-
Name
(S)-Amlodipine
- EINECS 1592732-453-0
- CAS No. 103129-82-4
- Article Data25
- CAS DataBase
- Density 1.227 g/cm3
- Solubility
- Melting Point 102-104°C
- Formula C20H25ClN2O5
- Boiling Point 527.2 °C at 760 mmHg
- Molecular Weight 408.882
- Flash Point 272.6 °C
- Transport Information
- Appearance white crystal powder
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms 3,5-Pyridinedicarboxylicacid, 2-[(2-aminoethoxy)methyl]-4-(2-chlorophenyl)-1,4-dihydro-6-methyl-,3-ethyl 5-methyl ester, (S)-;(-)-Amlodipine;(S)-(-)-Amlodipine;Levamlodipine;Levoamlodipine;Lodien;l-Amlodipine;
- PSA 99.88000
- LogP 3.29540
Synthetic route

-
-
103129-82-4
amlodipine

| Conditions | Yield |
|---|---|
| With sodium hydroxide In dichloromethane; water at 20℃; for 0.666667h; | 93.9% |

-
-
103129-82-4
amlodipine

| Conditions | Yield |
|---|---|
| With sodium hydroxide In dichloromethane; water at 20℃; for 0.5h; | 93% |

-
-
103129-82-4
amlodipine

| Conditions | Yield |
|---|---|
| With ammonia; water In dichloromethane for 0.5h; Product distribution / selectivity; | 92% |
-
-
88150-42-9
2-[(2-aminoethoxy)methyl]-4-(2-chlorophenyl)-3-ethoxycarbonyl-5-methoxycarbonyl-6-methyl-1,4-dihydropyridine

-
-
103129-82-4
amlodipine

| Conditions | Yield |
|---|---|
| Stage #1: 2-[(2-aminoethoxy)methyl]-4-(2-chlorophenyl)-3-ethoxycarbonyl-5-methoxycarbonyl-6-methyl-1,4-dihydropyridine With D-(+)-camphoric acid In isopropyl alcohol at 20℃; for 12.5h; Stage #2: With sodium hydroxide In dichloromethane; water for 1h; Reagent/catalyst; | 90.5% |
| With D-tartaric acid In dimethyl sulfoxide; acetone at 20 - 60℃; for 1.5h; Reagent/catalyst; Solvent; | 45.2% |
| Stage #1: 2-[(2-aminoethoxy)methyl]-4-(2-chlorophenyl)-3-ethoxycarbonyl-5-methoxycarbonyl-6-methyl-1,4-dihydropyridine With L-Tartaric acid In ISOPROPYLAMIDE at 5℃; for 2h; Resolution of racemate; Stage #2: With sodium hydroxide In dichloromethane; water for 0.666667h; | n/a |
| Multi-step reaction with 2 steps 1: water; iso-butanol / 1 h / 50 °C 2: sodium hydroxide / dichloromethane; water / pH 9 View Scheme |

-
-
103129-82-4
amlodipine

| Conditions | Yield |
|---|---|
| With sodium hydroxide In dichloromethane; water pH=9; | 90% |
| With sodium hydroxide In dichloromethane; water pH=9; | 90% |

-
-
103129-82-4
amlodipine

| Conditions | Yield |
|---|---|
| With ammonia; water In dichloromethane for 0.5h; Product distribution / selectivity; | 82% |
-
-
147-71-7
D-tartaric acid

-
-
88150-42-9
2-[(2-aminoethoxy)methyl]-4-(2-chlorophenyl)-3-ethoxycarbonyl-5-methoxycarbonyl-6-methyl-1,4-dihydropyridine

-
-
68-12-2, 33513-42-7
N,N-dimethyl-formamide

-
B
-
103129-82-4
amlodipine

| Conditions | Yield |
|---|---|
| In water at 20℃; for 4.5h; Product distribution / selectivity; | A 78.38% B n/a |


