-
Name
N-Boc-3-pyrrolidinone
- EINECS 600-204-5
- CAS No. 101385-93-7
- Article Data37
- CAS DataBase
- Density 1.133 g/cm3
- Solubility Insoluble in water.
- Melting Point 34-38 °C(lit.)
- Formula C9H15NO3
- Boiling Point 270.9 °C at 760 mmHg
- Molecular Weight 185.223
- Flash Point 117.6 °C
- Transport Information
- Appearance
- Safety 26-36/37/39
- Risk Codes 22-41-36/37/38-20/21/22
-
Molecular Structure
-
Hazard Symbols
 Xn,
Xn, Xi
Xi
- Synonyms 1,1-Dimethylethyl3-oxopyrrolidine-1-carboxylate;1-(tert-Butyloxycarbonyl)pyrrolidin-3-one;1-Pyrrolidinecarboxylicacid, 3-oxo-, 1,1-dimethylethyl ester;1-tert-Butoxycarbonyl-3-pyrrolidinone;3-Oxopyrrolidine-1-carboxylic acid tert-butyl ester;N-(tert-Butoxycarbonyl)-3-pyrrolidinone;N-tert-Butoxylcarbonyl-3-pyrrolidone;tert-Butyl 3-oxo-1-pyrrolidinecarboxylate;1-BOC-3-Pyrrolidinone;
- PSA 46.61000
- LogP 1.13420
Synthetic route
-
-
103057-44-9
N-(tert-butoxycarbonyl)-3-hydroxypyrrolidine

-
-
101385-93-7
3-oxo-pyrrolidine-1-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| Stage #1: N-(tert-butoxycarbonyl)-3-hydroxypyrrolidine With oxalyl dichloride; dimethyl sulfoxide In dichloromethane at -78℃; for 2.08333h; Stage #2: With triethylamine In dichloromethane at -78 - 20℃; for 3h; | 100% |
| With oxalyl dichloride; dimethyl sulfoxide; N-ethyl-N,N-diisopropylamine In dichloromethane at -60 - 20℃; for 1.5h; | 97% |
| With Dess-Martin periodane In dichloromethane | 97% |
-
-
101469-92-5
(S)-3-hydroxy-pyrrolidine-1-carboxylic acid tert-butyl ester

-
-
101385-93-7
3-oxo-pyrrolidine-1-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With Dess-Martin periodane In dichloromethane at 0 - 20℃; for 48h; | 81% |
| With Dess-Martin periodane In dichloromethane at 20℃; for 18h; Inert atmosphere; |
-
-
775-16-6
N-benzyl-3-pyrrolidinone

-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
101385-93-7
3-oxo-pyrrolidine-1-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With hydrogen; palladium 10% on activated carbon In ethanol at 20℃; under 760.051 Torr; | 79% |
-
-
109431-87-0
(R)-tert-butyl 3-hydroxypyrrolidine-1-carboxylate

-
-
101385-93-7
3-oxo-pyrrolidine-1-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With Dess-Martin periodane In dichloromethane at 0 - 20℃; for 0.2h; Inert atmosphere; | 77.3% |
| With Dess-Martin periodane In dichloromethane at 0 - 20℃; for 2h; Inert atmosphere; | 77.3% |
| With pyridine-SO3 complex; triethylamine In dimethyl sulfoxide at 20℃; | 34% |
| With [ruthenium(II)(η6-1-methyl-4-isopropyl-benzene)(chloride)(μ-chloride)]2; triethylamine In acetone at 150℃; Flow reactor; | 40 %Spectr. |
| With sulfur trioxide trimethylamine complex; triethylamine In dimethyl sulfoxide at 20℃; for 18h; |
-
-
146256-98-6
4-oxo-pyrrolidine-1,3-dicarboxylic acid 1-tert-butyl ester 3-ethyl ester

-
-
101385-93-7
3-oxo-pyrrolidine-1-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With water In dimethyl sulfoxide at 120 - 130℃; for 4h; Krapcho reaction; | 70% |
| With 2,6-di-tert-butyl-4-methyl-phenol In water; dimethyl sulfoxide; toluene for 6h; Reagent/catalyst; Solvent; | 10.8 g |
-
-
186550-13-0
3-amino-1-(t-butoxycarbonyl)pyrrolidine

