-
Name
1,2-Dimethyl-5-nitroimidazole
- EINECS 209-001-2
- CAS No. 551-92-8
- Article Data14
- CAS DataBase
- Density 1.36 g/cm3
- Solubility 9.69g/L(20 oC)
- Melting Point 177-182 °C
- Formula C5H7N3O2
- Boiling Point 313.7 °C at 760 mmHg
- Molecular Weight 141.129
- Flash Point 143.5 °C
- Transport Information
- Appearance solid
- Safety 26-36-36/37-22
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Imidazole,1,2-dimethyl-5-nitro- (7CI,8CI);5-Nitro-1,2-dimethylimidazole;Dimetridazol;Dimetridazole;Dimetrizadole;Dimetronidazole;Emtryl;Emtrylvet;Emtrymix;NSC 226253;
- PSA 63.64000
- LogP 1.15990
Synthetic route

| Conditions | Yield |
|---|---|
| With formic acid for 4h; Reflux; | 87% |
-
-
117836-28-9
2-bromomethyl-1-methyl-5-nitroimidazole

-
-
551-92-8
dimetridazole

| Conditions | Yield |
|---|---|
| With tri-n-butyl-tin hydride; 2,2'-azobis(isobutyronitrile) In toluene for 5h; Mechanism; Irradiation; | 63% |

| Conditions | Yield |
|---|---|
| With sodium perborate; acetic acid for 14h; Ambient temperature; | A 35% B 25% |
-
-
1739-84-0
1,2-dimethyl-1H-imidazole

-
A
-
13230-04-1
1,2-dimethyl-4-nitro-1H-imidazole

-
B
-
551-92-8
dimetridazole

| Conditions | Yield |
|---|---|
| With sulfuric acid; nitric acid anfangs bei 0grad, dann bei 100grad; |
-
-
696-23-1
2-methyl-5-nitro-1H-imidazole

-
-
77-78-1
dimethyl sulfate

-
A
-
13230-04-1
1,2-dimethyl-4-nitro-1H-imidazole

-
B
-
551-92-8
dimetridazole

-
-
696-23-1
2-methyl-4-nitro-1H-imidazole

-
-
77-78-1
dimethyl sulfate

-
A
-
13230-04-1
1,2-dimethyl-4-nitro-1H-imidazole

-
B
-
551-92-8
dimetridazole

-
-
175733-75-2
(1-Methyl-5-nitro-1H-imidazol-2-yl)-acetic acid

-
-
551-92-8
dimetridazole

| Conditions | Yield |
|---|---|
| at 110℃; |
-
-
1739-84-0
1,2-dimethyl-1H-imidazole

-
A
-
13230-04-1
1,2-dimethyl-4-nitro-1H-imidazole

-
B
-
551-92-8
dimetridazole

| Conditions | Yield |
|---|---|
| beim Nitrieren; |


| Conditions | Yield |
|---|---|
| With acetic acid; dimethyl sulfate In water |

| Conditions | Yield |
|---|---|
| In ethyl acetate for 2h; Menshutkin Reaction; Reflux; | 100% |
-
-
551-92-8
dimetridazole

-
-
13230-04-1
1,2-dimethyl-4-nitro-1H-imidazole

| Conditions | Yield |
|---|---|
| With methyl iodide In various solvent(s) at 156℃; for 48h; | 97% |
| With methyl iodide In various solvent(s) at 156℃; for 48h; Product distribution; Mechanism; variation of reagents, solvent, reaction time, and temperature;; | 97% |
-
-
551-92-8
dimetridazole

-
-
88-89-1
2,4,6-Trinitrophenol

-
-
24647-68-5
1,2-dimethyl-5-nitroimidazolium picrate

| Conditions | Yield |
|---|---|
| In ethanol; water at 40℃; for 10h; | 92% |
-
-
551-92-8
dimetridazole

-
-
98-88-4
benzoyl chloride

-
-
1448622-21-6
(Z)-2-(1-methyl-5-nitro-1H-imidazol-2-yl)-1-phenylvinylbenzoate

| Conditions | Yield |
|---|---|
| With triethylamine In acetone at 20℃; for 4h; Cooling with ice; | 92% |
-
-
551-92-8
dimetridazole