-
-
103129-82-4
amlodipine

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In dichloromethane; water at 5℃; for 0.333333h; | 69% |
-
-
143191-87-1
(-)-2-<<2-<(tert-butoxycarbonyl)amino>ethoxy>-methyl>-4-(2-chlorophenyl)-3-(ethoxycarbonyl)-5-(methoxycarbonyl)-6-methyl-1,4-dihydropyridine

-
-
103129-82-4
amlodipine

| Conditions | Yield |
|---|---|
| With hydrogenchloride In 1,4-dioxane; acetonitrile for 1h; | 64% |
-
-
125711-60-6
2-<(2-azidoethoxy)methyl>-4(S)-(2-chlorophenyl)-3-ethoxycarbonyl-5-methoxycarbonyl-1,4-dihydropyridine

-
-
103129-82-4
amlodipine

| Conditions | Yield |
|---|---|
| With hydrogen; Lindlar's catalyst In ethanol under 775.7 Torr; Yield given; | |
| With sodium tetrahydroborate; nickel dichloride In ethanol Ambient temperature; Yield given; |

-
A
-
103129-81-3
(R)-2-[(2-aminoethoxy)methyl]4-(2-chlorophenyl)-1,4-dihydro-6-methyl-3,5-pyridinedicarboxylic acid 3-ethyl 5-methyl ester

-
B
-
103129-82-4
amlodipine

| Conditions | Yield |
|---|---|
| With sodium hydroxide; water In dichloromethane |

-
-
103129-82-4
amlodipine

| Conditions | Yield |
|---|---|
| With sodium hydroxide In tert-butyl methyl ether at 20 - 25℃; for 0.5h; |
-
-
103069-49-4
2-<(2-azidoethoxy)methyl>-4-(2-chlorophenyl)-5-methoxycarbonyl-6-methyl-1,4-dihydropyridine

-
-
103129-82-4
amlodipine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 1.) carbonyldiimidazole / 1.) CH2Cl2, reflux, 2.) THF, RT, 1 h 2: NaOEt / bis-(2-methoxy-ethyl) ether / 1 h / Heating 3: H2 / 5percent Pd/CaCO3 / ethanol / 775.7 Torr View Scheme | |
| Multi-step reaction with 3 steps 1: 1.) carbonyldiimidazole 2: ethanol / 1 h / Heating 3: 6percent nickel chloride, sodium borohydride / ethanol / Ambient temperature View Scheme |
-
-
103094-34-4
(+)-2-<(2-azidoethoxy)methyl>-4-(2-chlorophenyl)-5-(methoxycarbonyl)-3-<(2(S)-methoxy-2-phenethoxy)carbonyl>-6-methyl-1,4-dihydropyridine

-
-
103129-82-4
amlodipine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: NaOEt / bis-(2-methoxy-ethyl) ether / 1 h / Heating 2: H2 / 5percent Pd/CaCO3 / ethanol / 775.7 Torr View Scheme | |
| Multi-step reaction with 2 steps 1: ethanol / 1 h / Heating 2: 6percent nickel chloride, sodium borohydride / ethanol / Ambient temperature View Scheme |
-
-
103069-47-2
2-cyanoethyl 4-(2-azidoethoxy)-3-oxobutanoate

-
-
103129-82-4
amlodipine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 41 percent / ethanol / 2 h / Heating 2: 74 percent / aq. NaOH / bis-(2-methoxy-ethyl) ether / 2 h / Ambient temperature 3: 1.) carbonyldiimidazole / 1.) CH2Cl2, reflux, 2.) THF, RT, 1 h 4: NaOEt / bis-(2-methoxy-ethyl) ether / 1 h / Heating 5: H2 / 5percent Pd/CaCO3 / ethanol / 775.7 Torr View Scheme |
-
-
103069-48-3
2-<(2-azidoethoxy)methyl>-4-(2-chlorophenyl)-3-(2-cyanoethoxycarbonyl)-5-methoxycarbonyl-6-methyl-1,4-dihydropyridine

