10-24 cm3
Surface Tension: 57.8 dyne/cm
Density: 1.206 g/cm3
Flash Point: 171.9 °C
Enthalpy of Vaporization: 63.98 kJ/mol
Boiling Point: 360.6 °C at 760 mmHg
Vapour Pressure: 7.87E-06 mmHg at 25°C
Categories: Amino Alcohols (Chiral);Asymmetric Synthesis;Chiral Building Blocks;Synthetic Organic Chemistry;Amino Alcohols;Chiral Building Blocks;Organic Building Blocks
Synonyms: (1R,2R)-(-)-2-AMINO-1-PHENYL-1,3-PROPANEDIOL;(1R,2R)-2-AMINO-1-PHENYLPROPANE-1,3-DIOL;(2R,3R)-3-PHENYLSERINOL;(2R,3S)-PHENYLSERINOL;[R(R*,R*)]-2-amino-1-phenylpropane-1,3-diol;(1R,2S)-3-Phenylserinol;1,3-Propanediol, 2-amino-1-phenyl-, [R-(R*,R*)]-;(1R,2R)-1-Phenyl-2-amino-1,3-propanediol
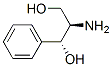
The Structure of (1R,2R)-(-)-2-Amino-1-phenyl-1,3-propanediol(46032-98-8):
(1R,2R)-(-)-2-Amino-1-phenyl-1,3-propanediol Safety Profile
 Xi
Xi Risk Statements: 36/37/38
36/37/38: Irritating to eyes, respiratory system and skin
Safety Statements: 26-36-37/39
26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
36: Wear suitable protective clothing
37/39: Wear suitable gloves and eye/face protection
RIDADR: UN 3259 8/PG 3
WGK Germany: 3
(1R,2R)-(-)-2-Amino-1-phenyl-1,3-propanediol Specification
Ingestion:
Get medical aid. Wash mouth out with water.
Inhalation:
Remove from exposure to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of soap and water for at least 15 minutes while removing contaminated clothing and shoes.
Eyes:
Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
2. Handling and Storage
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Handling:
Avoid breathing dust, vapor, mist, or gas. Avoid contact with skin and eyes.
Related Products
- (1R)-(-)-(10-Camphorsulfonyl)oxaziridine
- (1R)-(-)-Menthyl acetate
- (1R)-(-)-Menthyl chloroformate
- (1R)-(-)-Menthyl glyoxylate hydrate
- (1R)-(-)-Thiocamphor
- (1R)-(+)-alpha-Pinene
- (1R)-1-(3-fluorophenyl)-2-methylpropylamine
- (1R)-1-[4-(benzyloxy)-3-nitrophenyl]-2-{[(2R)-1-(4-methoxyphenyl)propan-2-yl]amino}ethanol
- (1R)-3-[3-amino-4-(benzyloxy)phenyl]-2-{[(2R)-1-(4-methoxyphenyl)propan-2-yl]amino}ethan-1-ol
- (1R)-3-Chloro-1-phenyl-propan-1-ol
- 460331-53-7
- 460-34-4
- 460345-16-8
- 460353-65-5
- 460-35-5
- 460-37-7
- 46038-83-9
- 460-39-9
- 460-40-2
- 460-43-5
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View