-
-
1188-33-6
N,N-dimethylformamide diethyl diacetal

-
-
57434-36-3
5-nitro-1-methyl-2-(2-dimethylaminovinyl)-imidazole

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid In N,N-dimethyl-formamide at 100℃; for 12h; Condensation; | 91% |
-
-
551-92-8
dimetridazole

-
-
87-13-8
diethyl 2-ethoxymethylenemalonate

-
-
145837-15-6
5-[2,2-bis(ethoxycarbonyl)-1-vinylamino]-1,2-dimethylimidazole

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal In 1,4-dioxane | 86% |
| Pd on carbon In 1,4-dioxane; hydrogenchloride; hydrogen | |
| With hydrogen; 5%-palladium/activated carbon In 1,4-dioxane under 1810.07 - 2068.65 Torr; for 29h; |
-
-
551-92-8
dimetridazole

-
-
195046-62-9
bis(1,2-dimethyl-5-nitro-imidazole)cobalt(II) chloride

| Conditions | Yield |
|---|---|
| In methanol stoichiometric ratio, refluxing (30 min); concn., crystn.; elem. anal.; | 85% |
-
-
551-92-8
dimetridazole

-
-
122-04-3
4-nitro-benzoyl chloride

-
-
92478-52-9
4-Nitro-benzoic acid (Z)-2-(1-methyl-5-nitro-1H-imidazol-2-yl)-1-(4-nitro-phenyl)-vinyl ester

| Conditions | Yield |
|---|---|
| With triethylamine In acetonitrile 1.) < 5 deg C, 2.) room temperature; | 84% |
-
-
551-92-8
dimetridazole

-
-
352464-95-0
1,2-dimethyl-5-nitroimidazolium nitrate

| Conditions | Yield |
|---|---|
| With nitric acid In ethanol; water at 40℃; for 10h; | 84% |
-
-
10025-99-7
potassium tetrachloroplatinate(II)

-
-
551-92-8
dimetridazole

-
-
97747-63-2, 89918-00-3, 97747-64-3
[(1,2-dimethyl-5-nitro-1H-imidazole)2PtCl2]

| Conditions | Yield |
|---|---|
| In water solid ligand added to aq. soln. of K2(PtCl4), stirred at 50 °C for 1 h; ppt. filtered, washed with EtOH/Et2O, Et2O, dried in vac.; elem. anal.; | 83% |
-
-
551-92-8
dimetridazole

-
-
98-88-4
benzoyl chloride

-
-
35636-14-7
1-benzoyloxy-2-(1-methyl-5-nitro-1H-imidazol-2-yl)-1-phenyl-ethene

| Conditions | Yield |
|---|---|
| With triethylamine In acetonitrile 1.) < 5 deg C, 2.) room temperature; | 80% |
| With TEA In acetone at 20℃; |
-
-
5271-67-0
2-Thiophenecarbonyl chloride

-
-
551-92-8
dimetridazole

-
-
92478-55-2
Thiophene-2-carboxylic acid (Z)-2-(1-methyl-5-nitro-1H-imidazol-2-yl)-1-thiophen-2-yl-vinyl ester

| Conditions | Yield |
|---|---|
| With triethylamine In acetonitrile 1.) < 5 deg C, 2.) room temperature; | 79% |
-
-
551-92-8
dimetridazole

| Conditions | Yield |
|---|---|
| With water-d2; sodium carbonate at 200℃; for 0.5h; Microwave irradiation; | 77% |
| With water-d2; aluminium; platinum on carbon at 200℃; for 1h; Microwave irradiation; | 57.2% |
| With deuteriated sodium hydroxide; 1,1,1-trideuteromethanol; water-d2 at 60℃; Rate constant; NaD2PO4-NaDCO3-K2CO3 buffer; half-live time; |
-
-
551-92-8
dimetridazole

-
-
13750-81-7
N-methyl-2-imidazolcarboxaldehyde

-
-
1161926-14-2
1-methyl-2-[(E)-2-(1-methyl-1H-imidazol-2-yl)vinyl]-5-nitro-1H-imidazole

| Conditions | Yield |
|---|---|
| With sodium ethanolate In ethanol at 65℃; Aldol condensation; | 77% |
-
-
551-92-8
dimetridazole