-
-
103129-82-4
amlodipine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 74 percent / aq. NaOH / bis-(2-methoxy-ethyl) ether / 2 h / Ambient temperature 2: 1.) carbonyldiimidazole / 1.) CH2Cl2, reflux, 2.) THF, RT, 1 h 3: NaOEt / bis-(2-methoxy-ethyl) ether / 1 h / Heating 4: H2 / 5percent Pd/CaCO3 / ethanol / 775.7 Torr View Scheme |
-
-
143290-24-8
2-<<2-<(tert-butoxycarbonyl)amino>ethoxy>-methyl>-4-(2-chlorophenyl)-3-(ethoxycarbonyl)-5-(methoxycarbonyl)-6-methyl-1,4-dihydropyridine

-
-
103129-82-4
amlodipine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 2: 64 percent / 6.7 M HCl / acetonitrile; dioxane / 1 h View Scheme |

-
-
103129-82-4
amlodipine

| Conditions | Yield |
|---|---|
| With sodium hydroxide In tert-butyl methyl ether; water; isopropyl alcohol | |
| With sodium hydroxide In tert-butyl methyl ether; water at 20 - 25℃; for 0.333333 - 0.5h; | |
| With sodium hydroxide In n-heptane; tert-butyl methyl ether; water at 20 - 40℃; for 2.5 - 3.5h; |

-
-
88150-42-9
2-[(2-aminoethoxy)methyl]-4-(2-chlorophenyl)-3-ethoxycarbonyl-5-methoxycarbonyl-6-methyl-1,4-dihydropyridine

-
-
103129-82-4
amlodipine

| Conditions | Yield |
|---|---|
| Stage #1: L-(+)-tartaric acid; 2-[(2-aminoethoxy)methyl]-4-(2-chlorophenyl)-3-ethoxycarbonyl-5-methoxycarbonyl-6-methyl-1,4-dihydropyridine With sodium hydroxide In water; butanone at 20℃; for 1h; Stage #2: With sodium hydroxide In dichloromethane; water for 0.5h; | n/a |
| Stage #1: L-(+)-tartaric acid; 2-[(2-aminoethoxy)methyl]-4-(2-chlorophenyl)-3-ethoxycarbonyl-5-methoxycarbonyl-6-methyl-1,4-dihydropyridine With sodium hydroxide In dichloromethane; butanone at 20℃; for 1h; Stage #2: With sodium hydroxide In dichloromethane; water for 0.5h; | n/a |
| Stage #1: L-(+)-tartaric acid; 2-[(2-aminoethoxy)methyl]-4-(2-chlorophenyl)-3-ethoxycarbonyl-5-methoxycarbonyl-6-methyl-1,4-dihydropyridine With sodium hydroxide In ethyl acetate; butanone at 20℃; for 1h; Stage #2: With sodium hydroxide In dichloromethane; water for 0.5h; | n/a |
| Stage #1: L-(+)-tartaric acid; 2-[(2-aminoethoxy)methyl]-4-(2-chlorophenyl)-3-ethoxycarbonyl-5-methoxycarbonyl-6-methyl-1,4-dihydropyridine With sodium hydroxide In ethanol; butanone at 20℃; for 1h; Stage #2: With sodium hydroxide In dichloromethane; water for 0.5h; | n/a |
| Stage #1: L-(+)-tartaric acid; 2-[(2-aminoethoxy)methyl]-4-(2-chlorophenyl)-3-ethoxycarbonyl-5-methoxycarbonyl-6-methyl-1,4-dihydropyridine With sodium hydroxide In acetone; butanone at 20℃; for 1h; Stage #2: With sodium hydroxide In dichloromethane; water for 0.5h; | n/a |

-
-
103129-82-4
amlodipine

| Conditions | Yield |
|---|---|
| With sodium hydroxide In dichloromethane; water for 0.5h; | n/a |
-
-
88150-42-9
2-[(2-aminoethoxy)methyl]-4-(2-chlorophenyl)-3-ethoxycarbonyl-5-methoxycarbonyl-6-methyl-1,4-dihydropyridine