-
A
-
101385-93-7
3-oxo-pyrrolidine-1-carboxylic acid tert-butyl ester

-
B
-
147081-49-0
(R)-3-aminopyrrolidine-1-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With ω-transaminase from Alcaligenes denitrificans; pyridoxal 5'-phosphate; 2-oxo-propionic acid In sodium phosphate buffer at 37℃; for 7h; pH=8; kinetic resolution; Enzymatic reaction; enantioselective reaction; | A 44% B 39% |
-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
101385-93-7
3-oxo-pyrrolidine-1-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: NaOH / H2O; tetrahydrofuran / 20 °C 2: triethylamine; trioxide-trimethylamine complex / dimethylsulfoxide / 20 °C View Scheme | |
| Multi-step reaction with 2 steps 1: NaHCO3 / tetrahydrofuran / 14 h 2: tetrapropylammonium perruthenate; NMO; 4A molecular sieves / CH2Cl2; acetonitrile / 1 h View Scheme | |
| Multi-step reaction with 3 steps 1: CH2Cl2 / 1 h / 20 °C 2: NaOEt / ethanol / 3 h / Heating 3: 70 percent / H2O / dimethylsulfoxide / 4 h / 120 - 130 °C View Scheme |
-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
101385-93-7
3-oxo-pyrrolidine-1-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: NaOH / H2O; tetrahydrofuran / 20 °C 2: DMSO; SO3*NMe3; triethylamine / 20 °C View Scheme |
-
-
146256-97-5
3-(tert-butoxycarbonyl-ethoxycarbonylmethyl-amino)-propionic acid ethyl ester

-
-
101385-93-7
3-oxo-pyrrolidine-1-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: NaOEt / ethanol / 3 h / Heating 2: 70 percent / H2O / dimethylsulfoxide / 4 h / 120 - 130 °C View Scheme |
-
-
58632-95-4
2-(tert-Butoxycarbonyloxyimino)-2-phenylacetonitrile

-
-
101385-93-7
3-oxo-pyrrolidine-1-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 79 percent / Et3N / dioxane; H2O / 2 h / Ambient temperature 2: 35 percent / PCC / CH2Cl2 / 4 h / Ambient temperature View Scheme |
-
-
40499-83-0
pyrrolidin-3-ol

-
-
101385-93-7
3-oxo-pyrrolidine-1-carboxylic acid tert-butyl ester
-
-
79-37-8
oxalyl dichloride

-
-
109431-87-0
(R)-tert-butyl 3-hydroxypyrrolidine-1-carboxylate

-
-
101385-93-7
3-oxo-pyrrolidine-1-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane; water; dimethyl sulfoxide; ethyl acetate; benzene | |
| With triethylamine In dichloromethane; water; dimethyl sulfoxide; ethyl acetate; benzene |
-
-
79-37-8
oxalyl dichloride

-
-
103057-44-9
N-(tert-butoxycarbonyl)-3-hydroxypyrrolidine

-
-
101385-93-7
3-oxo-pyrrolidine-1-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With dimethyl sulfoxide; triethylamine In tetrahydrofuran; water |
-
-
775-16-6
N-benzyl-3-pyrrolidinone

-
-
101385-93-7
3-oxo-pyrrolidine-1-carboxylic acid tert-butyl ester
-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
101385-93-7
3-oxo-pyrrolidine-1-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| Stage #1: pyrrolidin-3-one sulfate With sodium hydroxide In water at 0℃; pH=Ca. 7; Inert atmosphere; Glovebox; Stage #2: di-tert-butyl dicarbonate In dichloromethane; water pH=9 - 10; Inert atmosphere; Glovebox; | 564 mg |
-
-
103-67-3
benzyl-methyl-amine

