-
Name
1,4-Butane sultone
- EINECS 216-647-9
- CAS No. 1633-83-6
- Article Data17
- CAS DataBase
- Density 1.308 g/cm3
- Solubility Water solubility: 54 g/L (20 °C) decomposes
- Melting Point 12-15 °C(lit.)
- Formula C4H8O3S
- Boiling Point 299.9 °C at 760 mmHg
- Molecular Weight 136.172
- Flash Point 135.2 °C
- Transport Information
- Appearance clear colorless to yellowish liquid
- Safety 22-36/37-45-36
- Risk Codes 22-40-68-20/21/22
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms 1-Butanesulfonicacid, 4-hydroxy-, d-sultone (7CI);Butane sultone;NSC 71999;d-Butane sultone;d-Valerosultone;
- PSA 51.75000
- LogP 1.20740
Synthetic route

| Conditions | Yield |
|---|---|
| Stage #1: 4-Chlorobutyl acetate With sodium sulfite In water Reflux; Stage #2: With hydrogenchloride In methanol for 4h; Reflux; Stage #3: at 110℃; under 20 Torr; Temperature; Pressure; Solvent; | 92% |
-
-
26978-64-3
4-hydroxy-butane-1-sulfonic acid

-
-
1633-83-6
1,4-butane sultone

| Conditions | Yield |
|---|---|
| at 40 - 110℃; for 2h; Temperature; | 88% |
| In water; toluene at 85℃; Temperature; Solvent; | 82.77% |
| With ethanol; xylene Unter Entfernen des entstehenden Wassers; |

-
A
-
1633-83-6
1,4-butane sultone

-
B
-
113309-30-1
4-hydroxy-1-butanesulfonyl chloride

| Conditions | Yield |
|---|---|
| With chlorine In water | A n/a B 51% |
-
-
14970-83-3
4-Mercapto-1-butanol

-
A
-
1633-83-6
1,4-butane sultone

-
B
-
1633-84-7
4-chloro-1-butanesulfonyl chloride

| Conditions | Yield |
|---|---|
| With chlorine In chloroform Yields of byproduct given; | A 27% B n/a |
| With chlorine In chloroform Yields of byproduct given; | A n/a B 27% |
-
-
14970-83-3
4-Mercapto-1-butanol

-
A
-
1633-83-6
1,4-butane sultone

-
B
-
1633-84-7
4-chloro-1-butanesulfonyl chloride

-
C
-
113309-30-1
4-hydroxy-1-butanesulfonyl chloride

| Conditions | Yield |
|---|---|
| With chlorine In dichloromethane | A 12% B 14% C 27% |

-
-
61161-42-0
4,4'-oxy-bis-butane-1-sulfonic acid

-
-
1633-83-6
1,4-butane sultone

| Conditions | Yield |
|---|---|
| Unter vermindertem Druck; |
-
-
109-69-3
n-Butyl chloride

-
A
-
1633-83-6
1,4-butane sultone

-
B
-
1121-03-5
3-methyloxathiolane 2,2-dioxide

| Conditions | Yield |
|---|---|
| Yield given. Multistep reaction. Yields of byproduct given. Title compound not separated from byproducts; | |
| With sulfur dioxide; chlorine Im Gluehlampenlicht; Erwaermen des Reaktionsprodukts mit Wasser und Erhitzen des danach isolierten Reaktionsprodukts unter vermindertem Druck; |

| Conditions | Yield |
|---|---|
| (i) AcCl, (ii) aq. Na2SO3; Multistep reaction; |

| Conditions | Yield |
|---|---|
| With thionyl chloride In N,N-dimethyl-formamide for 22h; Ambient temperature; |
-
-
113309-30-1
4-hydroxy-1-butanesulfonyl chloride

-
-
1633-83-6
1,4-butane sultone

| Conditions | Yield |
|---|---|
| With butan-1-ol In chloroform-d1 Rate constant; Ambient temperature; further reagent and solvent : Et3N, EtOD; |
-
-
113309-31-2
sodium 4-hydroxy-1-butanesulfinate

-
A
-
1633-83-6
1,4-butane sultone

-
B
-
113309-30-1
4-hydroxy-1-butanesulfonyl chloride

| Conditions | Yield |
|---|---|
| With chlorine In dichloromethane Yield given. Yields of byproduct given; |
-
-
109-69-3
n-Butyl chloride