-
A
-
103129-81-3
(R)-2-[(2-aminoethoxy)methyl]4-(2-chlorophenyl)-1,4-dihydro-6-methyl-3,5-pyridinedicarboxylic acid 3-ethyl 5-methyl ester

-
B
-
103129-82-4
amlodipine

| Conditions | Yield |
|---|---|
| With chiral stationary phase including isopropyl-functionalized CF6 In methanol; acetic acid; triethylamine; acetonitrile at 0℃; Purification / work up; | |
| With isopropylamine; trifluoroacetic acid In methanol; isopropyl alcohol at 35℃; under 103432 Torr; Resolution of racemate; Supercritical conditions; | |
| With acetic acid; triethylamine In methanol at 5℃; Reagent/catalyst; Concentration; Temperature; Resolution of racemate; | |
| With L-glutamate based immobilized chiral ionic liquid on SBA-15 In methanol Resolution of racemate; |
-
-
103129-81-3
(R)-2-[(2-aminoethoxy)methyl]4-(2-chlorophenyl)-1,4-dihydro-6-methyl-3,5-pyridinedicarboxylic acid 3-ethyl 5-methyl ester

-
-
103129-82-4
amlodipine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: calcium hypochlorite / ethyl acetate; water / 20 - 30 °C 2: acetic acid; sodium cyanoborohydride / 2 h / 15 - 25 °C 3: D-tartaric acid / dimethyl sulfoxide; acetone / 1.5 h / 20 - 60 °C View Scheme |

| Conditions | Yield |
|---|---|
| In tert-butyl methyl ether; water; isopropyl alcohol at 0 - 50℃; for 4.5 - 5h; | 98.1% |
| In tert-butyl methyl ether; water; isopropyl alcohol at 50℃; for 1h; Product distribution / selectivity; | 98.1% |
| In tert-butyl methyl ether; water; isopropyl alcohol at 0 - 50℃; for 4h; Product distribution / selectivity; | 98.1% |

| Conditions | Yield |
|---|---|
| In ethanol at 20℃; for 1h; | 97% |

| Conditions | Yield |
|---|---|
| In ethanol at 20℃; for 1h; | 96% |
-
-
681-57-2
2,2-dimethylglutaric acid

-
-
103129-82-4
amlodipine

| Conditions | Yield |
|---|---|
| In ethanol at 20℃; for 1h; | 96% |

| Conditions | Yield |
|---|---|
| In ethanol; water | 93% |

| Conditions | Yield |
|---|---|
| In ethanol at 25℃; for 2h; Product distribution / selectivity; | 91% |
| In water at 25℃; for 2h; Product distribution / selectivity; | 89% |
| In ethanol; water | 82.88% |

| Conditions | Yield |
|---|---|
| In ethanol; isopropyl alcohol at 25℃; for 2h; Product distribution / selectivity; | 91% |
| In ethanol; isopropyl alcohol at 25℃; for 2h; Product distribution / selectivity; | 91% |
| In water for 5h; Product distribution / selectivity; | 89% |

| Conditions | Yield |
|---|---|
| In ethanol; water | 89.2% |

| Conditions | Yield |
|---|---|
| In water; isopropyl alcohol at 20℃; for 0.5h; Product distribution / selectivity; | 89% |
| In ethanol; water at 0 - 20℃; | 85% |
| In ethanol; water Product distribution / selectivity; | 82.88% |
| In ethanol; water at 20℃; for 24h; | 80.2% |
-
-
3144-16-9
(1S)-10-camphorsulfonic acid

-
-
103129-82-4
amlodipine

| Conditions | Yield |
|---|---|
| With water In isopropyl alcohol at 20℃; for 1h; | 88% |
-
-
3144-16-9
(1S)-10-camphorsulfonic acid