-
-
101385-93-7
3-oxo-pyrrolidine-1-carboxylic acid tert-butyl ester

-
-
862906-27-2
tert-butyl 3-(benzyl(methyl)amino)pyrrolidine-1-carboxylate

| Conditions | Yield |
|---|---|
| Stage #1: benzyl-methyl-amine; 3-oxo-pyrrolidine-1-carboxylic acid tert-butyl ester With sodium tris(acetoxy)borohydride In dichloromethane at 0 - 20℃; for 2h; Stage #2: With water; sodium hydrogencarbonate In dichloromethane | 100% |
| With sodium tris(acetoxy)borohydride; acetic acid In dichloromethane at 20℃; Inert atmosphere; | 76% |
| With sodium tris(acetoxy)borohydride; acetic acid In dichloromethane at 25℃; |

| Conditions | Yield |
|---|---|
| Stage #1: tryptamine; 3-oxo-pyrrolidine-1-carboxylic acid tert-butyl ester With acetic acid at 100℃; for 4.5h; Stage #2: With hydrogenchloride In 1,4-dioxane at 20℃; | 100% |
-
-
14490-05-2
7-methyltryptamine

-
-
101385-93-7
3-oxo-pyrrolidine-1-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| Stage #1: 7-methyltryptamine; 3-oxo-pyrrolidine-1-carboxylic acid tert-butyl ester With acetic acid at 100℃; for 4.5h; Stage #2: With hydrogenchloride In 1,2-dioxacyclohexane at 20℃; | 100% |
-
-
1950-68-1
4-methoxybenzenesulfonyl hydrazide

-
-
101385-93-7
3-oxo-pyrrolidine-1-carboxylic acid tert-butyl ester

-
-
1510865-63-0
tert-butyl 3-(2-((4-methoxyphenyl)sulfonyl)-hydrazono)pyrrolidine-1-carboxylate

| Conditions | Yield |
|---|---|
| In methanol at 20℃; Schlenk technique; | 100% |
-
-
5927-18-4
trimethyl phosphonoacetate

-
-
101385-93-7
3-oxo-pyrrolidine-1-carboxylic acid tert-butyl ester

-
-
441773-67-7
tert-butyl 3-(2-methoxy-2-oxoethylidene)pyrrolidine-1-carboxylate

| Conditions | Yield |
|---|---|
| Stage #1: trimethyl phosphonoacetate With sodium hydride In tetrahydrofuran; mineral oil at 0℃; for 0.333333h; Stage #2: 3-oxo-pyrrolidine-1-carboxylic acid tert-butyl ester In tetrahydrofuran; mineral oil at 0 - 20℃; for 16h; | 100% |
| Stage #1: trimethyl phosphonoacetate With potassium tert-butylate In tetrahydrofuran at 0℃; for 2.5h; Stage #2: 3-oxo-pyrrolidine-1-carboxylic acid tert-butyl ester In tetrahydrofuran at 0℃; for 1h; | 92.8% |
-
-
101385-93-7
3-oxo-pyrrolidine-1-carboxylic acid tert-butyl ester

-
-
103057-44-9
N-(tert-butoxycarbonyl)-3-hydroxypyrrolidine

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate; ethanol at 0 - 20℃; for 4h; | 99% |
| Stage #1: 3-oxo-pyrrolidine-1-carboxylic acid tert-butyl ester With sodium tetrahydroborate In methanol at 0℃; for 0.25h; Stage #2: With hydrogenchloride; water In methanol pH=~ 9; | 98.9% |
| With methanol; sodium tetrahydroborate In tetrahydrofuran at 0 - 20℃; for 0.666667h; | 98% |
| With sodium tetrahydroborate In methanol at 25℃; for 1h; | 91% |
| With sodium tetrahydroborate |
-
-
1576-35-8
toluene-4-sulfonic acid hydrazide

-
-
101385-93-7
3-oxo-pyrrolidine-1-carboxylic acid tert-butyl ester

-
-
1510865-62-9
tert-butyl 3-(2-tosylhydrazono)-pyrrolidine-1-carboxylate

| Conditions | Yield |
|---|---|
| In methanol at 20℃; Schlenk technique; | 99% |
| In methanol at 20℃; Inert atmosphere; | 92% |

-
-
101385-93-7
3-oxo-pyrrolidine-1-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With sodium tris(acetoxy)borohydride; acetic acid In methanol at 20℃; | 99% |
-
-
20870-79-5
5-nitrooxindole