-
-
71-36-3
butan-1-ol

-
A
-
1633-83-6
1,4-butane sultone

-
B
-
1121-03-5
3-methyloxathiolane 2,2-dioxide

| Conditions | Yield |
|---|---|
| Yield given. Multistep reaction. Yields of byproduct given. Title compound not separated from byproducts; |


-
A
-
1633-83-6
1,4-butane sultone

-
B
-
79917-90-1
1-butyl-3-methylimidazolium chloride

| Conditions | Yield |
|---|---|
| at 210℃; for 4h; |

-
A
-
1633-83-6
1,4-butane sultone

-
B
-
65039-09-0
1-ethyl-3-methyl-1H-imidazol-3-ium chloride

| Conditions | Yield |
|---|---|
| at 100℃; Vacuum; |

-
-
1374458-56-6
1-methyl-3-(3'-dimethylaminopropyl)imidazolium trifluoromethanesulfonate

-
A
-
1633-83-6
1,4-butane sultone

-
B
-
1374458-57-7
C13H26N3O3S(1+)*Cl(1-)

-
C
-
145022-44-2
1-ethyl-3-methylimidazolium triflate

| Conditions | Yield |
|---|---|
| at 100℃; for 2h; |


| Conditions | Yield |
|---|---|
| With potassium iodide; sodium sulfite In ethanol; water for 6h; Solvent; Reflux; |

| Conditions | Yield |
|---|---|
| at 40℃; for 10h; | 100% |
| at 70℃; | 97.6% |
| at 70℃; | 97.6% |
-
-
1633-83-6
1,4-butane sultone

-
-
1640-39-7
2,3,3-trimethylindoleniune

-
-
54136-26-4
4-(2,3,3-trimethyl-3H-indolium-1-yl)butane-1-sulfonate

| Conditions | Yield |
|---|---|
| With sodium acetate In acetonitrile for 12h; Reflux; | 100% |
| at 120℃; for 0.75h; Neat (no solvent); Microwave irradiation; | 99.8% |
| In 1,2-dichloro-benzene at 118℃; for 25h; | 97.4% |

| Conditions | Yield |
|---|---|
| In toluene at 60℃; for 24h; Inert atmosphere; | 100% |
| In toluene at 60℃; for 24h; Inert atmosphere; | 99.7% |
| In toluene for 24.0833h; Inert atmosphere; | 98% |
-
-
1633-83-6
1,4-butane sultone

-
-
80233-99-4
dimethyl(2-hydroxy-4,4,5,5,6,6,7,7,8,8,9,9,10,10,11,11,11-heptadecafluoroundecyl)amine

| Conditions | Yield |
|---|---|
| In acetonitrile for 20h; Heating; | 100% |

| Conditions | Yield |
|---|---|
| at 40℃; for 10h; | 100% |
| at 20℃; | 97.1% |
| at 20℃; for 48h; Inert atmosphere; | 96% |
-
-
1739-84-0
1,2-dimethyl-1H-imidazole

-
-
1633-83-6
1,4-butane sultone

-
-
1088461-53-3
1,2-dimethyl-3-(4-sulfonatobutyl)-1H-imidazol-3-ium

| Conditions | Yield |
|---|---|
| at 40℃; for 10h; | 100% |
| In ethanol at 20℃; for 48h; | 95% |
| In ethanol at 25℃; for 48h; | 95% |
-
-
1633-83-6
1,4-butane sultone

-
-
79917-90-1
1-butyl-3-methylimidazolium chloride

| Conditions | Yield |
|---|---|
| at 40℃; for 24h; Product distribution / selectivity; | 100% |
| In Nitroethane Kinetics; Activation energy; |
-
-
1633-83-6
1,4-butane sultone

-
-
25364-44-7
N-(2,4,6-trimethylphenyl)imidazole

-
-
1197988-21-8
1-(2,4,6-trimethylphenyl)-3-(4-sulfonatobutyl)imidazolidinium

| Conditions | Yield |
|---|---|
| In toluene for 48h; Reflux; Inert atmosphere; | 100% |
-
-
1633-83-6
1,4-butane sultone

