-
Name
N,N'-Diphenylurea
- EINECS 203-003-7
- CAS No. 102-07-8
- Article Data837
- CAS DataBase
- Density 1.25 g/cm3
- Solubility 150.3mg/L(temperature not stated)
- Melting Point 239-241 °C(lit.)
- Formula C13H12N2O
- Boiling Point 262 °C at 760 mmHg
- Molecular Weight 212.251
- Flash Point 91.147 °C
- Transport Information UN 2811
- Appearance solid
- Safety 22-24/25
- Risk Codes R22
-
Molecular Structure
- Hazard Symbols R22:Harmful if swallowed.;
- Synonyms Carbanilide(7CI,8CI);1,3-Diphenylcarbamide;AD 30;DPU;N,N'-Diphenylurea;N-Phenyl-N'-phenylurea;NSC 227401;NSC 8485;s-Diphenylurea;sym-Diphenylurea;
- PSA 41.13000
- LogP 3.47660
Synthetic route

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane | 100% |
| In acetic acid for 0.0833333h; | 98% |
| In hexane | 95% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide; copper(l) chloride In water; acetonitrile at 25℃; for 0.333333h; | 100% |
| With quinolinium monofluorochromate(VI) In acetonitrile for 3.5h; Heating; | 98% |
| With trans-3,5-dihydroperoxy-3,5-dimethyl-1,2-dioxolane; water; potassium hydroxide In acetonitrile at 20℃; for 0.0166667h; | 98% |
-
-
495-18-1
Benzohydroxamic acid

-
-
77219-88-6
bis(trimethylsilyl)benzohydroxamic acid

-
-
102-07-8
bis(diphenyl)urea

| Conditions | Yield |
|---|---|
| at 100℃; for 1.5h; | 100% |

| Conditions | Yield |
|---|---|
| Ambient temperature; prolonged holding in air; | 100% |
| With air (H2O) | 100% |
| With hydrogenchloride In dichloromethane at 0℃; for 0.5h; Yield given; | |
| With water |

| Conditions | Yield |
|---|---|
| With C24H28N8PrS4(1-)*Li(1+) In acetonitrile at 60℃; for 12h; Inert atmosphere; | 99% |
| With water; triethylamine In 1,4-dioxane at 20℃; for 0.05h; | 98% |
| at 20 - 50℃; Product distribution / selectivity; | 95% |

| Conditions | Yield |
|---|---|
| With pyridine at 120℃; under 3800.26 Torr; for 18h; | 99% |
| With pyridine; C14H34Cl2InNO2Si2 In toluene at 110℃; under 2280.15 Torr; for 24h; Catalytic behavior; Pressure; | 70% |
| dodecacarbonyl-triangulo-triruthenium at 150℃; under 10343 Torr; for 20h; | 48% |

| Conditions | Yield |
|---|---|
| With 1-butyl-3-methyl-1H-imidazole-2(3H)-selenone; oxygen at 90℃; under 9750.98 Torr; for 6h; Catalytic behavior; Reagent/catalyst; Temperature; Ionic liquid; Autoclave; | 99% |
| With palladium; oxygen; potassium iodide In 1,4-dioxane at 130℃; under 30003 Torr; for 24h; Autoclave; Green chemistry; | 99% |
| With potassium tetraiodopalladate(II) at 90℃; under 37503.8 Torr; for 24h; Neat (no solvent); Autoclave; | 96% |