-
-
101385-93-7
3-oxo-pyrrolidine-1-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With ammonia In methanol for 2h; Heating / reflux; | 97% |
-
-
101385-93-7
3-oxo-pyrrolidine-1-carboxylic acid tert-butyl ester

-
-
1066-54-2
trimethylsilylacetylene

| Conditions | Yield |
|---|---|
| Stage #1: trimethylsilylacetylene With n-butyllithium In tetrahydrofuran at -78℃; for 0.5h; Stage #2: 3-oxo-pyrrolidine-1-carboxylic acid tert-butyl ester In tetrahydrofuran at 20℃; for 0.666667h; | 97% |
| Stage #1: trimethylsilylacetylene With n-butyllithium In tetrahydrofuran; hexane at -78℃; for 1h; Stage #2: 3-oxo-pyrrolidine-1-carboxylic acid tert-butyl ester In tetrahydrofuran; hexane at -78 - 20℃; for 1.33333h; | 90% |
| Stage #1: trimethylsilylacetylene With n-butyllithium In tetrahydrofuran; hexane at -78℃; for 0.5h; Inert atmosphere; Stage #2: 3-oxo-pyrrolidine-1-carboxylic acid tert-butyl ester In tetrahydrofuran; hexane at -78 - 20℃; for 1.33333h; Inert atmosphere; | 90% |
-
-
1415318-48-7
C14H16F3N3O3*C2HF3O2

-
-
101385-93-7
3-oxo-pyrrolidine-1-carboxylic acid tert-butyl ester

-
-
1415318-53-4
C23H31F3N4O5

| Conditions | Yield |
|---|---|
| Stage #1: C14H16F3N3O3*C2HF3O2; 3-oxo-pyrrolidine-1-carboxylic acid tert-butyl ester With magnesium sulfate; N-ethyl-N,N-diisopropylamine In tetrahydrofuran at 20℃; for 0.0833333h; Stage #2: With sodium tris(acetoxy)borohydride In tetrahydrofuran | 95% |
-
-
553677-82-0
2-Amino-4-methoxy-5-oxazol-5-yl-benzamide

-
-
101385-93-7
3-oxo-pyrrolidine-1-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| In 1,2-dichloro-ethane at 80℃; for 3.5h; | 94% |
-
-
101385-93-7
3-oxo-pyrrolidine-1-carboxylic acid tert-butyl ester

-
-
765-38-8
2-methyl-1-pyrrolidine

-
-
1146415-31-7
2-methyl-[1,3']bipyrrolidinyl-1'-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| Stage #1: 2-methyl-1-pyrrolidine With hydrogenchloride In diethyl ether; 1,2-dichloro-ethane Stage #2: 3-oxo-pyrrolidine-1-carboxylic acid tert-butyl ester With sodium tris(acetoxy)borohydride In 1,2-dichloro-ethane at 20℃; Stage #3: With water; sodium hydrogencarbonate In 1,2-dichloro-ethane | 94% |
| Stage #1: 2-methyl-1-pyrrolidine With hydrogenchloride In diethyl ether; dichloromethane Stage #2: 3-oxo-pyrrolidine-1-carboxylic acid tert-butyl ester With sodium tris(acetoxy)borohydride In 1,2-dichloro-ethane at 20℃; Stage #3: With water; sodium hydrogencarbonate In 1,2-dichloro-ethane | 94% |
| Stage #1: 2-methyl-1-pyrrolidine With hydrogenchloride In diethyl ether; 1,2-dichloro-ethane Stage #2: 3-oxo-pyrrolidine-1-carboxylic acid tert-butyl ester With sodium tris(acetoxy)borohydride In 1,2-dichloro-ethane at 20℃; Stage #3: With water; sodium hydrogencarbonate In 1,2-dichloro-ethane | 94% |
| With sodium tris(acetoxy)borohydride In dichloromethane at 20℃; Inert atmosphere; | 94% |
| With sodium tris(acetoxy)borohydride In 1,2-dichloro-ethane at 20℃; | 84% |
-
-
872-32-2
2-methyl-1H-pyrroline

