-
Name
(S)-2-(3-((2-Isopropylthiazol-4-yl)methyl)-3-methylureido)-3-methylbutanoic acid
- EINECS 680-588-9
- CAS No. 154212-61-0
- Article Data11
- CAS DataBase
- Density 1.193 g/cm3
- Solubility
- Melting Point
- Formula C14H23N3O3S
- Boiling Point 532.573 °C at 760 mmHg
- Molecular Weight 313.421
- Flash Point 275.89 °C
- Transport Information
- Appearance
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms N-[2-Isopropylthiazol-4-ylmethyl(methyl)carbamoyl]-L-valine;
- PSA 110.77000
- LogP 2.90800
Synthetic route
-
-
154248-99-4
N-((N-methyl-N-((2-isopropyl-4-thiazolyl)methyl)amino)carbonyl)-L-valine methyl ester

-
-
154212-61-0
N-((N-Methyl-N-((2-isopropyl-4-thiazolyl)methyl)amino)-carbonyl)-L-valine

| Conditions | Yield |
|---|---|
| Stage #1: N-((N-methyl-N-((2-isopropyl-4-thiazolyl)methyl)amino)carbonyl)-L-valine methyl ester With lithium hydroxide In tetrahydrofuran; water at 20℃; for 1.5h; Stage #2: With hydrogenchloride; water In tetrahydrofuran | 93% |
| With lithium hydroxide In 1,4-dioxane for 0.5h; Ambient temperature; | |
| With lithium hydroxide; water In 1,4-dioxane at 25℃; for 1h; |
-
-
126147-70-4
(2S)-3-Methyl-2-(phenoxycarbonyl)aminobutyric acid

-
-
154212-60-9
N-methyl-N-<(2-isopropyl-4-thiazolyl)methyl>amine

-
-
154212-61-0
N-((N-Methyl-N-((2-isopropyl-4-thiazolyl)methyl)amino)-carbonyl)-L-valine

| Conditions | Yield |
|---|---|
| With NaH In tetrahydrofuran; water; toluene | 83% |
| With NaH In tetrahydrofuran; methanol; isopropyl alcohol | 24% |
| With NaH In tetrahydrofuran | |
| In tetrahydrofuran; water; acetonitrile |

-
-
126147-70-4
(2S)-3-Methyl-2-(phenoxycarbonyl)aminobutyric acid

-
-
154212-60-9
N-methyl-N-<(2-isopropyl-4-thiazolyl)methyl>amine

-
-
108-95-2
phenol

-
-
154212-61-0
N-((N-Methyl-N-((2-isopropyl-4-thiazolyl)methyl)amino)-carbonyl)-L-valine

| Conditions | Yield |
|---|---|
| With hydrogenchloride In tetrahydrofuran; water; toluene | 82% |

-
-
78221-33-7
(S)-3-methyl-2-[(2,2,2-trichloroethoxy)carbonylamino]butyric acid

-
-
154212-60-9
N-methyl-N-<(2-isopropyl-4-thiazolyl)methyl>amine

-
-
154212-61-0
N-((N-Methyl-N-((2-isopropyl-4-thiazolyl)methyl)amino)-carbonyl)-L-valine

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran at 50 - 60℃; for 6h; Green chemistry; | 81% |
-
-
126147-70-4
(2S)-3-Methyl-2-(phenoxycarbonyl)aminobutyric acid

-
-
154212-60-9
N-methyl-N-<(2-isopropyl-4-thiazolyl)methyl>amine

-
-
108-95-2
phenol

-
-
154212-61-0
N-((N-Methyl-N-((2-isopropyl-4-thiazolyl)methyl)amino)-carbonyl)-L-valine

| Conditions | Yield |
|---|---|
| With sodium hydroxide In tetrahydrofuran; n-heptane; water; toluene | 78% |

-
-
6306-52-1
L-valine methylester hydrochloride

-
-
154212-61-0
N-((N-Methyl-N-((2-isopropyl-4-thiazolyl)methyl)amino)-carbonyl)-L-valine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 4-methylmorpholine / CH2Cl2 / Ambient temperature 2: DMAP, Et3N / tetrahydrofuran / 2 h / Heating 3: 0.50 M aq. LiOH / dioxane / 0.5 h / Ambient temperature View Scheme |
-
-
154212-60-9
N-methyl-N-<(2-isopropyl-4-thiazolyl)methyl>amine