| Conditions | Yield |
|---|---|
| Stage #1: Benzohydroxamic acid With 4-methyl-morpholine; 1,3,5-trichloro-2,4,6-triazine In dichloroethane at 0℃; for 1.5h; Lossen rearrangement; Inert atmosphere; Stage #2: aniline In dichloroethane at 0 - 84℃; for 15h; Lossen rearrangement; Inert atmosphere; | 99% |
| Stage #1: Benzohydroxamic acid With ethyl 2-cyano-2-(4-nitrophenylsulfonyloxyimino)acetate; N-ethyl-N,N-diisopropylamine In tetrahydrofuran at 0℃; for 1.5h; Lossen Rearrangement; Stage #2: aniline In tetrahydrofuran at 20℃; for 4h; Lossen Rearrangement; | 83% |
| Stage #1: Benzohydroxamic acid With ethyl 2-(tert-butoxycarbonyloxyimino)-2-cyanoacetate; N-ethyl-N,N-diisopropylamine In dichloromethane at 0℃; for 0.25h; Green chemistry; Stage #2: aniline In dichloromethane for 6h; Lossen Rearrangement; Green chemistry; chemoselective reaction; | 82% |
| Stage #1: Benzohydroxamic acid With 2-chloro-1,3-dimethylimidazolinium chloride; triethylamine In dichloromethane at 20℃; for 15h; Rearrangement; Stage #2: aniline In dichloromethane at 20℃; for 4h; Addition; | 66% |
-
-
1202047-15-1
1-tert-butyl-1-ethyl-3-phenylurea

-
-
102-07-8
bis(diphenyl)urea

| Conditions | Yield |
|---|---|
| With water In toluene at 70℃; for 1h; | 99% |

| Conditions | Yield |
|---|---|
| Stage #1: bis(trichloromethyl) carbonate; aniline In acetonitrile at 0 - 5℃; for 1h; Stage #2: With ETS-10 molecular sieve catalyst In acetonitrile at 80℃; for 4h; Solvent; Temperature; | 99% |
| In acetonitrile at 20℃; for 4h; Sealed tube; | 38 %Chromat. |

| Conditions | Yield |
|---|---|
| With sodium acetate In methanol at 60℃; for 2h; Solvent; Reagent/catalyst; Temperature; Concentration; | 99% |

| Conditions | Yield |
|---|---|
| With trimethylsilylazide; triethylamine In toluene at 110℃; for 1h; Sealed tube; | 99% |
| With diphenyl phosphoryl azide; triethylamine In toluene at 110℃; Curtius Rearrangement; Sealed tube; | 96% |

| Conditions | Yield |
|---|---|
| With iodine at 90 - 95℃; for 0.0833333h; | 98% |
| With zinc(II) chloride at 80 - 85℃; for 0.0833333h; Neat (no solvent); | 98% |
| With PEG-400; cerium(III) chloride; potassium iodide In water for 0.266667h; microwave irradiation; | 97% |
-
-
64-17-5
ethanol

-
-
201230-82-2
carbon monoxide

-
-
62-53-3
aniline

-
A
-
101-99-5
N-carboethoxyaniline

-
B
-
102-07-8
bis(diphenyl)urea

| Conditions | Yield |
|---|---|
| With oxygen; sodium iodide; Pd-Se/Al2)3 at 170℃; under 49505 Torr; for 3h; Product distribution; Further Variations:; Catalysts; | A 97% B n/a |
| With oxygen; tertamethylammonium iodide; silica gel; palladium at 160 - 170℃; further catalyst systems; | A 94% B 0.5% |
| With selenium; oxygen; triethylamine at 180℃; under 15001.5 Torr; for 6h; Autoclave; | A 85% B 8% |
| With selenium; oxygen; triethylamine at 120℃; under 15001.5 Torr; for 6h; Autoclave; | A 35% B 46% |
| With oxygen; palladium on activated charcoal; tertamethylammonium iodide at 160 - 170℃; further catalyst systems; | A 42% B 11% |