-
-
101385-93-7
3-oxo-pyrrolidine-1-carboxylic acid tert-butyl ester

-
-
1146415-31-7
2-methyl-[1,3']bipyrrolidinyl-1'-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| Stage #1: 2-methyl-1H-pyrroline; 3-oxo-pyrrolidine-1-carboxylic acid tert-butyl ester With sodium tris(acetoxy)borohydride In 1,2-dichloro-ethane at 20℃; Inert atmosphere; Stage #2: With sodium hydrogencarbonate In water; 1,2-dichloro-ethane | 94% |
-
-
101066-87-9
4-iodo-2-(trifluoromethyl)benzonitrile

-
-
101385-93-7
3-oxo-pyrrolidine-1-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| Stage #1: 3-oxo-pyrrolidine-1-carboxylic acid tert-butyl ester With lithium hexamethyldisilazane In tetrahydrofuran at -78℃; for 0.333333h; Stage #2: With N,N-phenylbistrifluoromethane-sulfonimide In tetrahydrofuran at 20℃; for 1h; Stage #3: 4-iodo-2-(trifluoromethyl)benzonitrile Further stages; | 94% |
-
-
16182-15-3
2,4,6-trimethylbenzenesulfonohydrazide

-
-
101385-93-7
3-oxo-pyrrolidine-1-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| In methanol at 20℃; | 94% |
-
-
773837-37-9
sodium cyanide

-
-
101385-93-7
3-oxo-pyrrolidine-1-carboxylic acid tert-butyl ester

-
-
871115-54-7
HARV-120823-2

| Conditions | Yield |
|---|---|
| With ammonium acetate In methanol at 20℃; | 93% |
-
-
101385-93-7
3-oxo-pyrrolidine-1-carboxylic acid tert-butyl ester

-
-
74-89-5
methylamine

-
-
454712-26-6
tert‐butyl 3‐(methylamino)pyrrolidine‐1‐carboxylate

| Conditions | Yield |
|---|---|
| Stage #1: 3-oxo-pyrrolidine-1-carboxylic acid tert-butyl ester; methylamine In methanol at 0 - 20℃; for 2h; Stage #2: With methanol; sodium tetrahydroborate at 20℃; for 1h; | 92.5% |
| With 5%-palladium/activated carbon; hydrogen In methanol at 60℃; under 2585.81 Torr; | 52% |
| With sodium tris(acetoxy)borohydride; acetic acid In tetrahydrofuran at 20℃; for 16h; | |
| With D-glucose; glucose dehydrogenase CDX-901; imine reductase 91 from Kribbella flavida DSM 17836; nicotinamide adenine dinucleotide phosphate In aq. buffer at 25℃; for 24h; pH=9; Enzymatic reaction; | |
| Stage #1: 3-oxo-pyrrolidine-1-carboxylic acid tert-butyl ester; methylamine In methanol for 1h; Stage #2: With methanol; sodium tris(acetoxy)borohydride for 4h; Stage #3: With water; sodium hydrogencarbonate In dichloromethane |
-
-
101385-93-7
3-oxo-pyrrolidine-1-carboxylic acid tert-butyl ester

-
-
109431-87-0
(R)-tert-butyl 3-hydroxypyrrolidine-1-carboxylate

| Conditions | Yield |
|---|---|
| With recombinant Chryseobacterium sp. CA 49 ketoreductase; nicotinamide adenine dinucleotide In aq. phosphate buffer; isopropyl alcohol at 40℃; for 10h; pH=7.0; Reagent/catalyst; Enzymatic reaction; stereoselective reaction; | 92% |
| With Leifsonia sp. S749 cells alcohol dehydrogenase; nicotinamide adenine dinucleotide; isopropyl alcohol In phosphate buffer at 25℃; for 24h; pH=7.0; | 24% |
| With dichloro(pentamethylcyclopentadienyl)rhodium (III) dimer; 3,3'-[(1R,2R)-1-amino-2-(4-dodecylphenylsulfonamido)ethane-1,2-diyl]bis(N,N,N-trimethylbenzenaminium)diiodide; sodium formate In water at 5℃; for 48h; enantioselective reaction; | n/a |
-
-
75-16-1
methylmagnesium bromide

