-
Name
Estra-1,3,5(10)-triene-3,17-diol,7-[9-[(4,4,5,5,5-pentafluoropentyl)thio]nonyl]-,17-acetate,(7a,17b)-
- EINECS 1592732-453-0
- CAS No. 875573-69-6
- Article Data4
- CAS DataBase
- Density 1.19 g/cm3
- Solubility
- Melting Point
- Formula C34H49F5O3S
- Boiling Point 631.4±55.0 °C(Predicted)
- Molecular Weight 632.819
- Flash Point
- Transport Information
- Appearance
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms (7α,17β)-7-[9-(4,4,5,5,5-pentafluoropentyl)thio]nonyl]estra-1,3,5(10)- triene-3-diol-17oxyethyl;
- PSA 71.83000
- LogP 10.23790
Synthetic route
-
-
875573-66-3
(+)-(7α)-[9-bromononyl]estra-1,3,5(10)-triene-3-ol-17β-yl acetate

-
-
875573-69-6
17-O-acetyl-S-deoxo-fulvestrant

| Conditions | Yield |
|---|---|
| Stage #1: (+)-(7α)-[9-bromononyl]estra-1,3,5(10)-triene-3-ol-17β-yl acetate; C6H9F5N2S*ClH With potassium hydroxide In N,N-dimethyl-formamide at 0 - 10℃; for 1.5h; Stage #2: With acetic acid In ethyl acetate; N,N-dimethyl-formamide for 0.166667h; | 85% |
-
-
252947-01-6
1-methanesulfonyloxy-4,4,5,5,5-pentafluoropentane

-
-
875573-69-6
17-O-acetyl-S-deoxo-fulvestrant

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile for 48h; Inert atmosphere; Reflux; | 80% |
-
-
875573-66-3
(+)-(7α)-[9-bromononyl]estra-1,3,5(10)-triene-3-ol-17β-yl acetate

-
-
148757-88-4
4,4,5,5,5-pentafluoro-1-pentanethiol

-
-
875573-69-6
17-O-acetyl-S-deoxo-fulvestrant

| Conditions | Yield |
|---|---|
| With sodium hydroxide In N,N-dimethyl acetamide; water at 20℃; for 0.6h; Product distribution / selectivity; |
-
-
252947-01-6
1-methanesulfonyloxy-4,4,5,5,5-pentafluoropentane

-
-
875573-69-6
17-O-acetyl-S-deoxo-fulvestrant

| Conditions | Yield |
|---|---|
| With sodium hydroxide In N,N-dimethyl acetamide; water at 20℃; for 1h; Product distribution / selectivity; |

-
-
875573-69-6
17-O-acetyl-S-deoxo-fulvestrant

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: copper(I) bromide; lithium bromide / acetonitrile / 24 h / 20 °C 2: 2,2'-azobis(isobutyronitrile) / toluene / 1.5 h / Reflux 3: hydrazine hydrate / tetrahydrofuran / 4 h / 20 °C 4: potassium carbonate / acetonitrile / 48 h / Inert atmosphere; Reflux View Scheme |

-
-
875573-69-6
17-O-acetyl-S-deoxo-fulvestrant

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 2,2'-azobis(isobutyronitrile) / toluene / 1.5 h / Reflux 2: hydrazine hydrate / tetrahydrofuran / 4 h / 20 °C 3: potassium carbonate / acetonitrile / 48 h / Inert atmosphere; Reflux View Scheme |

-
-
875573-69-6
17-O-acetyl-S-deoxo-fulvestrant

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: hydrazine hydrate / tetrahydrofuran / 4 h / 20 °C 2: potassium carbonate / acetonitrile / 48 h / Inert atmosphere; Reflux View Scheme |
-
-
434-22-0
19-nortestosterone

-
-
875573-69-6
17-O-acetyl-S-deoxo-fulvestrant

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1.1: toluene-4-sulfonic acid / 1 h / Reflux 2.1: N-Bromosuccinimide / N,N-dimethyl-formamide; water / 1.25 h / 0 - 7 °C / Inert atmosphere 2.2: 2 h / 0 °C / Reflux; Inert atmosphere 3.1: copper(I) bromide / tetrahydrofuran / 0.75 h / -20 °C / Inert atmosphere 3.2: 0.5 h / -20 - 20 °C 4.1: copper(I) bromide; lithium bromide / acetonitrile / 24 h / 20 °C 5.1: 2,2'-azobis(isobutyronitrile) / toluene / 1.5 h / Reflux 6.1: hydrazine hydrate / tetrahydrofuran / 4 h / 20 °C 7.1: potassium carbonate / acetonitrile / 48 h / Inert atmosphere; Reflux View Scheme |
-
-
148043-73-6
4,4,5,5,5-pentafluorpentan-1-ol