-
-
1274701-95-9
2-tert-butoxy-6-hydroxy-N-phenylpiperidine-1-carboxamide

-
-
102-07-8
bis(diphenyl)urea

| Conditions | Yield |
|---|---|
| With water at 80℃; | 97% |

| Conditions | Yield |
|---|---|
| With benzoic acid In para-xylene at 130℃; for 8h; Inert atmosphere; Schlenk technique; | 96% |
| With graphene oxide In neat (no solvent) at 80℃; for 4h; Sealed tube; | 94% |
| With sulfated polyborate at 120℃; for 1h; | 93% |

| Conditions | Yield |
|---|---|
| With phenyl isocyanate; triethylamine In benzene | A 96% B n/a |


| Conditions | Yield |
|---|---|
| With iron(III) chloride; phenylsilane; 1,3,4,6,7,8-hexahydro-2H-pyrimido[1,2-a]pyrimidine In tetrahydrofuran at 110℃; under 760.051 - 3040.2 Torr; for 24h; Sealed tube; | 96% |
| With diethylene glycol dimethyl ether; cesium fluoride at 100℃; for 20h; Schlenk technique; Glovebox; | 96% |
| With iron(III) chloride; phenylsilane; 1,3,4,6,7,8-hexahydro-2H-pyrimido[1,2-a]pyrimidine In tetrahydrofuran at 110℃; under 760.051 Torr; for 24h; Reagent/catalyst; Solvent; Temperature; Sealed tube; | 92% |
-
-
1152-32-5
1-benzoyl-4-phenylsemicarbazide

-
-
62-53-3
aniline

-
A
-
613-94-5
benzoic acid hydrazide

-
B
-
102-07-8
bis(diphenyl)urea

| Conditions | Yield |
|---|---|
| In various solvent(s) at 150℃; for 1h; | A 94% B 96% |


| Conditions | Yield |
|---|---|
| With diphenyl phosphoryl azide; triethylamine In toluene at 100℃; for 0.0166667h; Curtius Rearrangement; Microwave irradiation; | 96% |
| With phenyl N-phenylphosphoramidoazidate; triethylamine In acetonitrile for 1.5h; Heating; | 94% |
| Stage #1: benzoic acid With sodium azide; phenyl chloroformate; sodium t-butanolate In 1,2-dimethoxyethane at 25℃; for 8h; Stage #2: aniline In 1,2-dimethoxyethane at 75℃; for 16h; | 89% |
-
-
62-53-3
aniline

-
-
122350-19-0
3,3'-carbonylbis<5-phenyl-1,3,4-oxadiazole-2(3H)-thione>

-
A
-
3004-42-0
5-phenyl-1,3,4-oxadiazole-2(3H)-thione

-
B
-
102-07-8
bis(diphenyl)urea

| Conditions | Yield |
|---|---|
| In various solvent(s) for 1h; Ambient temperature; | A n/a B 96% |


| Conditions | Yield |
|---|---|
| With sodium acetate In acetonitrile at 80℃; for 12h; Inert atmosphere; | 96% |
| With sodium acetate In acetonitrile at 80℃; under 760.051 Torr; for 12h; Schlenk technique; Inert atmosphere; | 95% |
| With sodium acetate In ethyl acetate at 75℃; for 24h; | 95% |
| With silver hexafluoroantimonate; bis[dichloro(pentamethylcyclopentadienyl)iridium(III)]; N-t-butylbenzamide In 1,2-dichloro-ethane at 90℃; for 24h; Curtius Rearrangement; | 35% |

| Conditions | Yield |
|---|---|
| dodecacarbonyl-triangulo-triruthenium at 100℃; under 15514.4 Torr; for 20h; | 95% |

| Conditions | Yield |
|---|---|
| With potassium phosphate; copper(l) iodide; N,N`-dimethylethylenediamine In N,N-dimethyl-formamide at 85℃; for 24h; | 95% |
| With potassium phosphate In N,N-dimethyl-formamide at 20 - 120℃; for 1h; Microwave irradiation; Sealed tube; Inert atmosphere; | 94% |
| With copper(I) oxide; potassium phosphate monohydrate at 120℃; for 0.666667h; Microwave irradiation; Inert atmosphere; Neat (no solvent); | 90% |