-
-
3484-22-8
2,3,3-trimethyl-5-nitro-3H-indole

-
-
1364016-68-1
2,3,3-trimethyl-1-(sulfonatobutyl)-5-nitroindolium

| Conditions | Yield |
|---|---|
| In 1,2-dichloro-benzene at 120℃; | 100% |
| at 110℃; under 0.225023 Torr; for 12h; Sealed tube; |
-
-
1633-83-6
1,4-butane sultone

-
-
717110-52-6
1-(2,4,6-trimethylphenyl)-4,5-dihydro-1H-imidazole

| Conditions | Yield |
|---|---|
| In toluene for 48h; Reflux; | 100% |

| Conditions | Yield |
|---|---|
| With sodium acetate In acetonitrile for 12h; Reflux; | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: 1,4-butane sultone; 1,8-diazabicyclo[5.4.0]undec-7-ene at 20℃; for 0.0833333h; Stage #2: With sulfuric acid at 20℃; | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: 1,4-butane sultone; 1,8-diazabicyclo[5.4.0]undec-7-ene at 20℃; for 0.0833333h; Stage #2: acetic acid at 20℃; | 100% |

-
-
1633-83-6
1,4-butane sultone

-
-
6674-22-2
1,8-diazabicyclo[5.4.0]undec-7-ene

-
-
76-05-1
trifluoroacetic acid

| Conditions | Yield |
|---|---|
| Stage #1: 1,4-butane sultone; 1,8-diazabicyclo[5.4.0]undec-7-ene at 20℃; for 0.0833333h; Stage #2: trifluoroacetic acid at 20℃; | 100% |


| Conditions | Yield |
|---|---|
| In 1,4-dioxane; water at 4 - 20℃; | 99% |
| In water at 20℃; for 24h; | 94% |
| In water at 20℃; for 24h; | 94% |
-
-
1633-83-6
1,4-butane sultone

-
-
41532-84-7
2,3,3-trimethylbenzo[e]indole

-
-
63149-24-6
4-(1,1,2-trimethyl-1H-benzo[e]indol-3-ium-3-yl)butane-1-sulfonate

| Conditions | Yield |
|---|---|
| at 120℃; for 2h; | 99% |
| In sulfolane at 120 - 130℃; | 92% |
| With 1,2-dichloro-benzene In diethyl ether at 120℃; for 18h; Inert atmosphere; Darkness; | 90% |
-
-
1633-83-6
1,4-butane sultone

-
-
80233-98-3
dimethyl(2-hydroxy-4,4,5,5,6,6,7,7,8,8,9,9,9-tridecafluorononyl)amine

| Conditions | Yield |
|---|---|
| In acetonitrile for 20h; Heating; | 99% |

| Conditions | Yield |
|---|---|
| In acetonitrile at 80℃; for 48h; | 99% |
| at 0 - 50℃; |
-
-
1633-83-6
1,4-butane sultone

| Conditions | Yield |
|---|---|
| at 25℃; for 72h; | 99% |

| Conditions | Yield |
|---|---|
| Stage #1: 6-phenyl-5H-[1,3]dioxolo[4,5-f]indole With sodium hydride In tetrahydrofuran; mineral oil for 0.666667h; Cooling with ice; Stage #2: 1,4-butane sultone In tetrahydrofuran; mineral oil at 100℃; for 4h; | 99% |
-
-
1633-83-6
1,4-butane sultone

| Conditions | Yield |
|---|---|
| In 1,2-dichloro-benzene at 118℃; for 25h; | 98.9% |
-
-
1633-83-6
1,4-butane sultone

-
-
65039-09-0
1-ethyl-3-methyl-1H-imidazol-3-ium chloride

| Conditions | Yield |
|---|---|
| In acetonitrile for 12h; Inert atmosphere; Reflux; | 98% |
| at 40℃; for 24h; neat (no solvent); |
-
-
1633-83-6
1,4-butane sultone

-
-
14753-51-6
2,5-dibromohydroquinone

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol at 80℃; for 21h; | 98% |
| With sodium hydroxide In ethanol at 90℃; for 20h; | 96% |
| With sodium hydroxide In ethanol for 20h; Reflux; | 96% |
| With sodium hydroxide In ethanol for 20h; Reflux; | 90.8% |
-
-
1633-83-6
1,4-butane sultone