-
-
875573-69-6
17-O-acetyl-S-deoxo-fulvestrant

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: triethylamine / acetonitrile / 4 h / 20 °C / Inert atmosphere 2: potassium carbonate / acetonitrile / 48 h / Inert atmosphere; Reflux View Scheme | |
| Multi-step reaction with 3 steps 1: triethylamine / dichloromethane / 2 h / 20 °C / Cooling with ice 2: ethanol / Reflux 3: sodium hydroxide / water; methanol / 24 h / Inert atmosphere; Reflux View Scheme | |
| Multi-step reaction with 3 steps 1.1: thionyl chloride; triethylamine / dichloromethane / 2 h / 0 - 20 °C 2.1: isopropyl alcohol / 80 - 85 °C 3.1: potassium hydroxide / N,N-dimethyl-formamide / 1.5 h / 0 - 10 °C 3.2: 0.17 h View Scheme |
-
-
4999-76-2
3,17β-diacetoxy-estra-3,5-diene

-
-
875573-69-6
17-O-acetyl-S-deoxo-fulvestrant

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1.1: N-Bromosuccinimide / N,N-dimethyl-formamide; water / 1.25 h / 0 - 7 °C / Inert atmosphere 1.2: 2 h / 0 °C / Reflux; Inert atmosphere 2.1: copper(I) bromide / tetrahydrofuran / 0.75 h / -20 °C / Inert atmosphere 2.2: 0.5 h / -20 - 20 °C 3.1: copper(I) bromide; lithium bromide / acetonitrile / 24 h / 20 °C 4.1: 2,2'-azobis(isobutyronitrile) / toluene / 1.5 h / Reflux 5.1: hydrazine hydrate / tetrahydrofuran / 4 h / 20 °C 6.1: potassium carbonate / acetonitrile / 48 h / Inert atmosphere; Reflux View Scheme |
-
-
2590-41-2
6-dehydro-19-nortestosterone acetate

-
-
875573-69-6
17-O-acetyl-S-deoxo-fulvestrant

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: copper(I) bromide / tetrahydrofuran / 0.75 h / -20 °C / Inert atmosphere 1.2: 0.5 h / -20 - 20 °C 2.1: copper(I) bromide; lithium bromide / acetonitrile / 24 h / 20 °C 3.1: 2,2'-azobis(isobutyronitrile) / toluene / 1.5 h / Reflux 4.1: hydrazine hydrate / tetrahydrofuran / 4 h / 20 °C 5.1: potassium carbonate / acetonitrile / 48 h / Inert atmosphere; Reflux View Scheme |

-
-
875573-69-6
17-O-acetyl-S-deoxo-fulvestrant

| Conditions | Yield |
|---|---|
| With sodium hydroxide In methanol; water for 24h; Inert atmosphere; Reflux; |
-
-
252947-01-6
1-methanesulfonyloxy-4,4,5,5,5-pentafluoropentane

-
-
875573-69-6
17-O-acetyl-S-deoxo-fulvestrant

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: ethanol / Reflux 2: sodium hydroxide / water; methanol / 24 h / Inert atmosphere; Reflux View Scheme |

-
-
875573-69-6
17-O-acetyl-S-deoxo-fulvestrant

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: isopropyl alcohol / 80 - 85 °C 2.1: potassium hydroxide / N,N-dimethyl-formamide / 1.5 h / 0 - 10 °C 2.2: 0.17 h View Scheme |
-
-
875573-69-6
17-O-acetyl-S-deoxo-fulvestrant

-
-
153004-31-0
(+)-(7α)-[9-(4,4,5,5,5-pentafluoropentylsulfanyl)nonyl]estra-1,3,5(10)-triene-3,17β-diol

| Conditions | Yield |
|---|---|
| Stage #1: 17-O-acetyl-S-deoxo-fulvestrant With potassium hydroxide In methanol at 40℃; for 2h; Stage #2: With acetic acid In methanol; ethyl acetate for 0.166667h; | 78.2% |
| With methanol; potassium hydroxide at 20℃; for 1 - 4h; Product distribution / selectivity; |
-
-
875573-69-6
17-O-acetyl-S-deoxo-fulvestrant

-
-
261506-24-5
ICI 182,780-17β-acetate

| Conditions | Yield |
|---|---|
| With sodium periodate In tetrahydrofuran; methanol; water at 5 - 20℃; |
-
-
875573-69-6
17-O-acetyl-S-deoxo-fulvestrant

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: pyridine / dichloromethane / -10 °C 2: potassium acetate; tricyclohexylphosphine; palladium diacetate / acetonitrile / 80 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 2 steps 1: pyridine / dichloromethane-d2 / -10 °C 2: palladium diacetate; tricyclohexylphosphine / acetonitrile / 80 °C View Scheme | |
| Multi-step reaction with 2 steps 1: pyridine / dichloromethane 2: palladium diacetate; tricyclohexylphosphine / acetonitrile / 80 °C View Scheme |
-
-
875573-69-6
17-O-acetyl-S-deoxo-fulvestrant