-
-
154212-61-0
N-((N-Methyl-N-((2-isopropyl-4-thiazolyl)methyl)amino)-carbonyl)-L-valine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: DMAP, Et3N / tetrahydrofuran / 2 h / Heating 2: 0.50 M aq. LiOH / dioxane / 0.5 h / Ambient temperature View Scheme | |
| Multi-step reaction with 2 steps 1: N-ethyl-N,N-diisopropylamine; 1,1'-carbonyldiimidazole 2: sodium hydroxide; methanol View Scheme | |
| Multi-step reaction with 2 steps 1: N-ethyl-N,N-diisopropylamine; 1,1'-carbonyldiimidazole 2: sodium hydroxide / methanol View Scheme |
-
-
162537-10-2
N-<<(4-nitrophenyl)oxy>carbonyl>-L-valine methyl ester

-
-
154212-61-0
N-((N-Methyl-N-((2-isopropyl-4-thiazolyl)methyl)amino)-carbonyl)-L-valine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: DMAP, Et3N / tetrahydrofuran / 2 h / Heating 2: 0.50 M aq. LiOH / dioxane / 0.5 h / Ambient temperature View Scheme |

-
-
154212-61-0
N-((N-Methyl-N-((2-isopropyl-4-thiazolyl)methyl)amino)-carbonyl)-L-valine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: H2O / 1 h 2: DMAP, Et3N / tetrahydrofuran / 2 h / Heating 3: 0.50 M aq. LiOH / dioxane / 0.5 h / Ambient temperature View Scheme |
-
-
1634-04-4
tert-butyl methyl ether

-
-
126147-70-4
(2S)-3-Methyl-2-(phenoxycarbonyl)aminobutyric acid

-
-
154212-60-9
N-methyl-N-<(2-isopropyl-4-thiazolyl)methyl>amine

-
-
154212-61-0
N-((N-Methyl-N-((2-isopropyl-4-thiazolyl)methyl)amino)-carbonyl)-L-valine

| Conditions | Yield |
|---|---|
| With hydrogenchloride; NaH In tetrahydrofuran; water; toluene |


-
-
126147-70-4
(2S)-3-Methyl-2-(phenoxycarbonyl)aminobutyric acid

-
-
154212-60-9
N-methyl-N-<(2-isopropyl-4-thiazolyl)methyl>amine

-
-
154212-61-0
N-((N-Methyl-N-((2-isopropyl-4-thiazolyl)methyl)amino)-carbonyl)-L-valine

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; n-heptane; water; toluene | 16.6 kg (83.8%) |


-
-
154248-99-4
N-((N-methyl-N-((2-isopropyl-4-thiazolyl)methyl)amino)carbonyl)-L-valine methyl ester

-
-
154212-61-0
N-((N-Methyl-N-((2-isopropyl-4-thiazolyl)methyl)amino)-carbonyl)-L-valine

| Conditions | Yield |
|---|---|
| With hydrogenchloride In 1,4-dioxane; dichloromethane | 1.1 g (81%) |
-
-
6306-52-1
L-valine methylester hydrochloride

-
-
154212-61-0
N-((N-Methyl-N-((2-isopropyl-4-thiazolyl)methyl)amino)-carbonyl)-L-valine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: N-ethyl-N,N-diisopropylamine; 1,1'-carbonyldiimidazole 2: sodium hydroxide; methanol View Scheme |
-
-
154212-61-0
N-((N-Methyl-N-((2-isopropyl-4-thiazolyl)methyl)amino)-carbonyl)-L-valine

| Conditions | Yield |
|---|---|
| With thionyl chloride In dichloromethane; N,N-dimethyl-formamide at 0 - 10℃; for 1h; | 98.1% |
| With thionyl chloride; triethylamine In ethyl acetate at 45℃; for 3h; Solvent; Temperature; Reagent/catalyst; |
-
-
154212-61-0
N-((N-Methyl-N-((2-isopropyl-4-thiazolyl)methyl)amino)-carbonyl)-L-valine

-
-
144164-11-4
(2S,3S,5S)-5-Amino-2-(N-((5-thiazolyl)-methoxycarbonyl)amino)-3-hydroxy-1,6-diphenylhexane