| Conditions | Yield |
|---|---|
| Stage #1: benzoyl chloride With pyridine; trimethylsilylazide In N,N-dimethyl-formamide at 20℃; Curtius rearrangement; Microflow reaction; Inert atmosphere; Stage #2: aniline With acetic acid In N,N-dimethyl-formamide at 110℃; Microflow reaction; Inert atmosphere; | 95% |
| Stage #1: benzoyl chloride With N-ethyl-N,N-diisopropylamine; hydroxylamine-O-sulfonic acid In dichloromethane Lossen Rearrangement; Inert atmosphere; Green chemistry; Stage #2: aniline In dichloromethane at 100℃; for 0.0833333h; Solvent; Reagent/catalyst; Microwave irradiation; Inert atmosphere; Green chemistry; | 80% |

| Conditions | Yield |
|---|---|
| With trichloroisocyanuric acid; triphenylphosphine In 1,4-dioxane at 80℃; for 4h; | 95% |
-
-
61650-22-4
N-(pivaloyloxy)benzamide

-
-
102-07-8
bis(diphenyl)urea

| Conditions | Yield |
|---|---|
| With [RhCl2(p-cymene)]2; silver(I) acetate In 1,4-dioxane at 80℃; for 12h; Catalytic behavior; | 95% |
-
-
21251-12-7
N-benzoyl-O-acetylhydroxylamine

-
-
102-07-8
bis(diphenyl)urea

| Conditions | Yield |
|---|---|
| With [RhCl2(p-cymene)]2; silver(I) acetate In 1,4-dioxane at 80℃; for 12h; | 94% |
| With potassium carbonate |

| Conditions | Yield |
|---|---|
| triethylamine In dichloromethane for 0.5h; Heating; | 94% |
-
-
13987-61-6
N-methylene-tert-butylamine

-
-
102-07-8
bis(diphenyl)urea

-
-
140367-33-5
5-tert-Butyl-1,3-diphenyl-[1,3,5]triazinan-2-one

| Conditions | Yield |
|---|---|
| for 4h; Heating; | 100% |
-
-
485-47-2
indan-1,2,3-trione hydrate

-
-
102-07-8
bis(diphenyl)urea

-
-
58137-72-7
3a,8a-dihydroxy-1,3-diphenyl-1,3,3a,8a-tetrahydro-indeno[1,2-d]imidazole-2,8-dione

| Conditions | Yield |
|---|---|
| In acetic acid for 0.25h; Heating; | 100% |
| In water at 50 - 60℃; | 64% |
| In benzene Reflux; regioselective reaction; |

| Conditions | Yield |
|---|---|
| 100% | |
| 98% | |
| With iodine; triethylamine | 98% |

| Conditions | Yield |
|---|---|
| In dichloromethane for 1.5h; Reflux; | 99% |
| In toluene at 50 - 85℃; for 2h; Temperature; | 91% |
-
-
64-17-5
ethanol

-
-
201230-82-2
carbon monoxide

-
-
102-07-8
bis(diphenyl)urea

-
-
101-99-5
N-carboethoxyaniline

| Conditions | Yield |
|---|---|
| With isocyanate de chlorosulfonyle; oxygen; palladium at 160 - 170℃; | 98% |
| With oxygen; rhodium contaminated with carbon at 160 - 170℃; under 63253.7 Torr; for 3h; |

-
-
102-07-8
bis(diphenyl)urea

-
-
864131-95-3
N,N'-diphenylformamidine

| Conditions | Yield |
|---|---|
| With iron(II) acetylacetonate; phenylsilane; tris(2-diphenylphosphinoethyl)phosphine In tetrahydrofuran at 100℃; for 24h; Reagent/catalyst; Inert atmosphere; Glovebox; Schlenk technique; | 98% |
| Stage #1: bis(diphenyl)urea With phenylsilane; tris(2-diphenylphosphinoethyl)phosphine; iron(II) diacetylacetonate In tetrahydrofuran at 100℃; for 24h; Stage #2: With water at 20℃; Reagent/catalyst; Solvent; | 98 %Chromat. |

| Conditions | Yield |
|---|---|
| With oxygen; sodium hydroxide In dimethyl sulfoxide at 60℃; for 4h; Reagent/catalyst; | 97.3% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide; potassium carbonate; tetra(n-butyl)ammonium hydrogensulfate In toluene for 3h; Alkylation; Heating; | 97% |
-
-
102-07-8
bis(diphenyl)urea