-
-
101385-93-7
3-oxo-pyrrolidine-1-carboxylic acid tert-butyl ester

-
-
412278-02-5
rac-tert-butyl 3-hydroxy-3-methylpyrrolidine-1-carboxylate

| Conditions | Yield |
|---|---|
| In diethyl ether at 20℃; for 1h; Inert atmosphere; | 92% |
| Stage #1: methylmagnesium bromide With lithium chloride; zinc(II) chloride In tetrahydrofuran; diethyl ether at -10℃; for 1h; Stage #2: 3-oxo-pyrrolidine-1-carboxylic acid tert-butyl ester In tetrahydrofuran; diethyl ether for 0.5h; | 83.8% |
| With lithium chloride; zinc(II) chloride In tetrahydrofuran; diethyl ether | 83.8% |
-
-
78797-58-7, 78797-59-8, 78797-62-3, 36520-39-5
azetidine hydrochloride

-
-
101385-93-7
3-oxo-pyrrolidine-1-carboxylic acid tert-butyl ester

-
-
1018442-99-3
tert-Butyl 3-azetidin-1-ylpyrrolidine-1-carboxylate

| Conditions | Yield |
|---|---|
| Stage #1: azetidine hydrochloride; 3-oxo-pyrrolidine-1-carboxylic acid tert-butyl ester With sodium tris(acetoxy)borohydride; acetic acid In dichloromethane at 20℃; Stage #2: With potassium carbonate In dichloromethane; water for 0.5h; | 92% |
-
-
1125-60-6
isoquinolin-5-ylamine

-
-
101385-93-7
3-oxo-pyrrolidine-1-carboxylic acid tert-butyl ester

-
-
1035096-80-0
N-(pyrrolidin-3-yl)isoquinolin-5-amine

| Conditions | Yield |
|---|---|
| Stage #1: isoquinolin-5-ylamine; 3-oxo-pyrrolidine-1-carboxylic acid tert-butyl ester With sodium tris(acetoxy)borohydride In acetic acid at 0 - 20℃; Stage #2: With hydrogenchloride In diethyl ether for 6h; | 92% |
-
-
593-56-6
N-methoxylamine hydrochloride

-
-
101385-93-7
3-oxo-pyrrolidine-1-carboxylic acid tert-butyl ester

-
-
654638-70-7
3-methoxyimino-pyrrolidine-1-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In tetrahydrofuran; ethanol at 40℃; for 1h; | 90% |
| With potassium carbonate In ethanol at 20℃; |
-
-
101385-93-7
3-oxo-pyrrolidine-1-carboxylic acid tert-butyl ester

-
-
150008-25-6
N-Boc-3-hydroxyiminopyrrolidine

| Conditions | Yield |
|---|---|
| With hydroxylamine hydrochloride; sodium hydrogencarbonate In tetrahydrofuran; ethanol at 20℃; for 1h; | 90% |
-
-
496-15-1
1-indoline

-
-
101385-93-7
3-oxo-pyrrolidine-1-carboxylic acid tert-butyl ester

-
-
1063408-78-5
3-(2,3-dihydro-1H-indol-1-yl)pyrrolidine-1-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With sodium tris(acetoxy)borohydride; acetic acid In dichloromethane for 24h; | 90% |
| Stage #1: 1-indoline; 3-oxo-pyrrolidine-1-carboxylic acid tert-butyl ester With sodium cyanoborohydride; acetic acid In methanol at 0 - 20℃; for 3h; Stage #2: With sodium hydroxide; water In methanol | 87% |
| With sodium cyanoborohydride; acetic acid In methanol at 0 - 20℃; for 3h; Inert atmosphere; | 87% |
-
-
7103-09-5
4-but-1-enylmagnesium bromide

-
-
101385-93-7
3-oxo-pyrrolidine-1-carboxylic acid tert-butyl ester

-
-
1446012-35-6
tert-butyl 3-(but-3-en-1-yl)-3-hydroxypyrrolidine-1-carboxylate

| Conditions | Yield |
|---|---|
| Stage #1: 4-but-1-enylmagnesium bromide With cerium chloride In tetrahydrofuran at 0℃; for 2h; Inert atmosphere; Stage #2: 3-oxo-pyrrolidine-1-carboxylic acid tert-butyl ester In tetrahydrofuran at 0 - 20℃; Inert atmosphere; | 90% |
-
-
870-46-2
t-butoxycarbonylhydrazine