-
-
155213-67-5
ritonavir

| Conditions | Yield |
|---|---|
| Stage #1: N-((N-Methyl-N-((2-isopropyl-4-thiazolyl)methyl)amino)-carbonyl)-L-valine With N-ethyl-N,N-diisopropylamine; diisopropyl-carbodiimide at 27℃; for 0.5h; Stage #2: (2S,3S,5S)-5-Amino-2-(N-((5-thiazolyl)-methoxycarbonyl)amino)-3-hydroxy-1,6-diphenylhexane for 7h; Temperature; | 91.5% |
| With diethylamine; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate In butanone at 41℃; for 0.666667h; Concentration; Temperature; | 87.4% |
| With N-ethyl-N'-(3-diethylaminopropyl)-carbodiimide; 1-hydroxybenzotriazol-hydrate In tetrahydrofuran for 16h; Ambient temperature; Yield given; | |
| Stage #1: N-((N-Methyl-N-((2-isopropyl-4-thiazolyl)methyl)amino)-carbonyl)-L-valine With pivaloyl chloride; triethylamine In dichloromethane at 0 - 10℃; Inert atmosphere; Large scale; Stage #2: With dmap In dichloromethane at 0 - 10℃; for 0.5h; Large scale; Stage #3: (2S,3S,5S)-5-Amino-2-(N-((5-thiazolyl)-methoxycarbonyl)amino)-3-hydroxy-1,6-diphenylhexane In dichloromethane at 25 - 35℃; Large scale; |
-
-
1004316-72-6
C19H27N3O2S

-
-
154212-61-0
N-((N-Methyl-N-((2-isopropyl-4-thiazolyl)methyl)amino)-carbonyl)-L-valine

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine In tetrahydrofuran | 87% |
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine |
-
-
144163-88-2
((1S,2S,4S)-4-amino-1-benzyl-2-hydroxy-5-phenyl-pentyl)-carbamic acid tert-butyl ester

-
-
154212-61-0
N-((N-Methyl-N-((2-isopropyl-4-thiazolyl)methyl)amino)-carbonyl)-L-valine

| Conditions | Yield |
|---|---|
| Stage #1: N-((N-Methyl-N-((2-isopropyl-4-thiazolyl)methyl)amino)-carbonyl)-L-valine With triethylamine; p-toluenesulfonyl chloride In dichloromethane at 0 - 10℃; for 3h; Green chemistry; Stage #2: ((1S,2S,4S)-4-amino-1-benzyl-2-hydroxy-5-phenyl-pentyl)-carbamic acid tert-butyl ester With triethylamine In dichloromethane at 0 - 20℃; for 6h; Green chemistry; | 86% |
-
-
1004318-14-2
C24H29N3O2S

-
-
154212-61-0
N-((N-Methyl-N-((2-isopropyl-4-thiazolyl)methyl)amino)-carbonyl)-L-valine

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine In tetrahydrofuran at 20℃; | 71% |
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine |
-
-
890904-63-9
N-((2R,3S)-3-amino-2-hydroxy-4-phenylbutyl)-4-(E-hydroxyiminomethyl)-N-(isobutyl)benzenesulfonamide

-
-
154212-61-0
N-((N-Methyl-N-((2-isopropyl-4-thiazolyl)methyl)amino)-carbonyl)-L-valine

| Conditions | Yield |
|---|---|
| With 4-methyl-morpholine In DMF (N,N-dimethyl-formamide); dichloromethane at 25℃; for 16h; | 53% |
-
-
154212-61-0
N-((N-Methyl-N-((2-isopropyl-4-thiazolyl)methyl)amino)-carbonyl)-L-valine

-
-
57260-71-6
1-t-Butoxycarbonylpiperazine

| Conditions | Yield |
|---|---|
| Stage #1: ((5-thiazolyl)methyl)-(4-nitrophenyl)carbonate; 1-t-Butoxycarbonylpiperazine With N-ethyl-N,N-diisopropylamine In acetonitrile at 25℃; for 12h; Stage #2: With hydrogenchloride; water In 1,4-dioxane; ethyl acetate at 25℃; for 12h; Stage #3: N-((N-Methyl-N-((2-isopropyl-4-thiazolyl)methyl)amino)-carbonyl)-L-valine With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine In tetrahydrofuran at 25℃; for 12h; | 45% |
-
-
1004317-04-7
C7H11N3O2S*ClH