-
-
80912-09-0
5-amino-1,2-dimethyl-1H-imidazole

| Conditions | Yield |
|---|---|
| With palladium on carbon (10%) In 1,4-dioxane at 20℃; Cooling with ice; Inert atmosphere; | 76% |
| With hydrogen; palladium on activated charcoal In 1,4-dioxane under 760 Torr; | 74% |
| With hydrogen; 5%-palladium/activated carbon In 1,4-dioxane under 760 Torr; | 74% |
-
-
551-92-8
dimetridazole

-
-
7205-98-3
chloromethyl phenyl sulfone

-
-
116248-42-1
1,2-dimethyl-4-phenylsulfonylmethyl-5-nitroimidazole

| Conditions | Yield |
|---|---|
| With potassium hydroxide In dimethyl sulfoxide for 0.25h; | 73% |
| With potassium hydroxide In dimethyl sulfoxide at 20℃; for 0.166667h; | 28% |
| With potassium hydroxide In dimethyl sulfoxide | 28% |

| Conditions | Yield |
|---|---|
| In toluene at 20 - 25℃; for 48h; | 72% |
-
-
551-92-8
dimetridazole

-
-
1122-91-4
4-bromo-benzaldehyde

-
-
1161925-98-9
2-[(E)-2-(4-bromophenyl)vinyl]-1-methyl-5-nitro-1H-imidazole

| Conditions | Yield |
|---|---|
| With sodium ethanolate In ethanol at 65℃; Aldol condensation; | 72% |
-
-
551-92-8
dimetridazole

-
-
81172-89-6
terephthalaldehyde mono(diethylacetal)

-
-
223482-25-5
2-{(E)-2-[4-(diethoxymethyl)phenyl]vinyl}-1-methyl-5-nitro-1H-imidazole

| Conditions | Yield |
|---|---|
| With sodium In ethanol at 60 - 65℃; for 3h; | 70% |
| With sodium ethanolate In ethanol at 65℃; Aldol condensation; | 24% |
-
-
551-92-8
dimetridazole

-
-
87-13-8
diethyl 2-ethoxymethylenemalonate

-
A
-
145837-19-0
diethyl (5-amino-1,2-dimethylimidazol-4-yl)methylenemalonate

-
B
-
145837-15-6
5-[2,2-bis(ethoxycarbonyl)-1-vinylamino]-1,2-dimethylimidazole

-
C
-
145837-52-1
4,4'-bis(5-diethoxycarbonylethyleneamino-1,2-dimethylimidazole)

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal In ethanol | A 5% B 65% C 1% |
| With hydrogen; palladium on activated charcoal In ethanol | A 5% B n/a C 1% |


| Conditions | Yield |
|---|---|
| In acetonitrile for 48h; Reflux; | 65% |
-
-
551-92-8
dimetridazole

-
-
54091-06-4
1-bromo-4-(chloromethylsulfonyl)benzene

| Conditions | Yield |
|---|---|
| With potassium hydroxide In dimethyl sulfoxide at 20℃; for 0.166667h; | 53% |
| With potassium hydroxide In dimethyl sulfoxide | 53% |
-
-
98-01-1
furfural

-
-
551-92-8
dimetridazole

-
-
1161926-15-3
2-[(E)-2-(2-furyl)vinyl]-1-methyl-5-nitro-1H-imidazole

| Conditions | Yield |
|---|---|
| With sodium ethanolate In ethanol at 65℃; Aldol condensation; | 53% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide In dimethyl sulfoxide at 20℃; for 0.166667h; | 49% |
| With potassium hydroxide In dimethyl sulfoxide | 49% |
-
-
108-86-1
bromobenzene

-
-
551-92-8
dimetridazole

-
-
1197836-47-7
1,2-dimethyl-5-nitro-4-phenyl-1H-imidazole

| Conditions | Yield |
|---|---|
| With palladium diacetate; potassium carbonate; tricyclohexylphosphine tetrafluoroborate In toluene at 120℃; for 18h; Inert atmosphere; regioselective reaction; | 44% |
-
-
551-92-8
dimetridazole