-
-
101385-93-7
3-oxo-pyrrolidine-1-carboxylic acid tert-butyl ester

-
-
1189770-77-1
C14H25N3O4

| Conditions | Yield |
|---|---|
| In ethanol at 20℃; for 2h; | 89% |

| Conditions | Yield |
|---|---|
| With triethylamine In methanol at 20℃; for 15h; | 88.9% |
-
-
7677-24-9
trimethylsilyl cyanide

-
-
5680-79-5
glycine ethyl ester hydrochloride

-
-
101385-93-7
3-oxo-pyrrolidine-1-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| Stage #1: glycine ethyl ester hydrochloride With triethylamine In dichloromethane for 0.166667h; Stage #2: trimethylsilyl cyanide; 3-oxo-pyrrolidine-1-carboxylic acid tert-butyl ester With acetic acid In dichloromethane at 15 - 60℃; for 2h; | 88.3% |

1-BOC-3-Pyrrolidinone Chemical Properties
Empirical Formula: C9H15NO3
Molecular Weight: 185.2203g/mol
Structure of 1-BOC-3-Pyrrolidinone (CAS NO.101385-93-7):
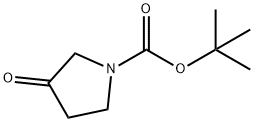
Index of Refraction: 1.486
Molar Refractivity: 46.95 cm3
Molar Volume: 163.4 cm3
Polarizability: 18.61×10-24cm3
Surface Tension: 41.2 dyne/cm
Density: 1.133 g/cm3
Flash Point: 117.6 °C
Enthalpy of Vaporization: 50.91 kJ/mol
Melting Point: 34-38 °C(lit.)
Boiling Point: 270.9 °C at 760 mmHg
Vapour Pressure: 0.00667 mmHg at 25°C
Product Categories: Amines and Anilines;Carbonyl Compounds;pharmacetical;Pyrrolidines;Pyrrole&Pyrrolidine&Pyrroline;Benzenes;CHIRAL CHEMICALS
Canonical SMILES: CC(C)(C)OC(=O)N1CCC(=O)C1
InChI: InChI=1S/C9H15NO3/c1-9(2,3)13-8(12)10-5-4-7(11)6-10/h4-6H2,1-3H3
InChIKey: JSOMVCDXPUXKIC-UHFFFAOYSA-N
1-BOC-3-Pyrrolidinone Uses
Application:Used in a study of asymmetric HYDROGEN-transfer bioreduction of KETONES with Leifsonia alcohol deHYDROGENase.
Packaging:1/10 g in glass btl
1-BOC-3-Pyrrolidinone Safety Profile
Hazard Codes:  Xn,
Xn, Xi
Xi
Risk Statements: 22-41-36/37/38-20/21/22
R22:Harmful if swallowed.
R41:Risk of serious damage to the eyes.
R36/37/38:Irritating to eyes, respiratory system and skin.
R20/21/22:Harmful by inhalation, in contact with skin and if swallowed.
Safety Statements: 26-36/37/39
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection.
WGK Germany: 3
Hazard Note: Irritant
1-BOC-3-Pyrrolidinone Specification
1-BOC-3-Pyrrolidinone , its cas register number is 101385-93-7. It also can be called 1-pyrrolidinecarboxylic acid, 3-oxo-, 1,1-dimethylethyl ester ; 2-Methyl-2-propanyl 3-oxo-1-pyrrolidinecarboxylate ; tert-Butyl 3-oxopyrrolidine-1-carboxylate ; tert-Butyl-3-oxopyrrolidin-1-carboxylat ; 1-(tert-Butoxycarbonyl)-3-pyrrolidinone ; 1-N-Boc-3-pyrrolidinone ; 1-N-boc-4-cyano-pyrrolidine-3-one .
Related Products
- 1-BOC-3-Pyrrolidinone
- 10138-59-7
- 10138-62-2
- 1013-88-3
- 10138-94-0
- 10138-99-5
- 10-13-9
- 10139-02-3
- 1013907-68-0
- 101-39-3
- 10139-47-6
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View