-
-
80-70-6
N,N,N',N'-tetramethylguanidine

-
-
1294516-89-4
4-(1,1,3,3-tetramethylguanidine-2-yl)butylsulfonic acid

| Conditions | Yield |
|---|---|
| In toluene at 80 - 85℃; for 4h; | 98% |
-
-
1633-83-6
1,4-butane sultone

-
-
1493-13-6
trifluorormethanesulfonic acid

-
-
54004-47-6
1-tetradecylimidazole

-
-
1374561-43-9
3-tetradecyl-1-(4-sulfobutyl)imidazolium trifluoromethanesulfonate

| Conditions | Yield |
|---|---|
| Stage #1: 1,4-butane sultone; 1-tetradecylimidazole at 40℃; for 10h; Stage #2: trifluorormethanesulfonic acid at 80℃; for 12h; | 98% |

-
-
1633-83-6
1,4-butane sultone

-
-
68971-82-4
5,11,17,23,29,35,41,47-octakis(tert-butyl)-49,50,51,52,53,54,55,56-octakis(hydroxy)calix[8]arene

| Conditions | Yield |
|---|---|
| Stage #1: 5,11,17,23,29,35,41,47-octakis(tert-butyl)-49,50,51,52,53,54,55,56-octakis(hydroxy)calix[8]arene With n-butyllithium In dimethyl sulfoxide at 0℃; Stage #2: 1,4-butane sultone at 25℃; for 96h; | 98% |
| Stage #1: 5,11,17,23,29,35,41,47-octakis(tert-butyl)-49,50,51,52,53,54,55,56-octakis(hydroxy)calix[8]arene With n-butyllithium In hexane; dimethyl sulfoxide at 0 - 25℃; for 24h; Inert atmosphere; Stage #2: 1,4-butane sultone In hexane; dimethyl sulfoxide at 25℃; Inert atmosphere; | 86% |
-
-
1633-83-6
1,4-butane sultone

-
-
82452-93-5
calix[8]arene

| Conditions | Yield |
|---|---|
| Stage #1: calix[8]arene With n-butyllithium In hexane; dimethyl sulfoxide at 0 - 25℃; for 24h; Inert atmosphere; Stage #2: 1,4-butane sultone In hexane; dimethyl sulfoxide at 25℃; Inert atmosphere; | 98% |
| Stage #1: calix[8]arene With n-butyllithium In dimethyl sulfoxide at 0℃; Stage #2: 1,4-butane sultone at 25℃; for 96h; | 86% |
-
-
1739-84-0
1,2-dimethyl-1H-imidazole

-
-
1633-83-6
1,4-butane sultone

-
-
1493-13-6
trifluorormethanesulfonic acid

-
-
1402671-71-9
1-butylsulfonic-2,3-dimethylimidazolium trifluoromethanesulfonate

| Conditions | Yield |
|---|---|
| Stage #1: 1,2-dimethyl-1H-imidazole; 1,4-butane sultone at 60℃; for 2h; Stage #2: trifluorormethanesulfonic acid at 60℃; for 1h; | 98% |
| Stage #1: 1,2-dimethyl-1H-imidazole; 1,4-butane sultone at 30℃; Stage #2: trifluorormethanesulfonic acid |


| Conditions | Yield |
|---|---|
| at 70℃; for 10h; Inert atmosphere; | 98% |
| In acetonitrile at 0 - 85℃; for 72h; | 63% |
| In acetonitrile at 85℃; for 120h; Inert atmosphere; | |
| In toluene at 60℃; for 24h; |

| Conditions | Yield |
|---|---|
| Stage #1: piperidine; carbon disulfide With potassium phosphate In water at 20℃; for 0.166667h; Stage #2: 1,4-butane sultone In water at 20℃; | 98% |