-
-
103129-82-4
amlodipine

| Conditions | Yield |
|---|---|
| In water; isopropyl alcohol at 40℃; Product distribution / selectivity; | 88% |
| In tert-butyl methyl ether; isopropyl alcohol at 25℃; for 2h; Product distribution / selectivity; | 84% |
| In water at 25℃; for 2h; Product distribution / selectivity; | 83% |
| In hexane; tert-butyl methyl ether; isopropyl alcohol at 20℃; for 2h; | 81.5% |
-
-
3144-16-9
(1S)-10-camphorsulfonic acid

-
-
103129-82-4
amlodipine

| Conditions | Yield |
|---|---|
| In water; isopropyl alcohol at 40℃; | 88% |
-
-
76-26-6, 3144-16-9, 5872-08-2, 35963-20-3
camphor-10-sulfonic acid

-
-
103129-82-4
amlodipine

| Conditions | Yield |
|---|---|
| With water In isopropyl alcohol at 15 - 20℃; for 5h; | 87.4% |

| Conditions | Yield |
|---|---|
| In methanol at 25℃; for 2h; Product distribution / selectivity; | 85% |
| In water at 20 - 25℃; for 2h; Product distribution / selectivity; | 85% |
-
-
35963-20-3
(R)-10-camphorsulfonic acid

-
-
103129-82-4
amlodipine

| Conditions | Yield |
|---|---|
| In tert-butyl methyl ether; isopropyl alcohol at 25℃; for 2h; Product distribution / selectivity; | 84% |
| In water at 25℃; for 2h; Product distribution / selectivity; | 83% |
| In water; isopropyl alcohol at 15 - 20℃; | 62.3% |
-
-
18069-17-5
2-methylglutaric acid

-
-
103129-82-4
amlodipine

| Conditions | Yield |
|---|---|
| In ethanol at 20℃; for 1h; | 84% |

| Conditions | Yield |
|---|---|
| In ethanol; water | 82.88% |

| Conditions | Yield |
|---|---|
| In Petroleum ether for 5h; Heating / reflux; | 79.3% |
-
-
125995-03-1
atorvastatin lactone

-
-
103129-82-4
amlodipine

| Conditions | Yield |
|---|---|
| In ethanol for 18h; Heating / reflux; | 64% |
-
-
39637-74-6
(1S)-(-)-camphanic chloride

-
-
103129-82-4
amlodipine

-
-
143191-86-0
(-)-2-<<2-(camphanoylamino)ethoxy>methyl>-4-(2-chlorophenyl)-3-(ethoxycarbonyl)-5-(methoxycarbonyl)-6-methyl-1,4-dihydropyridine

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane for 2h; Ambient temperature; Yields of byproduct given; |
(S)-Amlodipine Chemical Properties
Product Name: Levamlodipine besylate (CAS NO.103129-82-4)
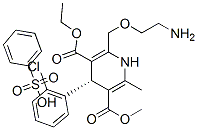
Molecular Formula: C20H25N2O5Cl
Molecular Weight: 408.88g/mol
Index of Refraction: 1.546
Molar Refractivity: 105.419 cm3
Molar Volume: 333.02 cm3
Surface Tension: 44.445 dyne/cm
Density: 1.228 g/cm3
Flash Point: 272.617 °C
Enthalpy of Vaporization: 80.168 kJ/mol
Product Categories: Pharmaceutical material and intermeidates
(S)-Amlodipine Uses
Levamlodipine besylate (CAS NO.103129-82-4) is mainly used as anti-cardiovascular drugs.
(S)-Amlodipine Analytical Methods
(S)-Amlodipine Specification
Levamlodipine besylate (CAS NO.103129-82-4) is also named as (S)-Amlodipine;3,5-Pyridinedicarboxylic acid, 2-[(2-aminoethoxy)methyl]-4-(2-chlorophenyl)-1,4-dihydro-6-methyl-, 3-ethyl 5-methyl ester, (4S)- ; 3,5-Pyridinedicarboxylic acid, 2-[(2-aminoethoxy)methyl]-4-(2-chlorophenyl)-1,4-dihydro-6-methyl-, 3-ethyl 5-methyl ester, (S)- and so on.
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View