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: pyridine / dichloromethane / -10 °C 2: potassium acetate; tricyclohexylphosphine; palladium diacetate / acetonitrile / 80 °C / Inert atmosphere 3: potassium hydroxide / methanol; tetrahydrofuran / 4 h / 0 - 20 °C View Scheme | |
| Multi-step reaction with 3 steps 1: pyridine / dichloromethane-d2 / -10 °C 2: palladium diacetate; tricyclohexylphosphine / acetonitrile / 80 °C 3: potassium hydroxide; methanol / tetrahydrofuran / 4 h / 0 - 20 °C View Scheme |
-
-
875573-69-6
17-O-acetyl-S-deoxo-fulvestrant

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: pyridine / dichloromethane / -10 °C 2: potassium acetate; tricyclohexylphosphine; palladium diacetate / acetonitrile / 80 °C / Inert atmosphere 3: potassium hydroxide / methanol; tetrahydrofuran / 4 h / 0 - 20 °C 4: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 0 °C View Scheme |
-
-
875573-69-6
17-O-acetyl-S-deoxo-fulvestrant

-
-
358-23-6
trifluoromethylsulfonic anhydride

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane at -10℃; | 0.7 g |
| With pyridine In dichloromethane-d2 at -10℃; | |
| With pyridine In dichloromethane |
-
-
875573-69-6
17-O-acetyl-S-deoxo-fulvestrant

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: pyridine / dichloromethane-d2 / -10 °C 2: palladium diacetate; tricyclohexylphosphine / acetonitrile / 80 °C 3: potassium hydroxide; methanol / tetrahydrofuran / 4 h / 0 - 20 °C 4: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 0 °C View Scheme | |
| Multi-step reaction with 4 steps 1: pyridine / dichloromethane 2: palladium diacetate; tricyclohexylphosphine / acetonitrile / 80 °C 3: methanol; potassium hydroxide / tetrahydrofuran 4: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 0 °C View Scheme |
-
-
875573-69-6
17-O-acetyl-S-deoxo-fulvestrant

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: pyridine / dichloromethane 2: palladium diacetate; tricyclohexylphosphine / acetonitrile / 80 °C 3: methanol; potassium hydroxide / tetrahydrofuran View Scheme |
-
-
875573-69-6
17-O-acetyl-S-deoxo-fulvestrant

-
-
129453-61-8
fulvestrant

| Conditions | Yield |
|---|---|
| With sodium periodate In methanol; water at 20℃; for 24h; Cooling with ice; |
(7a,17b)-7-[9-[(4,4,5,5,5-Pentafluoropentyl)thio]nonyl]-estra-1,3,5(10)-triene-3,17-diol 17-acetate Chemical Properties
Following is the structure of (7alpha,17beta)-7-[9-(4,4,5,5,5-pentafluoropentyl)thio]nonyl]estra-1,3,5(10)- triene-3-diol-17oxyethyl (CAS NO.875573-69-6):
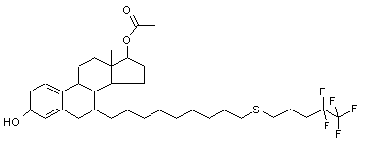
Empirical Formula: C34H49F5O3S
(7a,17b)-7-[9-[(4,4,5,5,5-Pentafluoropentyl)thio]nonyl]-estra-1,3,5(10)-triene-3,17-diol 17-acetate Specification
(7alpha,17beta)-7-[9-(4,4,5,5,5-pentafluoropentyl)thio]nonyl]estra-1,3,5(10)- triene-3-diol-17oxyethyl , its cas register number 875573-69-6. It also can be called (7α,17β)-7-[9-(4,4,5,5,5-pentafluoropentyl)thio]nonyl]estra-1,3,5(10)- triene-3-diol-17oxyethyl .
Related Products
- (7a,17a)-17-Hydroxy-3-oxo-pregna-4,9(11)-diene-7,21-dicarboxylicacid g-lactone methyl ester
- (7a,17b)-7-(9-Bromononyl)estra-1,3,5(10)-triene-3,17-diol
- (7a,17b)-7-(9-Bromononyl)-estra-1,3,5(10)-triene-3,17-diol 17-acetate
- (7a,17b)-7-[9-[(4,4,5,5,5-Pentafluoropentyl)thio]nonyl]-estra-1,3,5(10)-triene-3,17-diol
- (7a,17b)-7-[9-[(4,4,5,5,5-Pentafluoropentyl)thio]nonyl]-estra-1,3,5(10)-triene-3,17-diol 17-acetate
- (7alpha,14beta)-7-Methylestr-4-ene-3,17-dione
- 875576-30-0
- 87-55-8
- 87558-76-7
- 875-59-2
- 875-62-7
- 87562-76-3
- 875662-69-4
- 875664-27-0
- 875664-28-1
- 875664-30-5
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View