1,4-Butane sultone Chemical Properties
Molecular Formula: C4H8O3S
Molecular Weight: 136.18 g/mol
EINECS: 216-647-9
Index of Refraction: 1.47
Density: 1.308 g/cm3
Flash Point: 135.2 °C
Melting point: 12-15 °C(lit.)
Sensitive: Moisture Sensitive
Enthalpy of Vaporization: 51.84 kJ/mol
Boiling Point: 299.9 °C at 760 mmHg
Vapour Pressure: 0.00206 mmHg at 25 °C
Water solubility: 54 g/L (20 ºC) decomposes
Appearance: Clear colorless to yellowish liquid
Structure of 1,4-Butane sultone (CAS NO.1633-83-6):
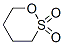
IUPAC Name: Oxathiane 2,2-dioxide
Product Category of 1,4-Butane sultone (CAS NO.1633-83-6): Heterocycles
1,4-Butane sultone Uses
1,4-Butane sultone (CAS NO.1633-83-6) is mainly used as the intermediates of organic sulfonation agent and fine chemicals. It is the raw material of electroplating intermediate, ande can be applicable to medicine, photographic materials, lithium battery, surface active agents.
1,4-Butane sultone Toxicity Data With Reference
| 1. | mmo-sat 100 µg/plate | JNCIAM Journal of the National Cancer Institute. 62 (1979),893. | ||
| 2. | dnr-esc 10 µL/disc | JNCIAM Journal of the National Cancer Institute. 62 (1979),873. | ||
| 3. | hma-mus/sat 138 mg/kg | JNCIAM Journal of the National Cancer Institute. 62 (1979),911. | ||
| 4. | orl-rat TDLo:1300 mg/kg/1Y-I:ETA,REP | ZEKBAI Zeitschrift fuer Krebsforschung. 75 (1970),69. | ||
| 5. | orl-rat LD50:500 mg/kg | ZEKBAI Zeitschrift fuer Krebsforschung. 75 (1970),69. | ||
| 6. | scu-rat LD50:350 mg/kg | ZEKBAI Zeitschrift fuer Krebsforschung. 75 (1970),69. | ||
| 7. | ivn-rat LD50:270 mg/kg | ZEKBAI Zeitschrift fuer Krebsforschung. 75 (1970),69. | ||
| 8. | ipr-mus LD50:138 mg/kg | JJIND8 JNCI, Journal of the National Cancer Institute. 62 (1979),911. |
1,4-Butane sultone Consensus Reports
EPA Genetic Toxicology Program. Reported in EPA TSCA Inventory.
1,4-Butane sultone Safety Profile
Suspected carcinogen with experimental tumorigenic data. Poison by subcutaneous, intravenous, and intraperitoneal routes. Moderately toxic by ingestion. Experimental reproductive effects. Human mutation data reported. See also SULFONATES. When heated to decomposition it emits toxic fumes of SOx.
Hazard Codes:  Xn
Xn
Risk Statements: 22-40-68-20/21/22
22: Harmful if swallowed
40: Limited evidence of a carcinogenic effect
68: Possible risk of irreversible effects
20/21/22: Harmful by inhalation, in contact with skin and if swallowed
Safety Statements: 22-36/37-45-36
22: Do not breathe dust
36/37: Wear suitable protective clothing and gloves
45: In case of accident or if you feel unwell, seek medical advice immediately (show label where possible)
36: Wear suitable protective clothing
1,4-Butane sultone Standards and Recommendations
DFG MAK: Animal Carcinogen, Suspected Human Carcinogen
1,4-Butane sultone Specification
1,4-Butane sultone , its cas register number is 1633-83-6. It also can be called 4-Hydroxy-1-butanesulfonic acid delta-sultone ; and Butanesultone .
Related Products
- 10,10'-Bis([1,1'-biphenyl]-4-yl)-9,9'-bianthracene
- 10,10'-Dibromo-9,9'-bianthryl
- 10,10-Dimethylanthrone
- 10,10-Oxybisphenoxarsine
- 10-[1,1'-Biphenyl]-4-yl-2-(1-methylethyl)-9-oxo-9H-thioxanthenium hexafluorophosphate
- 10,11-Dehydroimipramine
- 10,11-Dihydro-11-oxodibenzo[b,f][1,4]thiazepine
- 10,11-Dihydro-2-(4-methyl-1-piperazinyl)-11-(2-athiazolyl)-pyridazino(3,4-b)(1,4)benzoxazepine
- 10,11-DIHYDRO-2-(4-METHYL-1-PIPERAZINYL)-11-(3,4-XYLYL)PYRIDAZINO(3,4b)(1,4)-BENZOXAZEPINE MALEATE
- 10,11-Dihydro-5-(3-dimethylamino-2-methylpropyl)-5h-dibenz (b,f)azepine
- 16338-48-0
- 163400-20-2
- 1634-02-2
- 1634-04-4
- 163425-20-5
- 163428-90-8
- 16343-08-1
- 1634-34-0
- 1634-36-2
- 163437-14-7
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View