-
-
154212-61-0
N-((N-Methyl-N-((2-isopropyl-4-thiazolyl)methyl)amino)-carbonyl)-L-valine

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine In tetrahydrofuran at 25℃; for 12h; | 38% |
-
-
73874-95-0
(piperidin-4-yl)carbamic acid tert-butyl ester

-
-
154212-61-0
N-((N-Methyl-N-((2-isopropyl-4-thiazolyl)methyl)amino)-carbonyl)-L-valine

| Conditions | Yield |
|---|---|
| Stage #1: (piperidin-4-yl)carbamic acid tert-butyl ester; ((5-thiazolyl)methyl)-(4-nitrophenyl)carbonate With N-ethyl-N,N-diisopropylamine In acetonitrile at 25℃; for 12h; Stage #2: With hydrogenchloride; water In 1,4-dioxane; ethyl acetate at 25℃; for 12h; Stage #3: N-((N-Methyl-N-((2-isopropyl-4-thiazolyl)methyl)amino)-carbonyl)-L-valine With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine In tetrahydrofuran at 25℃; for 12h; | 36% |
-
-
75178-96-0
N-Boc-1,3-diaminopropane

-
-
154212-61-0
N-((N-Methyl-N-((2-isopropyl-4-thiazolyl)methyl)amino)-carbonyl)-L-valine

| Conditions | Yield |
|---|---|
| Stage #1: N-Boc-1,3-diaminopropane; ((5-thiazolyl)methyl)-(4-nitrophenyl)carbonate With N-ethyl-N,N-diisopropylamine In acetonitrile at 25℃; for 12h; Stage #2: With hydrogenchloride; water In 1,4-dioxane; ethyl acetate at 25℃; for 12h; Stage #3: N-((N-Methyl-N-((2-isopropyl-4-thiazolyl)methyl)amino)-carbonyl)-L-valine With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine In tetrahydrofuran at 25℃; for 12h; | 34% |
-
-
154212-61-0
N-((N-Methyl-N-((2-isopropyl-4-thiazolyl)methyl)amino)-carbonyl)-L-valine

-
-
162849-95-8
(2S,3S,5S)-5-(t-butyloxycarbonylamino)-2-(N-((5-thiazolyl)methoxycarbonyl)amino)-3-hydroxy-1,6-diphenylhexane

-
-
566939-06-8
(2S,3S,5S)-3-[O-[N-[N-methyl-N-[(2-isopropyl-4-thiazolyl)methyl]amino]carbonyl]-L-valyloxy]-5-[N-[(tert-butyloxy)carbonyl]amino]-2-[N-[(5-thiazolyl)methoxycarbonyl]amino]-1,6-diphenyl-3-hydroxyhexane

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In chloroform at 20℃; | 27% |
-
-
1004316-71-5
C19H27N3O2S

-
-
154212-61-0
N-((N-Methyl-N-((2-isopropyl-4-thiazolyl)methyl)amino)-carbonyl)-L-valine

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine In tetrahydrofuran | 20% |
-
-
1004317-31-0
C13H23N3O2S

-
-
154212-61-0
N-((N-Methyl-N-((2-isopropyl-4-thiazolyl)methyl)amino)-carbonyl)-L-valine

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine In tetrahydrofuran at 20℃; | 2% |
-
-
154212-61-0
N-((N-Methyl-N-((2-isopropyl-4-thiazolyl)methyl)amino)-carbonyl)-L-valine

-
-
159572-17-5
(S)-4-((2R,3S)-3-Amino-2-hydroxy-4-phenyl-butyl)-3-tert-butylcarbamoyl-piperazine-1-carboxylic acid tert-butyl ester

-
-
251112-23-9
(S)-3-tert-Butylcarbamoyl-4-((2R,3S)-2-hydroxy-3-{(S)-2-[3-(2-isopropyl-thiazol-4-ylmethyl)-3-methyl-ureido]-3-methyl-butyrylamino}-4-phenyl-butyl)-piperazine-1-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With TEA; N-(3-dimethylaminopropyl)-N-ethylcarbodiimide |
-
-
1004317-30-9
C11H19N3O2S

-
-
154212-61-0
N-((N-Methyl-N-((2-isopropyl-4-thiazolyl)methyl)amino)-carbonyl)-L-valine