-
-
60285-31-6
N,N'-diphenyl-N-nitroso-urea

| Conditions | Yield |
|---|---|
| With nitrosylchloride In N,N-dimethyl-formamide at 0℃; for 2h; | 96% |
| With cis-nitrous acid; acetic acid |
1,3-Diphenylurea Chemical Properties
Molecular Structure of CARBANILIDE(102-07-8):
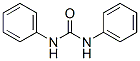
IUPAC Name:1,3-diphenylurea
Molecular Formula:C13H12N2O
Molecular Weight:212.247180 g/mol
Appearance:white crystalline solid
Melting Point:239-241 °C(lit.)
Density:1.249 g/cm3
Flash Point:91.1 °C
Enthalpy of Vaporization:49.98 kJ/mol
Boiling Point:262 °C at 760 mmHg
Vapour Pressure:0.0112 mmHg at 25 °C
Water Solubility:103.7 mg/L at 25 °C
BRN:782650
EINECS:203-003-7
Synonyms of CARBANILIDE(102-07-8):
CARBANILIDE;CARBANILLIDE;DIPHENYLCARBAMIDE;1,3-DIPHENYLUREA;AKOS B028830;PROXIMPHAM;N,N'-DIPHENYLUREA;N,N'-Diphenylurea
Categories of CARBANILIDE(102-07-8):
Biochemistry;Cytokinins;Plant Growth Regulators
1,3-Diphenylurea Uses
1,3-Diphenylurea Toxicity Data With Reference
| 1. | orl-rat LDLo:500 mg/kg | JPETAB Journal of Pharmacology and Experimental Therapeutics. 90 (1947),260. | ||
| 2. | ipr-mus LD50:200 mg/kg | NTIS** National Technical Information Service. (Springfield, VA 22161) (Formerly U.S. Clearinghouse for Scientific and Technical Information) AD277-689 . |
1,3-Diphenylurea Consensus Reports
1,3-Diphenylurea Safety Profile
Safety Statements:22-24/25
22:Do not breathe dust
24/25:Avoid contact with skin and eyes
RIDADR:2811
WGK Germany:3
RTECS:FD9800000
HazardClass:6.1(b)
PackingGroup:III
Poison by intraperitoneal route. Moderately toxic by ingestion. When heated to decomposition it emits toxic fumes of NOx.
1,3-Diphenylurea Specification
Keep in a tightly closed container, in a cool dry location. Store only with compatible chemicals.
Related Products
- 10,10'-Bis([1,1'-biphenyl]-4-yl)-9,9'-bianthracene
- 10,10'-Dibromo-9,9'-bianthryl
- 10,10-Dimethylanthrone
- 10,10-Oxybisphenoxarsine
- 10-[1,1'-Biphenyl]-4-yl-2-(1-methylethyl)-9-oxo-9H-thioxanthenium hexafluorophosphate
- 10,11-Dehydroimipramine
- 10,11-Dihydro-11-oxodibenzo[b,f][1,4]thiazepine
- 10,11-Dihydro-2-(4-methyl-1-piperazinyl)-11-(2-athiazolyl)-pyridazino(3,4-b)(1,4)benzoxazepine
- 10,11-DIHYDRO-2-(4-METHYL-1-PIPERAZINYL)-11-(3,4-XYLYL)PYRIDAZINO(3,4b)(1,4)-BENZOXAZEPINE MALEATE
- 10,11-Dihydro-5-(3-dimethylamino-2-methylpropyl)-5h-dibenz (b,f)azepine
- 102082-89-3
- 102082-98-4
- 1020-84-4
- 102087-56-9
- 102088-39-1
- 102-08-9
- 102089-50-9
- 102089-74-7
- 102-09-0
- 1020955-20-7
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View