-
-
433-13-6
1-chloromethanesulfonyl-4-fluoro-benzene

-
-
1146349-23-6
4-(4-fluorobenzenesulfonylmethyl)-1,2-dimethyl-5-nitro-1H-imidazole

| Conditions | Yield |
|---|---|
| With potassium hydroxide In dimethyl sulfoxide at 20℃; for 0.25h; | 40% |

| Conditions | Yield |
|---|---|
| With selenium(IV) oxide at 140℃; for 0.0833333h; | 39% |
| With selenium(IV) oxide at 140℃; for 0.0833333h; | 39% |
| With selenium(IV) oxide at 140℃; for 0.0833333h; Inert atmosphere; | 22.1% |
| Multi-step reaction with 2 steps 1.1: 91 percent / trifluoroacetic acid / dimethylformamide / 12 h / 100 °C 2.1: ozone / CH2Cl2; methanol / 2 h / -78 °C 2.2: 51 percent / dimethyl sulfide / CH2Cl2; methanol / -78 - 20 °C View Scheme | |
| With selenium(IV) oxide at 140℃; for 0.0833333h; chemoselective reaction; |
1,2-Dimethyl-5-nitroimidazole Chemical Properties
Molar mass:141.12798 g/mol
Structure :
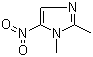
Synonyms of 1,2-Dimethyl-5-nitroimidazole(551-92-8):dimetridazole;Dimetridazol;1,2-dimethyl-5-nitro-1h-imidazole;1H-Imidazole, 1,2-dimethyl-5-nitro;Dimetridazolo;Dimetridazolum
Density:1.36 g/cm3
Flash Point:143.5 °C
Boiling Point:313.7 °C at 760 mmHg
Index of Refraction:1.598
Vapour Pressure:0.000901 mmHg at 25°C
Melting point:177-182°C
storage temp:2-8°C
Appearance:Light yellow needle-like crystalline powder
1,2-Dimethyl-5-nitroimidazole Uses
1,2-Dimethyl-5-nitroimidazole(551-92-8) is a livestock growth promoter and broad-spectrum antiprotozoal drug with efficient and low toxicity.
1,2-Dimethyl-5-nitroimidazole(551-92-8) can be used for pig feed, use 50-100g / t and also for chicken feed, use 125-200g / t.
1,2-Dimethyl-5-nitroimidazole Production
1. Synthesized with 2 - methyl-imidazole as the raw material, by nitration, esterification derived.
2. Using glyoxal as starting material,then cyclizes with acetaldehyde and ammonia . Then nitrification. The end, dimethyl sulfate methylation of imidazole derived Metronidazole.
1,2-Dimethyl-5-nitroimidazole Toxicity Data With Reference
| 1. | mmo-sat 25 µg/plate | MUREAV Mutation Research. 38 (1976),203. | ||
| 2. | bfa-rat/sat 800 mg/kg | MUREAV Mutation Research. 97 (1982),171. |
1,2-Dimethyl-5-nitroimidazole Consensus Reports
1,2-Dimethyl-5-nitroimidazole Safety Profile
Hazard Codes:Xi

Risk Statements:
36: Irritating to the eyes
37: Irritating to the respiratory system
38: Irritating to the skin
Safety Statements:
22: Do not breathe dust
26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
36: Wear suitable protective clothing
37: Wear suitable gloves
Related Products
- 10,10'-Bis([1,1'-biphenyl]-4-yl)-9,9'-bianthracene
- 10,10'-Dibromo-9,9'-bianthryl
- 10,10-Dimethylanthrone
- 10,10-Oxybisphenoxarsine
- 10-[1,1'-Biphenyl]-4-yl-2-(1-methylethyl)-9-oxo-9H-thioxanthenium hexafluorophosphate
- 10,11-Dehydroimipramine
- 10,11-Dihydro-11-oxodibenzo[b,f][1,4]thiazepine
- 10,11-Dihydro-2-(4-methyl-1-piperazinyl)-11-(2-athiazolyl)-pyridazino(3,4-b)(1,4)benzoxazepine
- 10,11-DIHYDRO-2-(4-METHYL-1-PIPERAZINYL)-11-(3,4-XYLYL)PYRIDAZINO(3,4b)(1,4)-BENZOXAZEPINE MALEATE
- 10,11-Dihydro-5-(3-dimethylamino-2-methylpropyl)-5h-dibenz (b,f)azepine
- 551-93-9
- 5519-50-6
- 55198-89-5
- 55199-59-2
- 55199-60-5
- 55199-72-9
- 55202-11-4
- 55203-24-2
- 55206-24-1
- 5520-66-1
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View