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine In tetrahydrofuran at 20℃; |
-
-
144186-34-5
(2R,5R)-1,6-diphenylhexane-2,5-diamine

-
-
154212-61-0
N-((N-Methyl-N-((2-isopropyl-4-thiazolyl)methyl)amino)-carbonyl)-L-valine

-
A
-
1004318-11-9
C32H45N5O2S

-
B
-
1004318-10-8
C46H66N8O4S2

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine In tetrahydrofuran for 12h; |


-
-
154212-61-0
N-((N-Methyl-N-((2-isopropyl-4-thiazolyl)methyl)amino)-carbonyl)-L-valine

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine In tetrahydrofuran at 20℃; | |
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine |
-
-
1004318-31-3
C9H15N3O2S

-
-
154212-61-0
N-((N-Methyl-N-((2-isopropyl-4-thiazolyl)methyl)amino)-carbonyl)-L-valine

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine In tetrahydrofuran at 20℃; | |
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine |
-
-
1004318-32-4
C23H27N3O2S

-
-
154212-61-0
N-((N-Methyl-N-((2-isopropyl-4-thiazolyl)methyl)amino)-carbonyl)-L-valine

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine In tetrahydrofuran at 20℃; | |
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine |
-
-
1004318-33-5
1-benzylamino-3-{benzyl[(1,3-thiazol-5-ylmethoxy)carbonyl]amino}propane

-
-
154212-61-0
N-((N-Methyl-N-((2-isopropyl-4-thiazolyl)methyl)amino)-carbonyl)-L-valine

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine In tetrahydrofuran at 20℃; |
-
-
154212-61-0
N-((N-Methyl-N-((2-isopropyl-4-thiazolyl)methyl)amino)-carbonyl)-L-valine

-
-
251112-24-0
N'-[(1S)-1-[[[(1S,2R)-3-[(2S)-2-[[(1,1-Dimethylethyl)amino]carbonyl]-1-piperazinyl]-2-hydroxy-1-(phenylmethyl)propyl]amino]carbonyl]-2-methylpropyl]-N-methyl-N-[2-(1-methylethyl)-4-thiazolylmethyl]urea

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: EDC, TEA 2: TFA View Scheme |
(S)-2-(3-((2-Isopropylthiazol-4-yl)methyl)-3-methylureido)-3-methylbutanoic acid Chemical Properties
Systematic Name: N-(Methyl{[2-(propan-2-yl)-1,3-thiazol-4-yl]methyl}carbamoyl)-D-valine
Synonyms of (S)-2-(3-((2-Isopropylthiazol-4-yl)methyl)-3-methylureido)-3-methylbutanoic acid (CAS NO.154212-61-0): N-[2-Isopropylthiazol-4-ylmethyl(methyl)carbamoyl]-L-valine
CAS NO: 154212-61-0
Molecular Formula: C14H23N3O3S
Molecular Weight: 313.42
Molecular Structure: 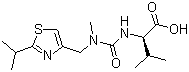
H bond acceptors: 6
H bond donors: 2
Freely Rotating Bonds: 6
Polar Surface Area: 110.77 Å2
Index of Refraction: 1.545
Molar Refractivity: 83.088 cm3
Molar Volume: 262.635 cm3
Surface Tension: 47.697 dyne/cm
Density: 1.193 g/cm3
Flash Point: 275.89 °C
Enthalpy of Vaporization: 85.085 kJ/mol
Boiling Point: 532.573 °C at 760 mmHg
Vapour Pressure: 0 mmHg at 25°C
SMILES: O=C(O)[C@H](NC(=O)N(Cc1nc(sc1)C(C)C)C)C(C)C
InChI: InChI=1/C14H23N3O3S/c1-8(2)11(13(18)19)16-14(20)17(5)6-10-7-21-12(15-10)9(3)4/h7-9,11H,6H2,1-5H3,(H,16,20)(H,18,19)/t11-/m1/s1
InChIKey: OSQWRZICKAOBFA-LLVKDONJBM
Std. InChI: InChI=1S/C14H23N3O3S/c1-8(2)11(13(18)19)16-14(20)17(5)6-10-7-21-12(15-10)9(3)4/h7-9,11H,6H2,1-5H3,(H,16,20)(H,18,19)/t11-/m1/s1
Std. InChIKey: OSQWRZICKAOBFA-LLVKDONJSA-N
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View